Abstract
No one can deny that pain serves as an alarm. Sometimes pain informs us about severe abnormalities inside our body. However, pain (in particular, chronic pain) does not always have the role of an alarm. Pain itself can be harmful, as in the case of patients with chronic pain. Thus, we should reveal the mechanisms of pain and control it even though this is a challenging task. Pain is always subjective to individuals, and we have no definitive and objective evaluation methods for measuring pain. Indeed, pain is very complex and difficult to understand. In this chapter, in order to better understand the complex phenomenon of pain, I first discuss the definitions and classifications of pain. Second, I explain methods for evaluating pain, including the methods of potential objective evaluation. Lastly, I describe the relationship between pain and the central nervous system, especially with respect to cognitive function, emotion, and psychiatric illness. I expect that there will be a paradigm shift in pain treatment in the future; studies of the affective components of pain will greatly progress, and drug discovery research will specifically aim at reducing pain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Definition and Classification of Pain
The International Association for the Study of Pain endorses the following definition of pain: “an unpleasant sensory and emotional experience that is associated with actual or potential tissue damage or that is described in terms of such damage.”
An important point about this definition is that pain does not always come with injuries. Even when patients do not have any tissue damage, if they say that they feel pain, then pain exists for them. Pain is a complex phenomenon involving multiple factors (Fig. 6.1). It is difficult to scientifically approach to pain and even to central pain mechanisms. Nevertheless, it is possible to define and classify different kinds of pain in terms of time course, pathology and underlying mechanism. The definitions and classifications provide the first and necessary step toward understanding and treating pain.
6.1.1 Classification Based on Time Course
Time course is an important factor in understanding the origin of the conditions in which subjects suffer from pain. Acute pain has an alarm function, which is essential to survive. However, chronic pain does not always have biological significance. The causes of chronic pain are mainly in the brain , and thus pain itself is not so important for survival, except in cases of severe depression in which it could be fatal.
Acute pain is generally regarded as lasting for no more than a month, whereas chronic pain is as lasting longer than 6 months. Bonica (1977) has suggested that chronic pain is defined as persisting for longer than a month beyond the normal healing period or as being associated with a pathological process that causes continuous or recurrent pain over months to years.
Pain that lies somewhere between acute and chronic pain is called ‘subacute.’ As shown in Fig. 6.2, it is not always easy to distinguish subacute pain from the other two types of pain. Subacute pain may require almost the same treatment as chronic pain.
6.1.2 Classification Based on Pathology (Table 6.1, Fig. 6.3)
6.1.2.1 Nociceptive
Nociceptive pain is visceral or somatic, and results from normal diseases or damages. Pain receptors in the body are located in the connective tissues of the skin, the subcutaneous tissues, the fascias, the periosteum, the inner bone membranes, and the joint capsules. Stimulation of these receptors leads to a localized dull or sharp pain. Visceral pain receptors are located in most organs and their surrounding connective tissues. Visceral pain, when induced by a hollow organ damage, feels severely cramping or tingling, and sometimes becomes referred pain. Visceral pain, when caused by a damage to the deep connective tissue or other visceral capsule may feel as a sharp localized pain.
6.1.2.2 Neuropathic
Neuropathic pain is caused by injury or dysfunction in the central nervous system or peripheral nervous system rather than by stimulation of pain receptors. Its diagnosis is made by evaluating the disproportionate pain relative to tissue damage, signs of nerve damage (e.g., burning or tingling pain), and abnormal sensations, in neurological examinations. Peripheral nerve damages or dysfunction can cause neuropathic pain. More specifically, the typical causes of neuropathic pain are nerve compression (e.g., neuroma or tumors due to disc herniation) and various metabolic neuropathies. Mechanisms underlying neuropathic pain are probably varying. Central neuropathic pain syndrome is involved in the process of the reorganization of central somatosensory processing. Deafferentation pain is due to partial or complete interruption of the afferent nerve activity of the peripheral or central nervous systems. The afferent nerve activity conveys information from the distal to the proximal.
6.1.2.3 Central-Induced
Central-induced pain is caused by the central nervous system, mainly in patients with brainabnormalities, such as central neuropathic pain and some kinds of psychiatric disease s. Patients with depression usually have complaints of pain, and they may suffer low back pain, headache, and neck stiffness. When patients with chronic pain suffer for a long time, it is mostly central-induced and its causes are brain network abnormalities or abnormalities in some brain regions.
If subjective patient symptoms outweigh what is consistent with the findings of objective evaluation s, clinicians should suspect that the pain might not just be caused by a somatosensory problem but also be central-induced.
6.2 Classification Based on Region and Each Mechanism
6.2.1 Peripheral Mechanisms of Nociception (Fig. 6.4)
Mechanical, chemical, or thermal nociceptive stimulation activates nociceptors: they conduct pain signals through the primary neurons to the dorsal horns of the spinal cord. Once information conducted by neurotransmitters, neuropeptides, or else arrives in the spinal cord, they make synaptic contact with the secondary neurons. The secondary neurons immediately cross the spinal cord by passing under the central canal to form the spinothalamic tract. The spinothalamic tract is located in a ventrolateral position relative to the spinal cord. It conducts information to different regions of the ventrobasal and the centromedian complex in the somatosensory thalamus. The secondary neurons also make synaptic contacts in different regions of the brain stem, e.g., with the periaqueductal grey matter (PAG) and the nucleus raphe magnus.
The initial nociceptive stimulus is not the only factor that contributes to perceived pain. There are four different steps that occur through a series of chemical and electrical reactions: transduction, transmission, modulation, and perception. In other words, even when a noxious signal is transmitted to the brain, if the signal is modulated as a non-painful state, people do not feel pain. Conversely, as is the case of chronic pain patients, if there are some brain abnormalities, when a non-noxious signal is received in the brain, people may feel pain.
6.2.1.1 Pain Fibers
There are many types of receptors in the skin. The free nerve endings are mainly responsible for pain perception. When signals are received by the nerve endings, they are transferred through the nerve fibers. The nerve fibers are divided into three classes: A-beta, A-delta, and C fibers (Fig. 6.5).
A-beta fibers are mechanoreceptors with a very low response threshold, and they respond to slight touch. Under persistent pain conditions, central nervous system sensitization will occur and result in abnormal connectivity between A-beta fibers and other fibers. The afferent impulses of A-beta fibers can be recognized as pain (allodynia). A-beta fibers have characteristic features, e.g., large diameter (from 6 to 12 μm) and significant myelination. These two features contribute to the rapid conduction capability of A-beta fibers; they convey impulses at speeds of around 35–75 m/s. A-beta fibers also have a significant inhibitory role in transferring nociceptive information. Selective stimulation of these fibers will lead to the recruitment of inhibitory interneurons in the substantia gelatinosa of the dorsal horn of the spinal cord, and these inhibitory interneurons block the transfer of nociceptive information originating from the same segment of the spinal cord.
A-delta fibers transfer sharp pain. They are myelinated, and most of them are nociptive in that most A-delta fibers have the essential characteristics that are necessary to be considered nociceptive. Their diameters range from 1 to 5 μm, and their conduction speeds are between 5 and 30 m/s. They are responsible for first pain, i.e., brief and extremely localized pain felt at the onset of stimulation. A-delta fibers are not spontaneously active. They are divided into two groups: mechanical receptors and mechanothermal receptors (Treede et al. 1998). Mechanonociceptors make up 20 % of all cutaneous A-delta fibers (Besson and Chaouch 1987). They are receptive to certain kinds of stimulation, such as stings and pinching. Under normal circumstances, they do not respond to chemical or thermal stimulation that is less than 53 °C. Mechanothermal nociceptors respond to both mechanical and thermal stimuli, and sometimes to chemical stimuli as well. Twenty percent to fifty percent of A-delta nociceptors are regarded to be mechanothermal, and some of them respond to cold. The latency is lower for mechanical nociceptors than for mechanothermal nociceptors (1 s vs. 0.2 s). This fact suggests that it is mainly mechanothermal nociceptors that are responsible for first pain (Treede et al. 1998).
C fibers are unmyelinated. Their diameters range from 0.2 to 1.5 μm, and their conduction speeds are slow (between 0.5 and 2 m/s). They are responsible for second pain, i.e., later and more diffuse pain than first pain. They play an important role in intensifying of pain (Ringkamp and Meyer 2008). C fibers represent nearly three-quarters of all peripheral nerve fibers, and more than 90 % of them are nociceptors. C fibers are polymodal receptors, and respond to mechanical, chemical, or thermal stimuli. In particular, they are excited by intense stimuli from sharp objects. C fibers can also be involved in non-nociceptive sensations (e.g., pruritus (itch) (Stander et al. 2003)) and in non-nociceptive sensations with a strong emotional component (e.g., caress). A study of patients with complete deafferentation of somesthetic myelinated fibers has shown that the patients do not feel the touch of a hand when gently stroked, while they report a pleasant sensation (Olausson et al. 2002).
6.2.2 Central Mechanisms of Pain (Fig. 6.6)
The brain mechanisms of pain may be investigated with regard to two different aspects: the emotional aspect and the sensory aspect (or the affective component and the sensory component ). There are very complex mechanisms involved in human pain, and their interactions are also complex. Human functional brain imaging has shown that pain affects various brain regions as well as the so-called pain matrix (Baranauskas and Nistri 1998).
6.2.2.1 Somatosensory Cortex
The somatosensory cortex is responsible for the sensory-discriminative aspect of pain. The entire noxious signal is transmitted from the peripheral nerves to the brain through the thalamus. Sensory information is transmitted to the primary somatosensory cortex (S1) and the secondary somatosensory cortex (S2) through a site called the ‘ventral basal complex’ in the thalamus.
6.2.2.2 Amygdala
The amygdala is located inside the temporal lobe, and it has the role of integrating emotional and instinctive behavior. On the basis of sensory inputs, the amygdala determines whether its stimulation is comfortable or uncomfortable. It then causes negative emotion s, such as fear and anger. The amygdala retrieves memorized information in accordance with nociceptive information. After pain is experienced, the information it carries is evaluated as negative or affective discomfort. Then, when pain is experienced again, negative emotional behaviors, e.g., fear, anger, anxiety, freezing or struggle, or escape responses, are induced.
6.2.2.3 Insula
The insular cortex (or the insula) is located on the outer surface of the brain in the outer groove of the lower halves of the parietal lobe and in the temporal lobe. The insular cortex receives a major input from the thalamus and S2, and it is in contact with the amygdala. Thus, the insula cortex is regarded to be responsible for both the sensory aspect and the emotional aspect of pain. The insular cortex is important and useful in many ways. First, it is regarded to be responsible for integrating and processing higher cognitive and emotional information that is related to the general (healthy) condition. Second, the insular cortex is related to the autonomic nervous system to maintain homeostasis. Third, the insula cortex is the part of the circuit system that is involved in the aversion and avoidance of painful situations. Last, the insula cortex is responsible for the prediction of pain, and it is also involved in placebo analgesia.
6.2.2.4 Anterior Cingulate Cortex (ACC)
The cingulate cortex is the cortical tissue that surrounds the corpus callosum. It is divided into three parts: ACC, midcingulate cortex, and posterior cingulate cortex. The ACC receives signal inputs not only from the amygdala but also from the thalamus, the PAG, and the locus coeruleus. The cingulate cortex is the central region of the noradrenergic descending pain inhibitory system, and anatomically divided into four regions: executive, evaluative, cognitive, and emotional. The emotional region, which is located in the ventral area, receives signal input from the amygdala. The cognitive part, which is located in the dorsal area, projects the signal to the spinal cord, and it is also involved in the avoidance of pain. The ACC plays a role in controlling emotional arousal produced in the limbic system.
6.2.2.5 Prefrontal Cortex (PFC)
The PFC is considered one of the regions in the so-called pain matrix, and it is connected with both the insular cortex and the amygdala. It is involved in the emotional aspect of pain.
6.2.2.6 Orbitofrontal Cortex (OFC)
The OFC is located at the back of the retina, and connects the PFC and the limbic system. It is involved in cognitive processing like decision-making and in the emotion and reward system. It is connected with the amygdala, which, too, is involved in the emotional system.
6.2.3 Pain and Cognitive Function
Pain and cognitive functions correlate with each other because of pain perception in the brain (Fig. 6.7). Suffering from pain for a long time may result in irreversible changes in neuroplasticity and/or activation or suppression of several neuromediators. Such results restrict the brain’s functional capacities.
6.3 Study Tools of Experimental Pain
Substantial evidence has shown that human pain perception can be revealed in experimental pain studies. Experimental pain studies use different patterns of stimulations: tonic pain and phasic pain. The strategy is used not only for examining subjective pain cognition , but also in physiological and imaging studies. Tonic stimulation can also be applied with evoked pain in functional magnetic resonance imaging (MRI) studies. The methods of stimulation include thermal and pressure. Phasic stimulation can also be used with event-related potentials, which are used to analyze the conditions by analyzing the brain. The methods of stimulations are varied and include thermal, electrical, and laser. When relatively higher temperatures of thermal phasic stimulation are used, the A-delta fibers are stimulated, while lower temperature around 42 °C stimulate C fibers. Laser stimulation is known to activate A-delta stimulation. In addition, electrical stimulation can selectively stimulate the three types of A-delta, A-beta, and C fibers separately (Fig. 6.8).
6.3.1 Quantitative Sensory Testing (QST)
Negative and positive sensory phenomena are assessed by neurologic bedside examinations and Quantitative Sensory Testing. Quantitative Sensory Testing (QST) is a psychophysical test of sensory perception during the administration of stimuli with predetermined physical properties and following specific protocols. QST is able to capture and quantify stimulus-evoked negative and positive sensory phenomena and, as such, should become a standard, if not critical, tool in neuropathic pain research and practice.
When we stimulate the injured area, we may find abnormal sensations or allodynia, and we can then diagnose the nerve injury. When we stimulate intact areas, we may find potential abnormalities (in cases of healthy subjects) or secondary or systemic hypersensitivities (in cases of patients with chronic pain).
6.3.1.1 Stimulation Detection Threshold
The stimulation detection threshold is the lowest intensity of stimulation that is perceptible to a subject. If the subject has an abnormal or damaged nerve, her pain threshold and pain tolerance may be affected. Thus, before measuring parameters, the detection threshold should be measured. In general, we can measure perception thresholds using mechanical and thermal stimulation.
6.3.1.2 Pain Detection Threshold
The pain detection threshold is the lowest level of stimulation that a subject feels as pain. In psychophysics, the pain threshold is measured by identifying the lowest intensity of stimulation that produces pain.
6.3.1.3 Tolerance Threshold
The tolerance threshold is the highest level of stimulation of pain that a subject is able to endure.
6.3.2 Event-Related Potential (ERP)
Event-related potentials (ERPs) are defined as the waveforms recorded on the scalp as a series of positive and negative peaks, which vary in polarity, amplitude, and duration over time. Because ERP data are objective, it is a promising tool for diagnosing neuropathy or other neurogenic disorders.
As we do in pain research, we can use ERPs as measures of brain reactions to noxious stimuli. ERP patterns differ from the ways that are used to stimulate fibers: A-delta, A-beta, and C-fibers. ERP latency varies in these fibers. As shown in Fig. 6.8, we are able to obtain separate ERP data for three different nerve stimulations, but, in fact, some of them are stimulated at the same time.
The problem with ERPs is how to determine the method for analyzing data. If the amplitudes of ERPs are small, we sometimes need to perform repeated stimulations and use computer summation techniques. However, latencies may be different even when the same stimulation is given. If we sum ERP data with the different latencies, the amplitudes of the ERPs are relatively smaller than the actual amplitudes.
6.4 Pain Evaluation Tools
6.4.1 Questionnaires
Pain is always subjective. Today, there are many tools that are used to evaluate answers to questionnaires. The most common way is to use a visual analog scale (VAS). A VAS is a 10-cm bar with the left end indicating “no pain” and the right end “the worst pain imaginable.” Participants rate the level of their pain on this scale. The results show a normal distribution, and parametric analyses can be performed on them. We commonly use a VAS to evaluate the intensity and unpleasantness of pain (Fig. 6.9).
In order to evaluating patients with chronic pain, the McGill Pain Questionnaire was developed by Melzack and Torgerson (1971). The Short Form McGill Pain questionnaire (SF-MPQ-2) is commonly used in clinical situations (Melzack 1987). Recently, the SF-MPQ-2 is expanded and revised so as to evaluate patients with neuropathic pain symptoms (Dworkin et al. 2009). The SF-MPQ-2 can divide the patients’ painful conditions into four categories: intermittent, continuous, affective, and neuropathic.
The Pain Catastrophizing Scale is known to be effective for evaluating patients’ abnormal thinking (Sullivan et al. 1995) The catastrophizing scale is a 13-item self-report scale created by Sullivan et al. (1995), and used to measure pain catastrophizing. Items are rated on a scale from 0 to 4, and they have three different categories: Rumination, Magnification, and Helplessness. It has been hypothesized (Osman et al. 1997) that pain catastrophizing is related to various levels of pain, physical disabilities, and psychological disabilities in clinical and nonclinical populations.
The Hospital Anxiety and Depression scale (HAD) is effective for evaluating depression and anxiety with physical symptoms (Zigmond and Snaith 1983). This scale consists of seven questions for anxiety and seven questions for depression.
6.4.2 The Amount of Pain as a Number by Electrical Stimulation
When patients evaluate their pain with a VAS, it is sometimes difficult for them to evaluate the effects of treatment. PainVision (Nipro Corporation, Osaka, Japan) is a pain-evaluating tool (Fig. 6.10). The machine applies gradually increasing electrical stimulation to A-beta fibers. When the patients feel the same pain as the electrical stimulation is meant to give, the patients inform us of the amount of the pain by pushing a button. As far as people can use this machine precisely, it can quantify the pain as a number. This is a breakthrough of pain treatment. However, it is not an objective tool, because the machine requires pushing the button intentionally; but if the patients understand how to use this machine, the data would be objective.
6.5 Pain and Emotion
6.5.1 What Is Emotion?
The definition of emotion is different in different academic fields, whereas emotion may be defined in a simple way. In his book Psychology, Schacter et al. (2011) defines emotion as “a positive or negative experience that is associated with a particular pattern of physiological activity”.
Emotions induce autonomic responses, such as hypertension, tachycardia, endocrinologic changes, and stress hormone responses. As shown in Fig. 6.11, kinds of emotions can be visualized with a hierarchical structure. It is divided into two broad categories of pleasant and unpleasant emotions, dominating smaller sub-categories of emotions.
6.5.2 Pain and Emotion
Felt pain intensity may vary from person to person, even when the amount of stimulation is the same. This is because emotion can modulate the perception of pain. There have been many studies of pain and emotion in various experimental and clinical situations (Table 6.2).
6.5.2.1 Anxiety and Pain
When one has anxiety about pain, one feels more pain. It is difficult to distinguish anxiety about pain from fear of pain. In other words, it is difficult to determine whether fear or anxiety reduces a pain threshold. It has been reported of surgical patients that when they are anxious, they experience more severe pain (Lautenbacher et al. 2010). It also has been reported that 7–28.8 % of chronic pain patients have comorbid anxiety disorders (Asmundson and Katz 2009). In an animal model of chronic pain, pain induces anxiety-like behavior (Narita et al. 2006; Suzuki et al. 2007). It also has been reported that it requires fewer amount of drugs to reverse anxiety-like behavior than to reverse pain-related behavior (Munro et al. 2007). These results suggest that anxiety and pain may be based on common mechanisms.
6.5.2.2 Fear and Pain
There are three stages of fear. At the first stage, we interpret stimulation as a threat. At the second stage, we strongly feel that the interpretation is correct, and, at the third stage, we exhibit defensive behaviors (Rachman and Hodgson 1974). Fear and anxiety interact with each other, and both have negative impacts on pain. In particular, for musculoskeletal pain, there is an excellent model called the “Fear-Avoidance Model” (Leeuw et al. 2007). The model explains how a disease or trauma-caused pain fails to become chronic.
In an experimental study of the relation of human pain to anxiety and fear, the pain threshold is increased with fear but decreased with anxiety (Rhudy and Meagher 2000). It is difficult to distinguish anxiety and fear as both may influence pain intensity. In an animal experiment of fear, defensive behaviors appear when an animal only sees a fearful scene; moreover, defensive behaviors are suppressed by experimentally restricting the role of the ACC (Jeon et al. 2010). When observing scary scenes, animals feel anxiety. Animal behaviors are controlled by the common brain region that regulates pain and fear. Another interesting point is that fear and anxiety interact with each other in animals as well.
6.5.2.3 Attention and Pain
In general, humans tend to forget pain when they concentrate on something else. However, patients with chronic pain feel more pain than it is predicted, because their attention is drawn to pain. In other words, the problem of chronic pain is the attention being directed toward it. When subjects with experimental pain are asked to concentrate on a task, a functional brain imaging study shows, they feel less pain than they do in the no-task condition. When they feel pain, the OFC and ACC (the areas related to emotion) are activated. This suggest that pain is reduced through the emotional aspect of pain (Bantick et al. 2002). In our recent experimental pain study, we assign a cognitive, working-memory task to subjects. The high performance subjects do not reduce their pain intensity when they concentrate on the task. By contrast, the low-performance subjects reduce their pain intensity (Nakae et al. 2013). The effects of attention to pain may be subject to individual differences, at least in normal subjects.
6.5.2.4 Social Isolation and Pain
It has been suggested in animal experiments that social isolation affects brain development and is involved at the onset of mental illness (Fone and Porkess 2008). An animal experiment shows that animal’s heat pain threshold decreases after being exposed to the stress of social isolation (Loeser 2000). At least, it seems certain that the stress of social isolation may influence pain in some sense.
6.5.2.5 Anger and Pain
Pain clinicians may see a patient who is angry with someone; in some cases, the patient may be a victim of an unfortunate accident. How does anger influence pain? Substantial evidence has revealed that the expression of anger affects the intensity of pain. One possible mechanism to explain this is the deficiency of the endogenous opioid system with regard to reduction of pain. In a functional brain imaging study, pain and anger both activate the rostral ACC, the OFC, the insular cortex, and the amygdala (Bruehl et al. 2009).
6.5.2.6 Sadness and Pain
Sadness and depression correlate well clinically. An experimental pain study shows that when participants see sad face pictures, they feel more pain, and the increase in pain activates the ACC (Yoshino et al. 2010).
6.5.2.7 Positive Emotion and Pain
How do humans feel experimental pain when they see a pleasant picture? A study has examined this question and obtained the results that when subjects see a pleasant picture, they feel less pain (Rhudy et al. 2010).
In addition, there is a study that analyzes the effects of pleasant emotion by distinguishing them from the effects of attention. The effects of pleasant emotion alleviate pain not just by distracting subjects to focus on comfortable stimuli. Pleasant emotion itself has the effect of pain relief (Villemure and Bushnell 2009). Positive emotion has been shown to influence coping efficacy and social functioning in patients with chronic pain (Park and Sonty 2010).
6.6 Mental Disorders as Pathological Models of Pain (Table 6.3)
The prevalence of mental illness has been shown to be significantly higher in patients with chronic pain (Turk et al. 2010). There is evidence that if patients’ functions are decreased by pain, they are more likely than otherwise to have comorbid mental illness (Chou and Shekelle 2010). The comorbidity of chronic pain and depression is especially high, and pain symptoms can be a main symptom of depression. It has been revealed in an animal study that when animals feel pain for a long time, they become depressive (Suzuki et al. 2007).
Pain is perceived in the brain. Thus, it is particularly necessary to consider the emotional aspect of pain in relation to the brain. There are some psychiatric illnesses that change pain sensitivity, and this fact suggests that the brain mechanisms of pain perception are involved in the mechanisms of psychiatric illnesses. If this is the case, then by studying alterations in pain sensitivity in psychiatric disorders, clues may appear that elucidate the relationship between pain sensitivity and emotional aspects of pain (Barlow 2001; Klossika et al. 2006).
6.6.1 Depression and Pain
Depression involves the symptoms of depressive mood and loss of appetite, and it remarkably decreases the entire brain function. Depression has a high incidence, and it is caused by genetic and environmental factors. Patients with depression have dysfunctional thoughts, emotions, and motivations. While one can be depressed due to a long exposure to pain, one of the typical symptoms of depression is pain. A positive correlation between depression and chronic post-surgical pain is reported to exist, and the correlation indicates that postoperative pain prolongs depressive states (Hinrichs-Rocker et al. 2009). A factor that is considered responsible for depression is dysfunction in noradrenergic and serotonergic systems. Serotonergic and noradrenergic systems are important systems that contribute to the descending pain inhibitory system in the spinal cord; dysfunction in serotonergic and noradrenergic systems may affect sensitivity to pain (Kundermann et al. 2009).
6.6.2 Borderline Personality Disorder and Pain
Borderline personality disorder is a mental illness that is known to be caused by emotional and cognitive abnormalities and characterized by frequently repeated self-injurious behavior. The main symptoms of borderline personality disorder are instability, impulsive actions, and feelings of depression accompanied by feelings of emptiness. In patients with borderline personality disorder, experimental pain thresholds are higher than normal, and there are no abnormal sensory-discriminative aspects. A functional brain imaging study on experimental heat pain has shown that patients with borderline personality disorder exhibit strong activation in the dorsolateral prefrontal cortex and weak activation in the amygdala and ACC. For this reason, the suppression of emotional aspects of pain is regarded as a cause of the repetition of self-injurious behavior (Schmahl et al. 2004).
6.6.3 Schizophrenia and Pain
Schizophrenia is a syndrome the main symptoms of which are delusions and hallucinations. Schizophrenic patients show decompensation when facing adverse life events. Substantial evidence has shown that schizophrenic patients have less sensitivity to pain. For example, it is reported that patients with schizophrenia do not recognize pain as an alarm signal (Murakami et al. 2010). The mechanisms underlying the decrease in pain sensitivity are unclear, but a phenomenon linked with attention dysfunction and impaired cognitive function in schizophrenia, as some have reported it, is relevant. There are two groups of schizophrenic patients: those with an extremely high pain threshold and those with a normal one. Pain thresholds do not correlate with the amount of antipsychotic medication and disease severity, according to our latest knowledge. A previous study has ruled out the possibility that high pain thresholds in schizophrenia are caused only by antipsychotic drugs (Potvin and Marchand 2008).
Even though some schizophrenic patients have normal sensitivity to experimental pain, it tends to feel less unpleasant to them, due to an abnormality of the emotional aspect of pain. In a recent functional MRI study, presenting experimental heat pain to schizophrenic patients, as compared to healthy volunteers, has resulted in less activation of the insular cortex and the other areas that have roles for the emotional aspect of pain (de la Fuente-Sandoval et al. 2010). In addition, patients have exhibited many other abnormalities in the insula (Wylle and Tregellas 2010). An animal model has been developed in schizophrenia pain research, with an expectation to clarify the mechanisms underlying the abnormal sensitivity to pain.
6.6.4 Pervasive Developmental Disorder and Pain
Developmental disorders are classified in complex ways. Autism, the most popular developmental disorder, exhibits three disorders: social disorders, communication disorders, and imagination disorders; behavioral disorders arise from these disorders. The communication impairments are mild in autism. Pervasive developmental disorders are those with relatively high performance. In epidemiological studies, the frequency of autism is reported to be as high as 1.7 %. Patients with autism spectrum disorders are known to have significant discomfort when they are touched; their sensory systems are generally sensitive, and more specifically, tactual and audiovisual systems are sensitive. Patients with autism have been subject to confusing reports on their sensitivity to pain; they have been reported to show hypersensitivity, to have normal sensation, and to have low sensitivity. It has been suggested that abnormal C-fiber pathways are explanatory of their sensitivity to pain, but the details of the explanation remains unrevealed. Mirror neurons are involved in the ability to sympathize with others, and a dysfunction in minor neurons may be responsible for autism. This explains the fact that autistic patients cannot empathize with the pain of others. Autism is also a syndrome, and its pathogenesis is influenced by a wide variety of biological backgrounds. It is possible that there are various types of these disorders of pain, such as hypersensitivity to pain due to abnormal neurotransmission or lower sensitivity to pain due to abnormal processing of the emotional aspects of pain.
6.6.5 Posttraumatic Stress Disorder (PTSD) and Pain
Posttraumatic stress disorder (PTSD) is a type of anxiety disorder. This disorder has been known since the 1970s as a syndrome associated with Vietnam veterans and rape victims. PTSD is caused after a serious, typically traumatic, experience, and it has three clusters of mixed symptoms: (1) symptoms of re-experiencing, e.g., nightmare flashbacks; (2) avoidance of trauma reminders and mental paralysis; and (3) hyperarousal, comorbid depression, alcohol and drug dependence, and anxiety. Generalized anxiety disorder is often comorbid with chronic pain (Asmundson and Katz 2009). Many patients with chronic pain may have comorbid PTSD. In imaging studies of patients with PTSD, the amygdala and the ACC are found to be hyperfunctional. In an experimental pain study of patients with PTSD, they feel more pain, while their sensitivity to experimental pain stimulation has declined. Thus, patients may fail to process the emotional aspects of pain normally (Defrin et al. 2008; Kraus et al. 2009).
6.6.6 Eating Disorder and Pain
An eating disorder is a disease that causes abnormal eating behavior. Patients with bulimia nervosa show overeating, while patients with anorexia nervosa show restricted eating. A variety of factors, such as genetic and social backgrounds, stress, and physical and mental changes during adolescence, are considered to be the causes of eating disorders. Children and young women are prone to eating disorders. It is reported that eating disorders change the functions of the ACC. There have been a few reports on the pain sensitivity of patients with eating disorders, and according to one of them, their pain sensitivity is reduced (Florin et al. 1988). The causes of the reduction of pain sensitivity is taken not to be a peripheral problem but to be a cognitive perceptual distortion (Klossika et al. 2006).
6.7 Conclusion
The conventional approach to pain treatment has been preoccupied with eliminating the cause of pain, because pain is regarded as a danger signal. However, the mechanisms of pain are more complex than has been imagined, and there is currently no pain relief drug that works for all kinds of pain. The current diagnostic system is insufficient to treat all patients suffering from pain. Currently, we have not established objective evaluation systems that use blood tests or physiological tests for pain. Furthermore, there is no system to evaluate the emotional aspects of pain. I expect that studies of the affective component of pain will greatly progress and advance also with regard to the affective component of pain, and drug discovery research will aim specifically at reducing pain suffering, resulting in a paradigm shift in pain treatment.
References
Abram, S.E., Haddox, J.D.: The Pain Clinic Manual, 2nd edn. Lippincott, Williams, and Wilkins, Philadelphia (2000)
Asmundson, G.J., Katz, J.: Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress. Anxiety. 26, 888–901 (2009)
Bantick, S.J., Wise, R.G., Ploghaus, A., Clare, S., Smith, S.M., Tracey, I.: Imaging how attention modulates pain in humans using functional MRI. Brain 125, 310–319 (2002)
Baranauskas, G., Nistri, A.: Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog. Neurobiol. 54, 349–365 (1998)
Barlow, D.H.: Clinical Handbook of Psychological Disorders: A Step-by-Step Treatment Manual, 3rd edn. The Guilford Press, New York (2001)
Beecher, H.K.: Pain in men wounded in battle. Ann. Surg. 123, 96–105 (1946)
Besson, J.M., Chaouch, A.: Peripheral and spinal mechanisms of nociception. Physiol. Rev. 67, 67–186 (1987)
Bonica, J.J.: Neurophysiologic and pathologic aspects of acute and chronic pain. Arch. Surg. 112, 750–761 (1977)
Bruehl, S., Burns, J.W., Chung, O.Y., Chont, M.: Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci. Biobehav. Rev. 33, 475–491 (2009)
Chou, R., Shekelle, P.: Will this patient develop persistent disabling low back pain? JAMA 303, 1295–1302 (2010)
de la Fuente-Sandoval, C., Favila, R., Gomez-Martin, D., Pellicer, F., Graff-Guerrero, A.: Functional magnetic resonance imaging response to experimental pain in drug-free patients with schizophrenia. Psychiatry Res. 183, 99–104 (2010)
Defrin, R., Ginzburg, K., Solomon, Z., et al.: Quantitative testing of pain perception in subjects with PTSD – implications for the mechanism of the coexistence between PTSD and chronic pain. Pain 138, 450–459 (2008)
Dworkin, R.H., Turk, D.C., Revicki, D.A., Harding, G., Coyne, K.S., Peirce-Sandner, S., Bhagwat, D., Everton, D., Burke, L.B., Cowan, P., Farrar, J.T., Hertz, S., Max, M.B., Rappaport, B.A., Melzack, R.: Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 144, 35–42 (2009)
Florin, I., Franzen, U., Meier, M., Schneider, S.: Pressure sensitivity in bulimic women; a contribution to research in body image distortion. J. Psychosom. Res. 32, 439–444 (1988)
Fone, K.C., Porkess, M.V.: Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 32, 1087–1102 (2008)
Franek, M., Vaculin, S., Yamamotova, A., Stastny, F., Bubenikova-Valesova, V., Rokyta, R.: Pain perception in neurodevelopmental animal models of schizophrenia. Physiol. Res. 59, 811–819 (2010)
Hinrichs-Rocker, A., Schultz, K., Jarvinen, I., Lefering, R., Simanski, C., Neugebauer, E.A.: Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur. J. Pain 13, 719–730 (2009)
Hirofumi, M., Yuji, S.: Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of pain catastrophizing scale. Jpn. J. Psychosom. Med. 47, 95–102 (2007)
Jeon, D., Kim, S., Chetana, M., et al.: Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010)
Klossika, I., Flor, H., Kamping, S., et al.: Emotional modulation of pain: a clinical perspective. Pain 124, 264–268 (2006)
Kraus, A., Geuze, E., Schmahl, C., et al.: Differentiation of pain ratings in combat-related posttraumatic stress disorder. Pain 143, 179–185 (2009)
Kundermann, B., Hemmeter-Spernal, J., Strate, P., et al.: Pain sensitivity in major depression and its relationship to central serotoninergic function as reflected by the neuroendocrine response to clomipramine. J. Psychiatr. Res. 43, 1253–1261 (2009)
Lautenbacher, S., Huber, C., Schofer, D., et al.: Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: a comparison with other psychological and physiological predictors. Pain 151, 722–731 (2010)
Leeuw, M., Goossens, M.E., Linton, S.J., Crombez, G., Boersma, K., Vlaeyen, J.W.: The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J. Behav. Med. 30, 77–94 (2007)
Loeser, J.D.: Perspectives on pain. In: Padgham, C., Hedges, A., Turner, P. (eds.) Clinical Pharmacology and Therapeutics, p. 314. University Press, Baltimore (1980)
Loeser, J.D.: Pain and suffering. Clin. J. Pain 16, S2–S6 (2000)
Melzack, R.: The short-form McGill pain questionnaire. Pain 30, 191–197 (1987)
Melzack, R., Torgerson, W.S.: On the language of pain. Anesthesiology 34, 50–59 (1971)
Munro, G., Erichsen, H.K., Mirza, N.R.: Pharamacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology 53, 609–618 (2007)
Murakami, H., Tamasawa, N., Yamashita, M., et al.: Altered pain perception in schizophrenia. Lancet 375, 864 (2010)
Nakae, A., Endo, K., Adachi, T., Ikeda, T., Hagihira, S., Mashimo, T., et al.: The influence of working memory capacity on experimental heat pain. J. Pain 14(10), 1088–1096 (2013)
Narita, M., Kaneko, C., Miyoshi, K., et al.: Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharamacology 31, 739–750 (2006)
Olausson, H., Lamarre, Y., Backlund, H., Morin, C., Wallin, B.G., Starck, G., et al.: Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 5, 900–904 (2002)
Osman, A., Barrios, F.X., Kopper, B.A., Hauptmann, W., Jones, J., O’Neill, E.: Factor structure, reliability, and validity of the pain catastrophizing scale. J. Behav. Med. 20, 589–605 (1997)
Park, S.H., Sonty, N.: Positive affect mediates the relationship between pain-related coping efficacy and interference in social functioning. J. Pain 11, 1267–1273 (2010)
Potvin, S., Marchand, S.: Hypoalgesia in schizophrenia is independent of antipsychotic drugs: a systematic quantitative review of experimental studies. Pain 138, 70–78 (2008)
Rachman, S., Hodgson, R.: I. Synchrony and desynchrony in fear and avoidance. Behav. Res. Ther. 12, 311–318 (1974)
Rhudy, J.L., Meagher, M.W.: Fear and anxiety: divergent effects on human pain thresholds. Pain 84, 65–75 (2000)
Rhudy, J.L., Bartley, E.J., Williams, A.E.: Habituation, sensitization, and emotional valence modulation of pain responses. Pain 148, 320–327 (2010)
Ringkamp, M., Meyer, R.A.: Physiology of nociceptors. In: Basbaum, A.I., Bushnell, C. (eds.) Science of Pain, pp. 105–107. Amsterdam, Elsevier (2008)
Schacter, D.L., Gilbert, D.T., Wegner, D.M.: Psychology, p. 310. Worth Publishers, New York (2011)
Schmahl, C., Greffrath, W., Baumgartner, U., et al.: Differential nociceptive deficits in patients with borderline personality disorder and self-injurious behavior: laser-evoked potentials, spatial discrimination of noxious stimuli, and pain ratings. Pain 110, 470–479 (2004)
Stander, S., Steinhoff, M., Schmelz, M., Weisshaar, E., Metze, D., Luger, T.: Neurophysiology of pruritus: cutaneous elicitation of itch. Arch. Dermatol. 139, 1463–1470 (2003)
Sullivan, M.J.L., Bishop, S.R., Pivik, J.: The pain catastrophizing scale: development and validation. Psychol. Assess. 7, 524–532 (1995)
Suzuki, T., Amata, M., Sakaue, G., et al.: Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth. Analg. 104, 1570–1577 (2007)
Treede, R.D., Meyer, R.A., Campbell, J.N.: Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J. Neurophysiol. 80, 1082–1093 (1998)
Turk, D.C., Audette, J., Levy, R.M., Mackey, S.C., Stanos, S.: Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin. Proc. 85, S42–S50 (2010)
Villemure, C., Bushnell, M.C.: Mood influences supraspinal pain processing separately from attention. J. Neurosci. 29, 705–715 (2009)
Wylle, K.P., Tregellas, J.R.: The role of the insula in schizophrenia. Schizophr. Res. 123, 93–104 (2010)
Yoshino, A., Okamoto, Y., Onoda, K., et al.: Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: an fMRI study. NeuroImage 50, 1194–1201 (2010)
Zigmond, A.S., Snaith, R.P.: The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Exercises
Exercises
-
1.
The figures below show the waveforms of evoked potentials .
What are the latency and amplitude of each evoked potential?
What fibers are stimulated?
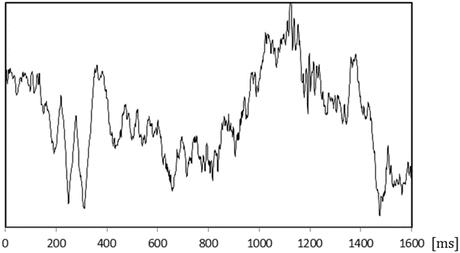
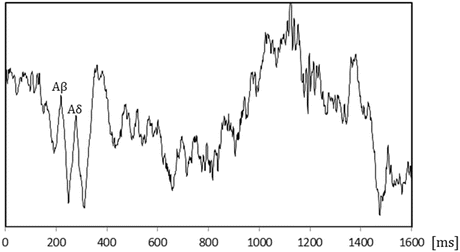
-
2.
State the correlation between pain perception and cognitive impairment.
-
3.
State the correlation between emotion and pain perception.
-
4.
What do you think about the next generation of analgesics?
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Nakae, A. (2016). Mechanisms of Pain. In: Kasaki, M., Ishiguro, H., Asada, M., Osaka, M., Fujikado, T. (eds) Cognitive Neuroscience Robotics B. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54598-9_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54598-9_6
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54597-2
Online ISBN: 978-4-431-54598-9
eBook Packages: EngineeringEngineering (R0)

















