Abstract
The neuroendocrine system is composed of the hypothalamus and pituitary gland; the nervous system controls the release of hormones from the pituitary gland. The secretory activity of the endocrine glands was formerly thought to be outside the direct control of the nervous system. Since the 1950s, the brain has been recognized as the center of the system controlling and regulating the physiological processes of the human body, and, currently, the neuroendocrine–immune network is proposed to mediate a bidirectional interaction between the neuroendocrine and immune systems. This network is responsible for maintaining homeostasis and orchestrating the essential responses to inflammation or injury through a tightly regulated network of neuropeptides, hormones, cytokines, and chemokines. Further investigation into neuroendocrine–immune crosstalk could shed light on the pathogenesis of diverse diseases, such as inflammatory and central nervous system diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The neuroendocrine system is composed of the hypothalamus and pituitary gland; the nervous system controls the release of hormones from the pituitary gland. The secretory activity of the endocrine glands was formerly thought to be outside the direct control of the nervous system. Since the 1950s, the brain has been recognized as the center of the system controlling and regulating the physiological processes of the human body, and, currently, the neuroendocrine–immune network is proposed to mediate a bidirectional interaction between the neuroendocrine and immune systems. This network is responsible for maintaining homeostasis and orchestrating the essential responses to inflammation or injury through a tightly regulated network of neuropeptides, hormones, cytokines, and chemokines.
2 Background
Neuroendocrinology is the study of the interactions between the nervous and endocrine systems, which regulate the physiological processes of the human body. Historically, the control of biological functions was thought to be independently controlled by the nervous and endocrine systems. The neuroendocrine system is composed of the hypothalamus and pituitary gland, with the nervous system controlling the release of hormones from the pituitary gland.
3 History of Neuroendocrinology
The concept of neurosecretion was first proposed by Ernst Scharrer and Wolfgang Bargmann in the 1950s [1, 2]. Scharrer and his wife, Berta, observed that certain neurons in the hypothalamus of vertebrates, as well as in invertebrates without a hypothalamus, secreted cytoplasmic granules that they thought to be hormones. Bargmann demonstrated the histology of the neurohypophysis and the nerve tracts that extended from the paraventricular and supraoptic nuclei to the neural lobe of the pituitary by using Gomori’s chrome alum hematoxylin–phloxine stain. Before their proposals, many endocrinologists generally ignored the observation that certain specialized nerve cells could secrete hormones, resulting in the belief that the nervous and endocrine systems were independent. Intensive studies on neurosecretory cells revealed that there are very close links between these two systems, which mainly control systemic hormonal functions. Geoffrey Harris, who is considered the “father” of neuroendocrinology, conducted extensive work on the hypothalamic control of pituitary function and demonstrated that the mammalian anterior pituitary gland is regulated by factors secreted into the hypothalamohypophysial portal circulation by hypothalamic neurons [3]. Since then, the brain has been recognized as the center of the system controlling and regulating the physiological processes of the human body.
4 Anatomy of the Neuroendocrine System
4.1 Hypothalamus
The hypothalamus, which is located anteroinferior to the thalamus and superior to the pituitary gland, is called the “master nerve control center” and encapsulates the ventral portion of the third ventricle. The anterior boundary is the anterior commissure and lamina terminalis, the mammillary bodies comprise the posterior boundary, and the superior boundary is the hypothalamic sulcus, which is the rostral continuation of the sulcus limitans. The hypothalamus is divided into four major groups of nuclei according to their location in the hypothalamic zones and regions. The anterior thalamic nuclei consist of the supraoptic and paraventricular nuclei, which are responsible for regulating blood pressure and fluid balance via the secretion of certain hormones, and the suprachiasmatic nucleus, which is involved in circadian timing. The medial thalamic nuclei consist of the ventromedial and arcuate nuclei, which are involved in the regulation of feeding behavior and body weight. The lateral nucleus includes the dorsomedial nucleus, which is involved in the control of food intake and water drinking behaviors. The posterior nucleus is the mammillary nucleus, which is involved in the control of emotional expression and the autonomic nervous system [4].
4.2 Pituitary Gland
The pituitary gland, weighing approximately 0.6 g in human adults, is called the “master gland” and is located within a recess in the median part of the middle cranial fossa of the sphenoid bone. This gland comprises two major components, the anterior lobe (adenohypophysis) and the posterior lobe (neurohypophysis). The anterior boundary is the optic chiasm, the posterior boundary is the dorsum sellae, which is continuous with the clivus, and the superior boundary is the hypothalamus and the floor of the third ventricle (Fig. 3.1). Other important boundaries of the pituitary gland include the cavernous sinus, laterally, which contains the internal carotid artery surrounded by sympathetic fibers, and cranial nerves III, IV, V (ophthalmic and maxillary branches), and VI. The optic chiasm is located anterosuperior to the gland and is separated from it by the cerebrospinal fluid-filled suprasellar cistern. The dural roof of the pituitary, the diaphragma sellae, covers the optic chiasm. The pituitary gland is connected to the hypothalamus through the pituitary stalk and controls homeostatic and endocrine functions.
Magnetic resonance image (MRI) (Lt) and corresponding schematic illustration (Rt) of the human hypothalamus and pituitary gland seen in the sagittal orientation. Note: The high intensity of the posterior pituitary (the so-called bright spot) in the MRI (shown by arrow), which is attributed to the normal representation of the functional storage of vasopressin in the posterior pituitary lobe
4.3 Hypothalamus and Its Connection to the Pituitary Gland
The blood supply of the pituitary stalk comes from the superior hypophyseal artery, whereas the blood supply to the neurohypophysis comes from the inferior hypophyseal artery. The cell bodies of hypothalamic secretory neurons are localized in areas protected by the blood–brain barrier (BBB), whereas their axon terminals are localized in the median eminence, which lacks a BBB. This implies a complex barrier system, allowing neurons of the central nervous system to secrete hormones into the bloodstream via the hypophyseal portal system without making the BBB leaky. The release of hypothalamic hormones promotes the secretion of anterior pituitary hormones that, in turn, regulate tissue function.
5 Hypothalamic Control of Pituitary Hormone
5.1 Anterior Pituitary
The anterior pituitary, an endocrine gland controlled by the hypothalamus, produces and secretes hormones. The anterior lobe contains glandular cells that secrete hormones directly into the bloodstream. This lobe is controlled by the hypothalamus through the vascular portal system. Hypothalamic hormones that are produced by the supraoptic, paraventricular, and arcuate nuclei of the hypothalamus control anterior pituitary hormone secretions. Neurosecretory cells send their axons into the tuberoinfundibular tract and terminate on the capillary bed of the superior hypophyseal artery and flow together into the hypophyseal portal veins. These veins drop through the infundibular stalk and form a second capillary plexus, finally connecting with the secretory cells of the anterior pituitary. The anterior pituitary secretes its hormones into the capillary net, which drains through the cavernous sinus and internal jugular vein into the systemic circulation to transport those hormones to their peripheral target tissues. The anterior pituitary is responsible for the production of six hormones, which are regulated by the hypothalamus.
-
(a)
Growth hormone
Growth hormone (GH) is under the dual regulation of two peptides, growth hormone-releasing hormone (GHRH) and somatostatin (growth hormone release inhibiting factor). Release of GH is determined by the balance of GHRH and somatostatin, which is controlled by stimulators (slow-wave sleep, exercise, nutrition) and inhibitors (hyperglycemia and free fatty acids). Growth hormone is necessary for cells to grow in size, an increase in protein synthesis, promotion of lipolysis, reduction of liver uptake of glucose, stimulation of the immune system, and more. With advancing age, there is a decline in GH secretion, which results in a reduction in insulin-like growth factor-1 (IGF-1) production in the liver, chondrocytes, kidney, muscle, and other tissues. The longer positive feedback loop, involving IGF-1 regulation at the hypothalamus, stimulates the secretion of growth hormone by the pituitary; a shorter negative feedback loop, demonstrated to involve direct IGF-1 action on the pituitary, leads to downregulation of GH secretion. Similar feedback loop systems exist for other major endocrine hormones. Dopamine agonist and alpha-2 adrenergic agonist are pharmacological stimulants of GH secretion, whereas beta-adrenergic agonists increase somatostatin release, inhibiting GH secretion.
-
(b)
Thyroid-stimulating hormone
Thyroid-stimulating hormone (TSH) release is controlled by thyrotropin-releasing hormone (TRH), which has a stimulant effect, and somatostatin and dopamine, which have inhibitory effects on its release. Thyroid-stimulating hormone is necessary to stimulate the production of thyroid hormone, iodine absorption by the thyroid gland, and thyroxine and triiodothyronine (T3) synthesis and release from the thyroid gland. Thyroxine and T3 inhibit TSH production and release at the level of the pituitary (direct long loop) and inhibit the release of TRH at the level of the hypothalamus (indirect long loop).
-
(c)
Adrenocorticotropic hormone
Adrenocorticotropic hormone (ACTH) is secreted from corticotrophs in response to bodily stress and circadian rhythm by corticotropin-releasing hormone (CRH) released by the hypothalamus. Circulating ACTH stimulates cortisol production in the adrenal glands. The secreted cortisol causes negative feedback on the hypothalamus and pituitary to inhibit further CRH/ACTH release. Stimulation of corticosteroid and androgen synthesis and release from adrenocortical cells requires ACTH.
-
(d)
Prolactin
Prolactin release is inhibited by dopamine and stimulated by TRH and vasoactive intestinal polypeptide. In contrast to other pituitary hormones, the hypothalamus strongly suppresses prolactin secretion from the pituitary. Prolactin is necessary for lactation, stimulation of milk synthesis, and its release from the mammary glands and is a mediator of sexual gratification.
-
(e)
Luteinizing hormone and follicle-stimulating hormone
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) control the gonads in males and females. In females, LH and FSH stimulate the ovaries to produce steroids, producing estradiol during the follicular phase and progesterone during the luteal phase. In addition, LH and FSH surge at midcycle, triggering ovulation; LH turns the follicle into the corpus luteum by triggering ovulation. In males, LH stimulates testosterone production from Leydig cells. Follicle-stimulating hormone stimulates Sertoli cells to produce inhibin, which acts in a negative feedback fashion to regulate FSH secretion and enhance the production of androgen-binding protein by Sertoli cells.
5.2 Posterior Pituitary
The posterior pituitary is called the neurohypophysis, which is a collection of axonal projections from the hypothalamus through the infundibulum and into the posterior pituitary. This simple arrangement is completely different from that of the system in the anterior pituitary. The posterior pituitary hormones are transported down the axons, from the magnocellular neurons in the supraoptic and paraventricular nuclei through the infundibulum to the neurohypophysis, where they are secreted into the blood circulation. The posterior pituitary is responsible for the production of two hormones, oxytocin and vasopressin.
-
(a)
Oxytocin
During lactation, oxytocin stimulates the myoepithelial cells of the mammary glands and causes milk letdown into the duct system. At parturition, oxytocin stimulates and enhances the contraction of the uterine myometrium for labor induction. An infant suckling at the breast and the stretching of the lower uterus and cervix by the pressure induced by the presence of the fetus are relayed by spinal nerves to the hypothalamus to cause the release of oxytocin.
-
(b)
Vasopressin
Vasopressin is known as an antidiuretic hormone, and is necessary for an increase in water permeability in the distal convoluted tubule and collecting ducts of kidney nephrons. This increase in water permeability promotes water reabsorption and increased blood volume. Vasopressin secretion is controlled by osmotic and nonosmotic stimulation by pathways that are anatomically separated. In osmotic stimulation, osmoreceptors are present in the anterior thalamic region, which lies outside the BBB. There is a positive relationship between plasma osmolality and circulating vasopressin concentrations, with vasopressin secretion being suppressed at levels below 280 mOSm/kg. In nonosmotic stimulation, both high-pressure (aortic and carotid) and low-pressure (left atrial) receptors function through parasympathetic pathways to provide for vasopressin release. Such pathways are activated in response to acute systemic hemodynamic changes, decreases in blood pressure, and a reduction in left atrial pressure.
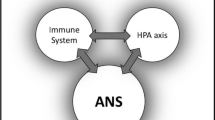
6 Neuroendocrine–Immune Network
The concept of the neuroendocrine–immune network has been proposed as a bidirectional interaction between the neuroendocrine and immune systems. This network is responsible for maintaining homeostasis and for orchestrating the essential responses to inflammation or injury through a closely regulated network of neuropeptides, hormones, cytokines, and chemokines.
That the immune system modulates brain activity, including body temperature, sleep, and feeding behavior, is well established [5]. For instance, interleukin 1 (IL-1) alerts the hypothalamus that there is “danger” in the periphery. This cytokine activates a febrile response through neurons in the preoptic area of the anterior hypothalamus. Considerable evidence suggests that IL-1 passes through the BBB and induces cyclooxygenase-2 and microsomal prostaglandin E synthase-1 activity. Interleukin-1β and prostaglandin E2, as proinflammatory stimuli, are secreted into the brain parenchyma and stimulate a temperature increase induced through the activity of preoptic neurons in the hypothalamus [6–8].
In addition, patients who experience acute ischemic injury to the central nervous system (CNS) present with moderate to severe hyperglycemia, and hyperglycemia following CNS injury is an independent risk factor for poor outcomes [9, 10]. Therefore, investigations into the basic mechanisms for both the induction of hyperglycemia and the consequences of it for ischemic outcomes are essential. Advanced glycation end products (AGE) and their receptors (RAGE) regulate inflammation and the dysfunction of glucose metabolism in response to CNS injury. Activation of RAGE induces inflammatory responses via the immune cells in the CNS, and consequent glucose dysregulation, reactive oxidant species production, and neuronal damage might cause tissue damage or poorer functional outcomes [11]. Therefore, the AGE–RAGE axis could be a therapeutic target for metabolic diseases and ischemia.
References
Scharrer E (1928) Die Lichtempfindlichkeit blinder Elritzen. I. Untersuchungen über das Zwischenhirn der Fishe. Z Vergl Physiol 7:1–38
Bargmann W (1949) Über die neurosekretorische Verknupfung von Hypothalamus und Neurohypophyse. Z Zellforsch 34:610–634
Harris GW (1955) Neural control of pituitary gland. Arnold, London
Swaab DF (ed) (2003) The human hypothalamus: basic and clinical aspects, Part I: Nuclei of Human Hypothalamus. Handbook of Clinical Neurology. Elsevier, Amsterdam, pp 1–476
Steinman L (2004) Elaborate interactions between the immune and nervous systems. Nat Immunol 5:575–581
Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A (2001) Inflammatory response: pathway across the blood–brain barrier. Nature 410:430–431
Hofstetter AO, Saha S, Siljehav V, Jakobsson PJ, Herlenius E (2007) The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc Natl Acad Sci U S A 104:9894–9899
Coceani F, Akarsu ES (1998) Prostaglandin E2 in the pathogenesis of fever. An update. Ann N Y Acad Sci 856:76–82
Capes SE, Hunt D, Malmberg K, Pathak PP, Gerstein HC (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients- a systematic overview. Stroke 32:2426–2432
Weir CJ, Murray GD, Dyker AG, Lees KR (1997) Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ 314:1303–1306
Weil ZM (2012) Ischemia-induced hyperglycemia: consequences, neuroendocrine regulation, and a role for RAGE. Horm Behav 62:280–285
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Nishiyama, Y., Katsura, Ki. (2015). The Neuroendocrine System and Its Regulation. In: Uchino, H., Ushijima, K., Ikeda, Y. (eds) Neuroanesthesia and Cerebrospinal Protection. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54490-6_3
Download citation
DOI: https://doi.org/10.1007/978-4-431-54490-6_3
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54489-0
Online ISBN: 978-4-431-54490-6
eBook Packages: MedicineMedicine (R0)