Abstract
Among various types of charged particles, protons and carbon ions have been most extensively used for cancer therapy around the world. In 1954, clinical application of proton beams was started for the first time in the world at Lawrence Berkeley National Laboratory (LBNL), which was shortly followed by Uppsala, Boston, Russia (three facilities), and Chiba. It is only after 1973, when computerized tomography (CT) was invented, that accurate calculation of dose distributions became feasible in clinical practice. In 1990, the world’s first hospital-based proton facility with a rotating gantry was built in Loma Linda. Since then, proton therapy has been applied to cancer treatment in an increasing number of facilities. Currently, there are about 36 facilities for proton beam therapy in operation, with still more facilities under construction or being planned in the world. As for the clinical application of heavier ions, the first patient was treated with helium ions in 1957 and with neon ions in 1975 at LBNL. Until it was closed in 1992, 2,054 patients were treated with helium ions and 433 patients with neon ions and other heavy ions. In 1994, the National Institute of Radiological Sciences (NIRS) in Japan started clinical application of carbon ions generated by Heavy Ion Medical Accelerator (HIMAC) in Chiba, which was the world’s first medically dedicated facility. Among various types of ion species, carbon ions were chosen for therapy because the biologically expressed dose distribution was assumed to be superior to other ions and the amount of high-LET components would be sufficient to ensure biological benefit in controlling photon-resistant tumors. By March 2013, more than 7,300 patients were treated at NIRS. In 1997, carbon-ion therapy was also initiated at Gesellschaft für Schwerionenforschung (GSI) in Germany to treat 440 patients until 2005, when it was closed and succeeded by Heidelberg University. Currently, there are six carbon therapy facilities in operation and several other facilities under construction in the world.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Within 2 months of the discovery of X-rays by Wilhelm Conrad Röntgen in 1895, X-rays were used to take pictures for diagnostic purposes as well as to treat a wide variety of diseases including malignant tumors. Since then, the primary principle of radiotherapy (RT) has been extensively pursued, which lies in precise dose localization in the target lesion and minimal damage to the surrounding normal tissues. In this sense, the era of the 1950s, when high-energy accelerators such as the tele-cobalt machine and linear accelerator were developed and employed in clinical practice, marked the beginning of modern RT.
In the late twentieth century, high-technology approaches including the development of intensity-modulated RT (IMRT) and three-dimensional stereotactic RT (SBRT) became available and contributed to the improvement of treatment results as well as extended their applicability to a wider range of tumors. IMRT delivers photon beams aimed at the target from many different directions, thereby permitting high dose concentration in the target while diluting unwanted doses outside the treatment volume [1, 2]. Currently, these photon treatments are widely available and are often called “conventional” RT to distinguish them from the new charged particle RT such as proton and carbon-ion RT.
Particle RT has a history of 60 years or more, and it has enhanced the clinical possibilities of RT. Among a wide variety of particles, particular attention has been focused on protons and carbon ions, which have been the front-runners around the world. This is based on the fact that, when compared to photons, they provide beneficial dose distribution and, in the case of carbon ions being heavier than protons, larger relative biological effectiveness (RBE), leading to a higher probability of tumor control while sparing surrounding normal tissues [3, 4].
In this chapter, the history of charged particle radiotherapy with special emphasis on carbon-ion RT is presented.
2 The Dawning of Particle Beam Radiotherapy
2.1 Invention of Accelerators for Particle Beam Radiotherapy
The success of radiation therapy largely depends on the performance of accelerators, beam delivery system, treatment planning system, and many other related devices. This becomes particularly clear when we take note of the evidence of the higher energy of photons reaching the order of MV in the 1950s contributing significantly to the improvement of therapeutic outcomes (Table. 1.1) [5]. Then, extrapolating from this, if charged particles were to be used for cancer therapy, treatment results could be expected to be improved even more.
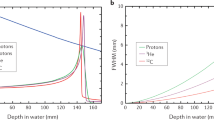
Among various types of charged particles, protons and carbon ions have been most widely employed for cancer therapy in the world. The energetic ion beam deposits much of its energy at the end of its range, resulting in what is called the Bragg peak (Fig. 1.1), so-named after Sir William Henry Bragg, a British physicist. He reported this phenomenon in 1904 [6]. Realizing the advantage of the Bragg peak, Robert R. Wilson (1914–2000) published his seminal paper on the rationale of using accelerated protons and heavier ions for human cancers in 1946 [7]. This was the first proposal to apply charged particles for medical use. He participated in the development of the atomic bomb in Los Alamos during World War II. After the war, he returned to Berkeley to look for peaceful atomic-energy projects, and he wrote a historic paper on the potential benefit of high-energy protons in cancer therapy. He later became the first director of the Fermi Laboratory, where clinical research of fast neutron therapy was conducted on more than 3,100 patients. Compared to conventional photon treatment, charged particle beams appeared to promise higher cure rates with smaller complications as they could deliver sufficient doses precisely, while lowering unwanted doses to normal tissues adjacent to the tumor. Wilson also hypothesized that carbon ions might be superior to proton beams.
Prior to Wilson’s proposal, Ernest Orlando Lawrence (1901–1958) discovered a method of accelerating particles to very high energy without the use of high voltage, which was actually the development of the cyclotron in 1929. The first model of Lawrence’s cyclotron was made of brass, wire, and sealing wax and was only 4 in. in diameter—it could literally be held in one hand. A photograph of Lawrence holding the first cyclotron in his hand appeared on the front cover of “Time” newsmagazine. Subsequently, higher-energy cyclotrons 11, 27, and 37 in. in diameter were constructed at Berkeley.
In 1938, the world’s first particle therapy was conducted on 24 patients with a single fraction of fast neutrons generated by the historic 37-inch cyclotron. This treatment was judged as successful, and then from 1938 to 1943 a total of 226 patients were treated with fractionated fast neutrons generated by a 60-inch cyclotron. Together with the clinical effects of fast neutron therapy, however, long-term side effects on normal tissues deemed were too high, and in 1948 Dr. Stone concluded that fast neutrons should not be used for cancer therapy [8].
A British group, however, evaluated fast neutron therapy again, and in 1965 Mary Catterall at Hammersmith Hospital in London once more began this therapy. By 1969, it was shown that for certain tumors, better local control could be achieved with neutron irradiation. Encouraged by these results, many other institutes in Europe, the USA, and Japan began neutron therapy research in the 1970s. However, the demand for fast neutron therapy decreased thereafter, and most of the institutions abandoned this therapy. Fast neutron therapy is currently conducted at only a few facilities for the treatment of selected tumors.
Incidentally, in the same year (1938) of the start of fast neutron therapy, Gerald Kruger, a physicist at the University of Illinois, came up with a new idea for cancer therapy using alpha particles emitted from boron when it captured neutrons. He proposed to saturate the tumor with a boron compound and then exposed it to a neutron beam. The boron capture cross section for thermal neutrons was about 100 times higher than that of other tissue compounds. This method came to be known as “neutron capture therapy.”
2.1.1 First Phase of Proton Beam Radiotherapy
In 1947, E. Lawrence completed construction of the 184-inch synchrocyclotron at the University of California (UC) Berkeley, making it possible to accelerate protons, deuterons, and helium nuclei to energies of several hundred MeV/u. Protons and heavier ions, being much more massive than electrons, require bigger accelerators to accelerate them to produce enough kinetic energy to treat deep-seated tumors. For example, a proton is 1,836 times heavier than an electron. E. Lawrence suggested that Cornelius A. Tobias and John H. Lawrence at UC Berkeley jointly use the 184-inch cyclotron to test the scientific validity of Wilson’s ideas [9].
Prior to irradiation of human patients, they decided to deliver deuteron beams to the pituitary of a dog patient with breast cancer in 1954 [10]. The dog’s tumor was ulcerated, bleeding and oozing milk continuously. Within a few days, pituitary irradiation caused a noticeable effect and the bleeding stopped. Within 2 weeks, the secretion of milk dried up completely and the animal had regained some strength. The dog was well and her tumor remained in remission for several months before death due to tumor relapse.
In 1954, encouraged by this favorable effect on the animal tumor, the world’s first human patient with disseminated breast cancer had pituitary irradiation with protons generated from the 184-inch synchrocyclotron [11]. Therapeutic exposure was performed with ion beams for a total of 50 human patients (deuterons, protons, and helium ions). The first human patient with widespread breast cancer was given pituitary proton irradiation, about half of what they actually expected to be an effective dose. She showed almost immediate improvement but eventually died several months later. Although it was impossible to determine who might benefit from the treatment, the earliest optimistic sign was the reduction of both skin temperature and swelling. After pituitary irradiation, more than half of the patients treated exhibited some beneficial effects, although in some there was no effect at all. As for toxicities, the most prominent side effect was the development of diplopia. It was thought that protons and helium ions were most likely spread too wide inside the head due to multiple scattering, and such effect could be minimized if carbon or oxygen beams could be used.
The second series of pituitary irradiation were for patients with acromegaly. Eventually about 700 patients were treated. Unlike with other treatments, acromegaly remained in regression and the growth hormone levels stayed within normal limits for many years [12]. In 1957, the 184-inch synchrocyclotron was modified to accelerate helium nuclei. By the time of the closure of the facility in 1992, a total of 2,054 patients had been treated with helium ions. These initial treatments with protons in the 1950s and 1970s had been mainly aimed at pituitary tumors, as they could be localized by orthogonal plane X-ray films and rigid immobilization of the skull. This was the manner of proton therapy before the invention of computed tomography (CT).
2.1.2 Second Phase of Proton Beam Radiotherapy
Soon after the initiation of clinical studies at UC Berkeley, programs of proton therapy also began at other proton facilities. They were originally constructed for nuclear physics research, including Uppsala, Sweden (1957), Cambridge, Massachusetts (1961), Dubna (1967), Moscow (1969), St Petersburg (1975), Chiba (1979), Tsukuba (1983), and Villigen (1984) [13].
Charged particle therapy was only made practical for cancer therapy with the advent of CT scanning in 1973, which could accurately determine the beam path in a patient. In 1974, Suit et al. initiated studies of fractionated RT with protons [14]. In the 1970s and 1980s, however, throughout the world the tumors treated with protons were mostly choroidal melanoma, skull base tumors, and intracranial tumors (Table 1.2). Among them, the largest number of patients treated with proton beams had choroidal melanomas, the first tumor treated safely with a large dose of 60–70 GyE in 4 to 5 fractionations in 1 week. At that time, only at Tsukuba University were deep-seated tumors such as lung, esophagus, liver, uterine cervix, prostate, and head and neck malignancies extensively treated by Tsujii et al. [15].
In 1990, a hospital-based proton facility was commissioned by James Slater at the Loma Linda University Medical Center in California [16], the historic world’s first facility to employ a 250 MeV proton accelerator dedicated to medical service and research. It had passive beam nozzles and four treatment rooms with three rotating gantries and one fixed beam line. Since then, an increasing number of facilities have begun proton therapy throughout the world (Fig. 1.2). As of April 2013, around 35 industry-built proton therapy facilities are operational and about 30 more facilities are under construction or being planned around the world.
3 Carbon-Ion Radiotherapy
3.1 Initiation of Carbon-Ion Radiotherapy
High-energy heavy-ion beams were obtained at the Berkeley Bevatron, a synchrotron-based facility, which was constructed in 1954. The injector was designed to obtain carbon, oxygen, and neon particles. During the almost 40 years since its commissioning, the venerable Bevatron made major contributions to four distinct areas of research: high-energy particle physics, nuclear heavy-ion physics, medical research and therapy, and space-related studies of radiation damage and heavy particles in space. The Bevatron was later given a productive new lease on life through the invention of the Bevalac, in which the Bevatron was linked to the SuperHILAC linear accelerator in 1974.
The Berkeley research teams embarked on helium-ion therapy in 1957 and then neon-ion RT in 1975. During the 1970s and 1980s, the use of Bevalac beams in medical and biological research became an important part of the program. In 1977, the first carbon-ion patient was treated in Phase I trials at LBNL [17–19]. At that time, however, only a small number of patients were treated with carbon ions, with the majority being treated with neon ions together with helium ions. Unfortunately, after 17 years and more than 2,000 patients treated, Berkeley terminated all radiotherapy programs in 1992.
Encouraged by the clinical studies at LBNL, other facilities sprang up around the world. As if a baton had been passed from the West Coast across the Pacific Ocean (Fig. 1.3), the National Institute of Radiological Sciences (NIRS), Japan, built the first heavy-ion accelerator in the world, called HIMAC in Chiba (Fig. 1.4), and started the clinical application of carbon ions in 1994 [20, 21]. Many of the Berkeley experiences with ion beam therapy were transferred to NIRS (Fig. 1.3). Following NIRS, the Gesellschaft für Schwerionenforschung (GSI), Germany, also started clinical studies with carbon-ion RT in 1997 [22], but then clinical usage was terminated and succeeded by Heidelberg Ion-Beam Therapy Center (HIT) in 2009 [23, 24]. HIT is the world’s first particle therapy facility for treatment using protons and carbon ions with a scanning beam delivery system.
At this writing, there are six facilities in operation for carbon-ion RT in the world: three in Japan, one in Germany, one in Italy, and one in China. The four facilities besides NIRS and HIT are Hyogo Ion Beam Medical Center, Hyogo, Japan (2002); Institute of Modern Physics (IMP), Lanzhou, China (2006); Gunma University Heavy Ion Medical Center, Gunma, Japan (2010); National Center of Oncological Hadrontherapy (CNAO), Pavia, Italy (2011). At present, two facilities in Japan, one in Germany, one in Austria, and two in China are under construction. In the USA, only proton facilities are in operation or under construction, but increasing interest in C-ion RT has recently emerged.
3.2 Clinical Experiences of Carbon-Ion RT
3.2.1 Clinical Experiences at LBNL
At LBNL, a total of 433 patients were treated with neon ions by the end of 1988. Among them, 239 patients received a minimum neon physical dose of 10 Gy (median follow-up for survivors, 32 months). According to Castro et al. and Linstadt et al. [25, 26], as compared with historical results, favorable results of neon-ion therapy were observed in several types of tumors, including advanced or recurrent macroscopic salivary gland carcinomas, paranasal sinus tumors, advanced soft tissue sarcomas, macroscopic sarcomas of the bone, locally advanced prostate carcinomas, and biliary tract carcinomas. However, the treatment results of malignant gliomas, pancreatic cancer, gastric cancer, esophageal cancer, lung cancer, and advanced or recurrent head and neck cancer appeared no better than those achieved with conventional X-ray therapy. Unfortunately, clinical research at LBNL was terminated in 1992 because of budget constraints and aging of the machine.
3.2.2 Clinical Experiences at NIRS, GSI, and Other Facilities
The number of patients treated with proton beams and C-ion beams in the world is shown in Figs. 1.5 and 1.6. The largest numbers of patients were treated by proton therapy in the USA (47 %) and by carbon-ion RT in Japan (91 %).
At NIRS, clinical trials were initiated in June 1994 with Phase I/II dose-escalation studies on various types of tumors, aiming to confirm the safety of carbon-ion RT and to evaluate its antitumor effects. Carbon-ion RT has been given at 4 times per week while keeping both the fraction number and treatment period fixed in all tumor-specific protocols. For the initial clinical study, locally advanced head and neck tumors were chosen, after which the range of application was expanded to many other tumors. As of March 2013, more than 7,300 patients with various types of tumors had been treated with carbon ions based on more than 50 protocols at NIRS [3, 27].
Experiments with high-LET radiations including carbon ions and fast neutrons demonstrated that increasing their fraction dose tended to lower the RBE for both tumor and normal tissues, but the RBE for tumor did not decrease as rapidly as the RBE for normal tissues. These results substantiated that the therapeutic ratio could increase rather than decrease even though the fraction dose was increased. In carbon-ion RT at NIRS, it has been possible to complete a treatment course with an average of 13 fractions over approximately 3 weeks. This means that the carbon therapy facility can be operated more efficiently, offering treatment for a larger number of patients than is possible with other modalities over the same period of time.
At GSI, the raster-scan method (active beam scanning) was developed by Haberer et al. [22], allowing the narrow carbon beam to precisely and selectively scan the tumor volume. By this method, the speed of ions and consequently their penetration depth is controlled by varying the energy levels of the accelerator, and the whole tumor is scanned layer by layer. Scanning is performed by deflecting the beam horizontally and vertically within each layer using magnets, similar to electron beams in a cathode ray tube. Even an irregular shape of tumors within the body can thus be uniformly irradiated to the nearest millimeter, with the damage to healthy tissue being minimized.
Until termination of its clinical study, more than 450 patients were treated at GSI using active beam scanning. Main indications treated at GSI were patients with chordomas and chondrosarcomas of the skull case, locally advanced adenoid cystic carcinomas (ACC), as well as chordomas and chondrosarcomas of the sacrum and prostate cancer [23]. Carbon-ion RT offered an effective treatment option for these tumors with acceptable toxicity. Based on these experiences at GSI and the overall need for particle therapy in Germany, the Heidelberg Ion-Beam Therapy Center (HIT) was constructed as a university hospital-based center.
Facilities other than NIRS and GSI/HIT are also accumulating experiences of C-ion RT for various types of cancers.
4 Conclusions
The principle of RT is to concentrate a sufficient dose to the target while minimizing radiation to the surrounding normal tissues. Historically, there have been technological developments for improving the spatial dose distribution, and in the late twentieth century, high-precision 3D conformal RT was developed, including SBRT, IMRT, and charged particle radiotherapy, by which dose concentrations have become significantly improved. Among them, C-ion RT has been employed for almost 20 years in Japan and Europe. It has demonstrated a clinical advantage in terms of high physical dose concentration with a narrow penumbra and, as high-LET radiations, clinical gain from high radiobiological effectiveness. Additionally, it has been confirmed that hypofractionation is feasible in treatment of almost all types of tumors. Hopefully, the next generation will be so advanced in this specialty that our present high technology of treatment planning and delivery can be considered obsolete. Our expectation is that within three decades, a very large proportion of definitive radiation treatment will be based on particle beams and feature 4D image-guided radiation therapy to maintain the target correctly positioned in the beam throughout each pencil beam scanning treatment session. A responsibility for the present generation should be to further develop radiotherapeutic techniques and deepen radiation oncology expertise.
References
Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Rad Oncol Biol Phys. 2003;56:83–8.
Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Rad Oncol Biol Phys. 2006;65:1–7.
Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42:670–85.
Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:853–64.
Conquest of Cancer. Report of the National Panel of Consultants of the Committee on Labor and Public Welfare United States Senate; 1970. p. 51.
Bragg WH, Kleeman R. On the ionization curves of radium. Philos Mag. 1904;8:726–38.
Wilson RR. Radiological use of fast protons. Radiol. 1946;47:487–91.
Stone RS. Neutron therapy and specific ionization. Am J Roentgenol. 1948;59:771–81.
Tobias CA, Anger HO, Lawrence JH. Radiological use of high-energy deuterons and alpha particles. Am J Roentgenol. 1952;67:1–27.
Cornelius, Ida Tobias. People and particles. San Francisco Press, Inc. CA; 1997. p. 121.
Tobaias CA, et al. Pituitary irradiation with high-energy proton beams. Cancer Res. 1958;18:121.
Laramore JH, et al. Heavy-particle therapy in acromegaly and Cushing’s disease. J A Med Assoc. 1976;235:2306.
Raju MR. The history of ion beam therapy. In: Ute Lintz, editor. Ion beams in tumor therapy. Chapman & Hall; 1995. p. 3–9.
Suit HD, Goitein M, Munzenrider J, et al. Definitive radiation therapy for chordoma and chondrosarcoma of the base of the skull and cervical spine. J Neurosurg. 1982;56:377.
Tsujii H, Tsuji H, Inada T, et al. Clinical results of fractionated proton therapy. Int J Radiat Oncol Biol Phys. 1993;25:49–60.
Slater JM, Daniel W, Archambeau JO, et al. Development of a hospital-based proton beam treatment center. Int J Radiat Oncol Biol Phys. 1988;14:761–75.
Castro JR, Quivey JM, Lyman JT, et al. Radiotherapy with heavy charged particles at Lawrence Berkeley Laboratory. J Can Assoc Radiol. 1980;31:30–4.
Chen GTY, Castro JR, Quivey JM, et al. Heavy charged particle radiotherapy. Ann Rev Biophys Bioeng. 1981;10:499–529.
Chatterjee A, Alpen EL, Tobias CA, et al. High energy beams of radioactive nuclei and their biomedical applications. Int J Radiat Oncol Biol Phys. 1981;7:503–7.
Tsujii H, Minohara S, Noda K. Heavy-particle radiotherapy: system design and application. In: Chao AW, editor. Reviews of accelerator science and technology, vol. 2. UK: Imperial College Press; 2009. p. 1–19.
Tsujii H, Mizoe J, Kamada T, et al. Overview of experiences on carbon ion radiotherapy at NIRS. Radiother Oncol. 2004;73 Suppl 2:S41–9.
Kraft G. Tumor therapy with heavy charged particles. Prog Part Nucl Phys. 2000;45:S473–544.
Haberer T, Becher W, Schardt D, Kraft G. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods. 1993;A330:296–305.
Combs SE, Jaekel O, Heberer T, Debus J. Particle therapy at the Heidelberg Ion Therapy Center (HIT) – integrated research-driven university-hospital-based radiation oncology service in Heidelberg, Germany. Radiother Oncol. 2010;95:41–4.
Castro JR, Saunders WM, Tobias CA, et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys. 1982;8:2191–8.
Linstadt DE, Castro JR, et al. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys. 1991;20:761–9.
Tsujii H, Kamada T, Baba M, et al. Clinical advantages of carbon-ion radiotherapy. New J Phys. 2008;10:1367–2630.
Acknowledgements
The author is thankful for the support of the Chang Yung-Fa Foundation and the NIRS International Open Laboratory (IOL).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Tsujii, H. (2014). History of Charged Particle Radiotherapy. In: Tsujii, H., Kamada, T., Shirai, T., Noda, K., Tsuji, H., Karasawa, K. (eds) Carbon-Ion Radiotherapy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54457-9_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-54457-9_1
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54456-2
Online ISBN: 978-4-431-54457-9
eBook Packages: MedicineMedicine (R0)