Abstract
Purpose: Vasogenic edema on glioblastoma multiforme (GBM) or a metastatic brain tumor (METS) may have different T2 relaxation time values because it involves an increased water component. In this study, we assessed the diagnostic utility of T2 mapping techniques in distinguishing GBM from METS.
Materials and Methods: We studied a glioblastoma (GBM) patient and a metastatic brain tumor (METS) patient who had not undergone previous surgery or treatment. All MR imaging was carried out using a 3.0-T whole-body unit, and axial T2 maps were generated with five TEs (TE = 20, 40, 60, 80, and 100 ms). Data were analyzed by using image processing and analysis software.
Results: The T2 map of a GBM case showed that the peritumoral area at a T2 relaxation time of 120–160 ms is prominent compared with the area at 210–240 ms. In contrast, the peritumoral area at 210–240 ms was prominent compared with the area at 120–160 ms in a METS case.
Conclusion: The distribution of T2 relaxation time in the peritumoral area shows different patterns in glioblastomas and metastatic brain tumors.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Conventional radiological characteristics that are thought to favor cerebral lesions being metastatic as opposed to primary brain cancer include a peripheral location, spherical shape, ring enhancement, and multiple lesions [1]. Gliomas may, however, be multifocal (showing gross or microscopic continuity or evidence of cerebrospinal fluid spread and/or local metastases) or multicentric (no macroscopic or microscopic connection) and are therefore potentially indistinguishable on conventional imaging from metastatic disease with multiple enhancing lesions [2].
Currently, conventional MRI consists of a combination of proton density-, T1-, and T2-weighted sequences. T2-weighted imaging is sensitive to the identification of pathological change, and T2 relaxation is governed by both the total amount of water and the ratio of free to bound water, which is itself dependent on the macromolecular environment. A disturbance to this environment, such as neuronal loss or demyelination, results in an increase in free water, with a longer T2 relaxation time and greater signal intensity on a T2-weighted image. Quantitative evaluation of T2-weighted images is more sensitive and objective than visual assessment for the identification of subtle cerebral pathology. Quantitative T2 mapping has been applied to cerebral neoplasia [3], neurodegenerative conditions [4], ischemia [5], head injury [6], encephalitis [7], and, most frequently, multiple sclerosis (MS) [8], where increased T2 signal has been observed within lesions as well as in cerebral tissue that appeared normal on conventional MRI.
Most of the new MRI techniques, with reported use in distinguishing glioma from metastatic disease, rely on detecting differences in the peritumoral region. In metastases, this area consists of vasogenic edema, whereas in glioma, neoplastic cells may also be present. As a result, a relative reduction in the peritumoral T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintense signal is expected in glioma, in contrast to cerebral metastases. Many exciting new developments in MR imaging techniques have been used to differentiate between a solitary metastasis and a high-grade glioma in the peritumoral region, including the use of spectroscopy, diffusion and perfusion imaging, and absolute apparent diffusion coefficient (ADC) measurements. Magnetic resonance spectroscopy alone has been shown to allow discrimination between metastases and glioblastomas [9, 10]. Diffusion tensor imaging has also shown promise in this distinction [11, 12]. Tang et al. suggested that non-enhancing adjacent cortical signal abnormality detected by FLAIR has the potential to differentiate between solitary gliomas and metastases [13]. These studies have all involved differentiating solitary cerebral lesions. No study to date has specifically assessed the T2 relaxation time and the detection of non-enhancing adjacent cortical signal abnormality for differentiating between multicentric and/or multifocal gliomas and other cerebral tumors with multiple enhancing foci.
The key to making the distinction between these two entities appears to lie in detecting the changes within the peritumoral area, the area beyond the enhancing margin on imaging. In metastases, this consists essentially of vasogenic edema, while in glioma, this may also contain neoplastic cells. Our hypothesis is that vasogenic edema on glioblastoma multiforme (GBM) and that on a metastatic brain tumor (METS) should show different T2 relaxation time values because it involves an increased water component. In this study, we investigated the diagnostic utility of T2 mapping in assessing non-enhancing signal intensity abnormality to distinguish GBM from METS. To the best of our knowledge, no previous similar studies have considered the implications of these findings.
Materials and Methods
Subjects
We studied a patient with glioblastoma (GBM) and a patient with metastatic brain tumor (METS) who had not undergone any previous surgery or treatment. Diagnosis was confirmed by histological examination.
Magnetic Resonance Imaging
All MR imaging was carried out using a 3.0-T whole-body unit (Achieva3T; Philips, Eindhoven, The Netherlands) with a SENSE-head-8 coil.
Axial T2 maps with fat saturation were generated by using a multishot GRASE protocol with 5 TEs (TE = 20, 40, 60, 80, and 100 ms). Other sequence parameters were TR = 3,705 ms (shortest); field of view (FOV), 230 × 183 mm; section thickness, 2 mm; section gap, 0 mm. and number of acquisitions = 1.
Image Interpretation
Data were analyzed sequentially by one author (Kanji Nakai) using image processing and analysis software (Image J, version 1.45n, available at http://imagej.nih.gov/ij/index.html; MRI Analysis Calculator plugin, available at http://imagej.nih.gov/ij/plugins/mri-analysis.html). T2 maps were calculated on a pixel-by-pixel basis in the transverse plane. The ROI was obtained by the primary author (K.Nakai) by manually tracing the outline of the peritumoral area where T2/FLAIR was hyperintense. The tumoral area, where the borderline was obvious with contrast enhancement and compatible with the tumor margin seen by a T2/FLAIR image, was excluded from the ROI. Addition to the ROI of the whole slices including perifocal edema on the T2 map generated a histogram of T2 distribution for each case. As patients can have different edema volumes, the histogram was normalized according to each ROI.
Results
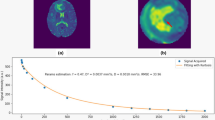
Normalized percentage of pixels of T2 relaxation time showed a marked peak at around 80 ms in both tumors. In the GBM case, it decreased with prolongation of the T2 relaxation time, whereas in the METS case, it decreased to around 160 ms and then increased to a second small peak at 190 ms, indicating that the profile of normalized pixels had a bimodal distribution (Fig. 1). The T2 map of the GBM case showed that the peritumoral area at T2 relaxation time of 120–160 ms is prominent compared with the area at 210–240 ms (Fig. 2). In contrast, the peritumoral area at 210–240 ms was prominent compared with the area at 120–160 ms in the METS case (Fig. 3). The ratio of 160–230 ms at T2 relaxation time of the GBM case was higher than that of the METS case (Table 1).
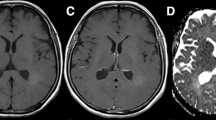
A 70-year-old man with surgically confirmed glioblastoma multiforme. (a) Axial fluid attenuated inversion recovery (FLAIR) image shows a hyperintense mass surrounded by a moderate degree of edema. (b) Axial post-contrast T1-weighted image shows enhancement of the solid part. (c), (d) T2 maps show a larger area at 120–160 ms (c) than at 210–240 ms (d)
Conclusion
In our study, the T2 mapping technique showed that there was a significant difference in the distribution of T2 relaxation time in the peritumoral area between the GBM group and the METS group.
The key to differentiating between the two neoplasm types appears to lie in the peritumoral area. In a glioblastoma, the peritumoral region may be infiltrated by malignant cells in addition to vasogenic edema [14], whereas in a metastatic deposit, the surrounding peritumoral area comprises predominantly vasogenic edema. By appearance alone, these peritumoral changes cannot generally be used to differentiate glioma from metastatic disease; however, vasogenic edema involves an increased water component. Using diffusion tensor imaging (DTI), Lu et al. demonstrated that there are clear differences in the diffusion characteristics of the vasogenic edema surrounding brain tumors compared with those of normal-appearing white matter [12].
Tang et al., who examined solitary enhancing cerebral lesions using FLAIR to detect non-enhancing adjacent cortical signal abnormality, reported a sensitivity of 44 % in distinguishing glioma [13], whereas Stuckey et al. reported that this sign was present in all multicentric/multifocal glioma patients presenting with more than one cerebral enhancing lesion [15]. Thus, it appears that non-enhancing adjacent cortical FLAIR signal abnormality is more common in multicentric/multifocal disease; however, this is not entirely unexpected as, by definition, these patients have more than one enhancing lesion to evaluate for the presence of this sign. In a larger series, 100 % sensitivity would presumably not be maintained. In contrast, the positive predictive values in the studies by Stuckey et al. and Tang et al. were 67 and 84 % respectively [13, 15]. The presence of this sign is not necessarily as good a predictor of multicentric/multifocal glioma when multiple enhancing lesions are present as it is for glioma when a solitary lesion is present. The difference is presumably due to the assumption made by the treating doctors that multicentric/multifocal cerebral lesions (in the context of proven malignancy at other sites) reflect metastatic disease, lessening the perceived clinical need for a histological diagnosis.
In conclusion, the distribution of T2 relaxation time in the peritumoral area shows different patterns in glioblastoma and metastatic brain tumors. T2 mapping may be useful for differentiating glioblastoma from metastasis in patients.
References
Soffietti R, Ruda R, Mutani R (2002) Management of brain metastases. J Neurol 249:1357–1369. doi:10.1007/s00415-002-0870-6
Prather JL, Long JM, van Heertum R, Hardman J (1975) Multicentric and isolated multifocal glioblastoma multiforme simulating metastatic disease. Br J Radiol 48:10–15
Kurki T, Lundbom N, Valtonen S (1995) Tissue characterisation of intracranial tumours: the value of magnetisation transfer and conventional MRI. Neuroradiology 37:515–521
Laakso MP, Partanen K, Soininen H, Lehtovirta M, Hallikainen M, Hanninen T, Helkala EL, Vainio P, Riekkinen PJ Sr (1996) MR T2 relaxometry in Alzheimer’s disease and age-associated memory impairment. Neurobiol Aging 17:535–540
Jacobs MA, Mitsias P, Soltanian-Zadeh H, Santhakumar S, Ghanei A, Hammond R, Peck DJ, Chopp M, Patel S (2001) Multiparametric MRI tissue characterization in clinical stroke with correlation to clinical outcome: part 2. Stroke 32:950–957
Yanagawa Y, Tsushima Y, Tokumaru A, Un-no Y, Sakamoto T, Okada Y, Nawashiro H, Shima K (2000) A quantitative analysis of head injury using T2*-weighted gradient-echo imaging. J Trauma 49:272–277
Thurnher MM, Thurnher SA, Fleischmann D, Steuer A, Rieger A, Helbich T, Trattnig S, Schindler E, Hittmair K (1997) Comparison of T2-weighted and fluid-attenuated inversion-recovery fast spin-echo MR sequences in intracerebral AIDS-associated disease. AJNR Am J Neuroradiol 18:1601–1609
Stevenson VL, Parker GJ, Barker GJ, Birnie K, Tofts PS, Miller DH, Thompson AJ (2000) Variations in T1 and T2 relaxation times of normal appearing white matter and lesions in multiple sclerosis. J Neurol Sci 178:81–87
Opstad KS, Murphy MM, Wilkins PR, Bell BA, Griffiths JR, Howe FA (2004) Differentiation of metastases from high-grade gliomas using short echo time 1H spectroscopy. J Magn Reson Imaging 20:187–192. doi:10.1002/jmri.20093
Ishimaru H, Morikawa M, Iwanaga S, Kaminogo M, Ochi M, Hayashi K (2001) Differentiation between high-grade glioma and metastatic brain tumor using single-voxel proton MR spectroscopy. Eur Radiol 11:1784–1791
Tsuchiya K, Fujikawa A, Nakajima M, Honya K (2005) Differentiation between solitary brain metastasis and high-grade glioma by diffusion tensor imaging. Br J Radiol 78:533–537. doi:10.1259/bjr/68749637
Lu S, Ahn D, Johnson G, Cha S (2003) Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 24:937–941
Tang YM, Ngai S, Stuckey S (2006) The solitary enhancing cerebral lesion: can FLAIR aid the differentiation between glioma and metastasis? AJNR Am J Neuroradiol 27:609–611. doi: 27/3/609[pii]
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874. doi:10.3171/jns.1987.66.6.0865
Stuckey SL, Wijedeera R (2008) Multicentric/multifocal cerebral lesions: can fluid-attenuated inversion recovery aid the differentiation between glioma and metastases? J Med Imaging Radiat Oncol 52:134–139. doi: ARA1931[pii]10.1111/j.1440-1673.2008.01931.x
Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this paper
Cite this paper
Nakai, K., Nawashiro, H., Shima, K., Kaji, T. (2013). An Analysis of T2 Mapping on Brain Tumors. In: Katayama, Y., Maeda, T., Kuroiwa, T. (eds) Brain Edema XV. Acta Neurochirurgica Supplement, vol 118. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1434-6_36
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1434-6_36
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1433-9
Online ISBN: 978-3-7091-1434-6
eBook Packages: MedicineMedicine (R0)