Abstract
Recent studies on mice experimentally infected with scrapie suggested that large increase in the levels of manganese ion occurs in blood and brain prior to the onset of symptoms of the prion disease, and the observed elevated manganese ion in several central nervous systems implies that the prion diseases should be considered to be one of the manganism. We have observed that oxidation of Mn(III) ion in several manganese chelates occurs in the presence of apo-transferrin, giving a di-μ-oxo bridged Mn(III/IV) species (hereafter we will call these Mn(III) and Mn(IV) ions to be labile plasma manganese ions), and at the same time facile uptake of manganese ions by apo-transferrin proceeds. This clearly shows that most manganese ions can be transported to the brain in a facile manner by transferrin under certain conditions. There are many iron-containing enzymes in the brain, and substitution of iron ion in these enzymes with other metal ions such as manganese ion results in complete or partial loss of enzymatic activity, and this is because the reactivity of the iron ion towards oxygen molecule is quite different from that of the manganese ions. Thus, the excess accumulation of the manganese ion in the brain should lead to (a) abnormality in iron metabolism, i.e., the increase of the labile plasma iron (or non-transferrin-bound iron, NTBI), which is in fact observed for the certain regions of the brain of scrapie strain infected mice; these iron ions are not transferred to transferrin, giving to the iron-deficiency state in the brain which leads to the defect of neurotransmitters such as dopamine and serotonin and (b) the abnormalities of the brain functions due to the toxicity of the labile plasma iron ions, which leads to neural cell death. Based on the above facts, and that (1) the labile plasma iron can in a facile manner produce the hydrogen peroxide and (2) the prion diseases can be elucidated by the “gain-of-function” of the prion proteins as copper(II)-containing enzyme in the presence of excess hydrogen peroxide, we have concluded that the prion diseases including both the sporadic and infected types should be elucidated by the combined toxicities due to the both labile plasma manganese and iron ions. Very recently we have succeeded in obtaining the chelate which captures both the labile plasma iron and manganese ions effectively and removes these ions without toxicity from the solution in vitro. Thus, we can hope that our new chelates should make notable contribution to the prevention and therapeutics for the prion disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, schizophrenia, and dementia, which are now in progress in Japan.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Prion diseases and transition metal ions
Prion diseases and prion protein
Between 1980 and roughly 1996, about 750,000 cattle infected with BSE (bovine spongiform encephalopathy) were slaughtered for human consumption in Great Britain, and it is now clear that BSE, also known as “mad cow disease” is neither merely a UK phenomenon nor is it merely an economic nuisance. In fact, it may be an impending worldwide health crisis, and in recent months several other European countries have found BSE in their cattle herds, and over the past few years about 100 mostly young individuals have fallen victim to a fatal condition known as new variant Creutzfeldt–Jacob disease (vJCD) [1–3]. BSE and vJCD are one of the transmissible spongiform encephalopathies (TSEs, or prion disease) which are a group of fatal neurodegenerative disorders that include BSE, vJCD, scrapie of sheep, chronic wasting diseases (CWD) of mule deer and elk, as well as Gestmann–Straussler–Scheinker disease (GSS) and fatal familial insomnia (FFI) of humans [4, 5].
Approximately 250 years ago, a sheep disease that presented with excitability, itching, ataxia, and finally paralysis and death was recognized and this is known today as scrapie in English-speaking countries, “the trembles” in France, “trotting disease” in Germany, and “itching disease” in Japan, reflecting the gamut of its symptoms. The first major advance in scrapie research took place in 1936 when Cuille and Chelle succeeded in transmitting the disease to sheep and goats by inoculating them with lumbar cord of diseased animals. Subsequently, transmission to mice and hamsters provided more-convenient experimental models. It was soon recognized that the transmissible agent had quite extraordinary properties, such as unusually long incubation periods, measured in months to years, and uncommon resistance to high temperature, formaldehyde treatment, and UV irradiation.
Enriching fractions from Syrian hamster (SHa) brain for scrapie infectivity led to the discovery of the prion protein (PrP), and at present it is generally accepted that the central event in TSEs is the post-translational conversion of the normal cellular prion protein (PrPC) into a abnormal isoform called scrapie PrP (PrPSc) that has a high-β-sheet content and is associated with the transmissible disease [6]. These misfolded prions (PrPSc) ultimately kill neurons and leave the brain riddled with holes, like a sponge, and the 1997 Nobel Prize in Physiology and Medicine was awarded to Professor S. Prusiner of the University of California, San Francisco, for his contributions towards the identification of the infectious agent that causes TSEs.
The normal cellular prion protein PrPC is a glycoprotein expressed on the surface of many cell types (see Fig. 1) and the fact that the protein is expressed in neurons at higher levels than in any other cell types suggests that PrPC has special importance for neurons; PrPC is highly concentrated at the synapse and there is evidence for intense localization not only at central nerves synapse but also at endplates. PrPC is linked to the cell membrane by glycosylphosphatidylinositol (GPI) anchor (Fig. 1) [7], and has one or two sugar chains that are closely linked to the C terminus and also exists in a non-glycosylated form. Recent studies have showed that PrPC not only binds copper (Cu) within the octarepeat region located in the unstructured N terminus, but under certain specific circumstances may bind along the C-terminal structured domain of protein fragments. It has been demonstrated that both recombinant and brain-derived PrP have superoxide dismutase (SOD)-like activity when Cu is bound to the octarepeat region [8].
Model of PrPC structural domains. The folded C-terminal portion of PrPC t contains the short β-sheet strands and the α-helix is based on a model derived from NMR-based coordinates of residues of hamster PrP [7]
The misfolded prion protein, PrPSc is extracted from affected brains as highly aggregated, detergent-insoluble materials that is not amenable to high-resolution structural technique and is covalently indistinguishable from PrPC. During infection, the underlying molecular events that lead to the conversion of PrPC to the scrapie agent remain ill defined.
Prion protein and transition metal ions
At present it is generally recognized that BSE may have originated from a scrapie agent infecting small ruminants, which have been recycled through cattle and disseminated through the use of contaminated meat and bone meal. Although BSE may spread among cattle by the feeding of infected offal, the majority of the cases of naturally occurring prion diseases arise sporadically with no known cause. Thus, the most important problem to be solved is to elucidate the intrinsic origin, i.e., the precise chemical mechanism of the prion diseases which arise sporadically.
Investigations of scrapie, CJD, and chronic wasting disease clusters in Iceland, Slovakia, and Colorado, respectively, have indicated that the soil in these regions is low in copper and high in manganese, and Brown et al. observed striking elevation of manganese ion accompanied by significant reduction of copper ion bound to purified PrP in all sCJD (sCJD = sporadic CJD) variants [9]. Brown et al. have reported that it loses the SOD-like activity when Cu is replaced with Mn in recombinant PrP, and Cu binding to PrP purified from sporadic CJD was significantly decreased while the binding of Mn and Zn was markedly increased [9]. These results suggest that altered metal-ion occupancy of PrP plays a pivotal role in the pathogenesis of prion diseases.
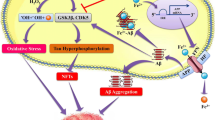
Elevated occupational exposures to manganese are known to cause significant neurotoxicity, and epidemiologic studies have suggested a relationship between elevated manganese exposure and an increased risk for parkinsonian disturbances, called manganism [10, 11], although the exact mechanisms underlying the neurotoxic effects of manganese remain unclear. Recent studies on mice experimentally infected with scrapie suggested that large increase in the levels of manganese ion occurs in blood and in several central nervous systems prior to the onset of symptoms of the prion disease [12]. It is quite likely that the excess accumulation of the manganese ion in the brain may lead to (a) abnormalities in iron metabolism, i.e., increase in the labile plasma iron (or non-transferrin-bound iron, NTBI), which is in fact observed in certain regions of the brain of scrapie strain infected mice [13, 14]; these iron ions are not transferred to transferrin, giving to a iron-deficiency state in the brain which leads to the defect of the neurotransmitters such as dopamine and serotonin and (b) abnormalities of the brain functions due to the toxicity by the labile plasma iron ions, which lead to neural cell death [15–17].
The sporadic neurodegenerative diseases are in general endemic; many years ago, amyotrophic lateral sclerosis (ALS) patients were collectively found in the New Guinea and Papua islands, and its origin has been attributed to drinking subterranean water, which contains much Al3+ and Mn2+ ions, and in these regions many patients of Alzheimer’s and Parkinson’s diseases were found [18], and increased aluminum levels were reported in the hippocampus of patients with Alzheimer’s disease [19, 20]. In Alzheimer’s disease, specific regions such as the hippocampus and the motor cortex contain elevated iron levels relative to normal, and abnormalities in brain iron metabolism have been described for several neurodegenerative disorders, including Alzheimer’s diseases, Parkinson’s disease, Huntington’s, and prion diseases [17].
The above facts clearly indicate that the prion diseases should be considered to be one of manganism, and the sporadic neurodegenerative diseases are closely related to the abnormal functions of metal ions such as manganese and iron. Based on these facts we will show the new mechanism for pathogenesis of prion diseases including both sporadic and infected types and would like to postulate the new countermeasures to prevent the prion diseases, Parkinson’s diseases, Alzheimer’s diseases, schizophrenia, and other neurodegenerative disorders.
Manganism and labile plasma manganese ions
Manganism
As a nutrient, manganese is an essential component of several enzymes; a deficiency can lead to heart and bone problems and in children, stunted growth; however, since 1937 elevated occupational exposures to manganese are known to cause significant neurotoxicity, and epidemiologic studies have suggested a relationship between elevated manganese exposure and an increased risk for parkinsonian disturbances, called manganism [10, 11]. It has been reported that rats exposed to very elevated manganese levels via drinking water from an early age displayed increased brain manganese levels and altered copper and iron levels in the striatum and in the basal ganglia; when manganese (this should be manganese oxide) is inhaled, blood ferries it from the lungs to the brain, where it can readily cross the blood–brain barrier.
Transport of manganese ions into the central nervous system has been directly investigated in a limited number of studies. It is generally believed that iron and manganese ions are able to be complexed and carried by transferrin/transferrin receptor, with iron being far more prevalent under normal circumstances [21]. Several authors suggest transport of trivalent manganese complexed to transferrin into the brain capillary endothelium, but the exact mechanism underlying the transport of manganese by transferrin to the brain has been elucidated very recently by us, which will be described below.
Transport of manganese ion by transferrin to brain
We have investigated the uptake of manganese ion by apo-transferrin in terms of the capillary electrophoresis method [22] and obtained clear evidence that (1) Mn(II) ion in several chelates is not transported to transferrin, (2) oxidation state, +3, and binuclear unit of a manganese chelate are critical factors for the facile uptake by apo-transferrin, similar to that observed for Fe(III) species [23], and (3) the facile oxidation of Mn(III) ion to a Mn(IV) state with simultaneous formation of a di-μ-oxo-bridged Mn(III/IV) species in several manganese chelates occurs in the reaction mixture containing Mn(III) chelates and apo-transferrin.
In our study the manganese compounds shown below were used: MnII(dpea)Cl2, MnII(dpa)ClClO4, MnII(dpal)Cl2, MnII(Me-en-py)Cl2, Mn III/IV2 O2(dpa)2(ClO4), Mn III/IV2 O2(Me-en-py)2(ClO4)3, and μ-oxo-μ-acetato-bridged binuclear manganese(III) complex, Mn III2 (Me-en-py)2(O)(CH3COO)(PF6)2; the chemical structures of the ligands are illustrated in Fig. 2 [24].
All the manganese(II) complex solutions used in our study are colorless, and solutions of these Mn(II) complexes with (dpa), (dpal), and (Me-en-py) are stable in the air under our experimental conditions (in tris-buffer solution, pH 7.3). The capillary electrophoresis method indicated that all the manganese(II) ions in the Mn(II) chelates used in this study are not transported to transferrin, because no change in the electrophoresis of the apo-transferrin occurs by the addition of Mn(II)(dpal) chelate. However, the shift and increase in the intensity of peak due to the apo-transferrin were observed when the solution of Mn III/IV2 O2(dpa)2(ClO4) was added to the solution of apo-transferin as illustrated in Fig. 3. These changes demonstrate that the Mn(III) ion is introduced to transferrin [22, 23], and a similar uptake of Mn(III) ion by apo-tremasferrin was observed for other di-μ-oxo-bridged binuclear Mn(III/IV) compounds.
The colorless Mn(II)(dpea)Cl2 in the buffer solution became pale brown when the solution was kept standing for more than 1 day, demonstrating that Mn(II) ion in the Mn(II)(dpea) chelate is readily oxidized to a Mn(III) ion in the atmosphere [22]. The addition of colorless apo-transferrin to the aged-pale-brown Mn(dpea) complex solution immediately induced the color change to dark brown, and uptake of the manganese ion by apo-transferrin proceeded.
In order to obtain more information regarding the above process, we have measured the ESR spectra of the solutions. The freshly prepared coloress Mn(II)(dpea) solution is ESR detectable, exhibiting the multiline ESR signal characteristic for the high-spin type Mn(II) ion. However, no ESR signal was observed for the aged-pale-brown Mn(dpea) complex solution (see Fig. 4(A)), consistent with the general concept that almost all Mn(III) species are ESR-non-detectable [25]. On the other hand, we have found 16 lines in the ESR spectrum of the dark-brown solution described above, as illustrated in Fig. 4(B). The same 16-line ESR signals was also observed in the solution containing apo-transferrin and the binuclear Mn(III) complex with (Me-en-py), Mn III2 (Me-en-py)2(O)(CH3COO)(PF6)2, but no 16-line ESR signal was detected in the solutions containing apo-transferrin and Mn(II) chelates with (dpa), (dpal), and (Me-en-py) under the same conditions.
As it has been pointed out that 16-line ESR signal observed in the figure is diagnostic for the binuclear Mn(III)/Mn(IV) species with di-μ-oxo bridge [26], the present results are clearly implying that in the solution containing apo-transferrim, the Mn(III) ion of the chelates is readily oxidized to a Mn(IV) state to give a binuclear di-μ-oxo bridged Mn(III)/Mn(IV) species. Because the facile uptake of manganese ion by apo-transferrin was observed in the solution containing the di-μ-oxo-bridged binuclear Mn(III)/Mn(IV) complexes with (dpa) and (Me-en-py) [22], it seems very reasonable to assume that formation of a di-μ-oxo-bridged Mn(III/IV) species in the solution plays a key role in the transport of manganese ion in the manganese chelates to apo-transferrin.
Labile plasma manganese ion
It is generally believed that iron and manganese ions are able to be complexed and carried by transferrin/transferrin receptor, with iron being far more prevalent under normal circumstances [21]. This is only true under the circumstance that the manganese ion exists as manganese(II) ion, and in fact uptake of manganese ion by apo-transferrin does not proceed in the solutions containing manganese(II) compounds with (dpa), (dpal), and (Me-en-py). The prevalence of the iron ion over the manganese ion is not valid when the Mn(III) or Mn(IV) ions are present in the plasma, and such condition may be induced in a facile manner as described above. Thus our results may give important information to understand the mechanism of the manganese ion transport to the brain, which should induce manganism, and we will call these Mn(III) and Mn(IV) ions to be labile plasma manganese ions.
The solutions of Mn(II) complexes with (dpa), (dpal) and (Me-en-py) are stable in the air, but these are converted to di-μ-oxo-bridged binuclear Mn(III)/Mn(IV) complexes by hydrogen peroxide [26]. Thus, it seems quite likely that the presence of hydrogen peroxide is closely related to the formation of the labile plasma manganese ions and thus with the pathogenesis of manganism, and it has been pointed out that formation of hydrogen peroxide is readily induced by the labile plasma iron ions (see sections “Hydrogen peroxide formation by binuclear iron(III) species”, and also “New chelation therapy for the neurodegeneration”), which are observed in a certain region of the brain of scrapie strain infected mice [13, 14].
Recent studies on mice experimentally infected with scrapie suggested that large increase in the levels of manganese ion occurs in blood and brain prior to the onset of symptoms of the prion disease and the observed elevated manganese and iron ions in several central nervous systems of scrapie strain infected mice imply that prion diseases should be considered to be one of manganism [12–14]. Thus, it seems reasonable to point out that the best way to prevent the prion diseases is to remove both the labile plasma manganese and iron ions from the plasma (see section “New chelation therapy for the neurodegeneration”).
Difference between Fe(II) and Mn(II) ions for oxidation reaction
The α-keto acid-dependent enzymes are distinguished from other non-heme iron enzymes by the requirement of an α-keto acid cofactor as well as Fe(II) and O2 for reactivity (see Fig. 5) [27]; the reactions catalyzed by these enzymes involve the oxidation of an unactivated C–H bond to give either of the hydroxylated products. In general, these enzymes require 1 equiv of Fe(II), and α-keto acid and ascorbate for full activity, and α-keto acid and ascorbate may behave as pterin of reduced form in tyrosine hydroxylase (TH) as illustrated in Fig. 6.
Substitution with other divalent metal ions (Zn(II), Mn(II), Co(II), Mg(II), and Ni(II)) results in complete loss of enzymatic activity [27]. These facts clearly show that oxygenase activity by the Fe(II) ion is completely different from those of other metal ions, especially Mn(II). This should be because Mn(II) compounds are in favor of the “two-electron oxidation” reaction and readily oxidized to a Mn(IV) ion, whereas “one-electron reaction” for the Fe(II) species. This is clearly exemplified in our recent results, i.e., Mn(II) ion of the Mn(II) complex, Mn(ntb)ClCl4, which is very stable in air, is readily oxidized to a Mn(IV) ion in the presence of reducing agent, cycloyhexane-carboxyaldehyde in air (see Fig. 7) [28].
These are indicating that excess accumulation of manganese ion gives a serious damage to the function of the biological iron-containing hydroxylases such as tyrosine or triphtophan hydroxylases, which are necessary for the synthesis of transmitters such as dopamine and serotonin, and this will induce abnormalities in iron metabolism, leading to increasing labile plasma iron in brain (see section “Labile plasma iron: formation and toxicity”), which is consistent with the observed facts [12–14]. Similar abnormalities in iron metabolism were also induced by the excess accumulation of Al3+ ion in the brain [15, 16].
Labile plasma iron and iron-overload syndrome
Labile plasma iron: formation and toxicity
Human iron metabolism and absorption have been the subject of a recent review. Normal human males contain 3–5 g of iron (often less in females) and of this, two-thirds is in circulating red cells as hemoglobin and 15–25 % in storage as ferritin and hemosiderin [29]. The remaining iron is in muscle myoglobin (about 8 %) and in cytochromes and iron-containing enzymes. Plasma transferrin accounts for only 3 mg of Fe, but the daily exchange of iron through plasma transferrin is ten times this account. Transferrin, therefore, plays a central role in iron distribution. Iron delivery by transferrin to erythroid and many non-erythroid cells involves interaction of transferrin with specific receptors followed by endocyosis and recycling of apotransferrin and receptor. These receptors are present in low amounts on phagocytosis cells which receive their iron from degraded red cell hemoglobin. Genetic haematochromatosis is one of the most common genetic disorder in western populations, particularly among Celtic peoples. This disease is associated with greatly increased (sometimes 50-fold) deposits of storage iron, predominantly as haemosiderin, in the liver and other tissues due to abnormally high absorption from the gut, and the excess iron cannot be eliminated and the elevated body iron leads to increased iron in storage (called iron-overload syndrome).
Several data support the hypothesis that haemosiderin is a degradation product of ferritin, and this has been confirmed for phytosiderin, an insoluble iron-containing product from pea seed, which was found to contain a peptide derived from the ferritin subunit [29]. Haemosiderin is typically insoluble, as isolated, in contrast to the soluble ferritin, but it can be solubilized by the several amino acids or small peptides to form a soluble iron(III) chelate; these are called as labile plasma iron or non-transferrin-bound iron (NTBI) [13–17], which may exist as soluble dimeric (or polymeric) compounds in brain, similar to Fe2(HPTP)Cl +4 (Fig. 8), or to Fe(III)-(nta) chelate; the latter complex is of a μ-oxo bridged dimeric structure, [Fe2O(nta)2(CO3)]2−, as illustrated in Fig. 9 [15, 30]. It should be noted here that these binuclear iron(III) compounds promote the formation of an iron(III)-oxygen species in the presence of hydrogen peroxide or of both oxygen and reducing agents such as glutathione reductase, and the notable reactivity of an iron(III)-oxygen species, similar to singlet oxygen (1 Δ g ), is induced through the interaction with substrate and the peripheral organic groups [15, 17, 30].
Toxicity due to the binuclear iron(III) species: 1
We have found that the binuclear iron(III) complexes, such as Fe2(HPTP)Cl +4 and [Fe2(HPTB)(OH)(NO3)2]2+, give much TBARS in the reaction with linolenic acid under aerobic conditions [15, 30]; here TBARS are malondialdehyde (which gives pink products with TBA (TBA = 2-thiobarbituric acid; see below); λ max = 532 nm) or monoaldehyde derivatives (gives orange products), which are formed from the peroxidation of linolenic acid.
The above results have been elucidated on the assumption that oxygenation of linolenic acid by O2 proceeds without the change of oxidation state of Fe(III) by forming an intermediate containing two iron(III) ions, O2, and linolenic acid, as illustrated in Fig. 10; in this case, the substrate is linolenic acid. It should be noted that the interaction between the two unpaired electrons of two iron(III) atoms and O 2 is necessary to activate the O 2 , which is promoted through interaction with the linolenic acid, and the reactivity of oxygen molecule in the intermediate is similar to that of singlet oxygen (1Δg) [15, 17, 30, 31].
The binuclear Al(III) complex, Al2(HPTP)(OH)Cl2(ClO4)2 was also isolated, and the activity for the oxygenation of linolenic acid was compared with that of the binuclear Fe(III) complex by measuring the quantities of the TBARS compounds. The result clearly shows that Al(III) complex exhibits no activity for the oxygenation of linolenic acid, and this can be attributed to the absence of unpaired d-electron in Al(III) complex, and this also supports the important role of unpaired d-electron of Fe(III) ion in the activation of O2 in Fig. 10.
A similar fact was also observed when DMPO, which is one of the famous spin-trapping agents for OH∙ radical, was added to the solution containing binuclear iron(III) complex, Fe2(HPTP)(OH)(NO3) 2+2 ; strong four signals which correspond to the formation of DMPO-OH have appeared as illustrated in Fig. 11 [15, 32], whereas no such signal was detected by the addition of DMPO to the solutions of Fe(edta)− and Al2(HPTP)(OH)Cl2(ClO4)2. This clearly indicates that the formation of DMPO-OH is not due to the presence of OH∙ in the solution. The above mysterious fact was elucidated in a similar way as described for the oxygenation of linolenic acid through the formation of the intermediate illustrated in Fig. 10 (in this case, substrate is DMPO); O2 is activated to interact with DMPO, leading to DMPO-OH formation. These clearly demonstrate that the presence of DMPO induced the formation of an iron(III)-oxygen species to give a DMPO-OH in the reaction with DMPO, and thus the results reported hitherto on the formation of DMPO-OH should be reinvestigated.
ESR spectra of the solutions containing DMPO and A: Fe2(HPTP)(OH)(NO3) 2+2 (upper) B: Fe(edta)Na (lower) [32]
Toxicity due to the binuclear iron(III) species: 2
We have observed that in the solution containing binuclear iron(III) compound [Fe2(HPTB)(OH)(NO3)2]2+ and hydrogen peroxide, the peroxide adduct shown in Fig. 12 forms in a facile manner [15, 31].
It should be noted that the peroxide ion captured in the binuclear iron(III)-peroxide adduct exhibits high reactivity towards several organic compounds; its reactivity is similar to that of singlet oxygen (1Δg) [15, 30, 31], which is known to react with several organic compounds as illustrated in Fig. 13.
Thus, the labile plasma iron ions exhibit high toxicity towards human body when hydrogen peroxide is present in the plasma.
Hydrogen peroxide formation by binuclear iron(III) species
It is noteworthy that the oxidation of the TMPD, one of the famous one-electron donor, is greatly accelerated in the presence of the binuclear iron(III) complex, Fe2(HPTP)Cl +4 , or binuclear copper(II) complex [15, 32], to give higher quantity of hydrogen peroxide according to the similar reaction scheme shown in Fig. 10; this indicates that the soluble dimeric iron(III) species of labile plasma iron can produce hydrogen peroxide in the presence of reducing agent and O2. These facts suggest that hydrogen peroxide may readily form in patients with iron overload, and this hydrogen peroxide should be one of serious origin in the pathogenesis of sporadic scrapie and BSE as described in the next section.
Copper(II)-hydroperoxide adduct in sporadic amyotophic lateral sclerosis and sporadic prion diseases
“Gain-of function” of copper(II)-hydroperoxide adduct in mutant SOD enzyme
ALS is a progressive paralytic disease characterized by selective degeneration of the upper and lower motor neurons [17, 33, 34]. Although ALS is predominantly a sporadic disease, ~10 % cases are inherited in an autosomal dominant manner (familial ALS (fALS)) and a subset of the fALS cases are caused by mutations in the SOD1 gene. The gene product of SOD1, cytoplasmic Cu, Zn-superoxide dismutase (SOD1), is a ubiquitously expressed enzyme that catalyzes the disproportionation reaction of superoxide radicals (see the Eqs. (1) and (2)) [35].
There are several lines of evidence suggesting that SOD1 mutations result in a gain, rather than loss of function that causes ALS. One hypothesis of the gain-of-function of SOD1 is that misfolding of the mutant alters the catalytic mechanism to allow the production of oxidants such as peroxynitrite and possibly hydrogen peroxide. Another major hypothesis is toxicity caused by intracellular aggregation of SOD1. SOD1 inclusion bodies, which also react with anti-ubiquitous antibodies, are a common pathological finding in motor neurons and neighboring astrocytes of ALS patients [33, 34]. Although SOD1 aggregates may be inherently toxic or cause motor neuron toxicity by sequestering chaperons and blocking proper functioning of the proteasome, origin of toxicity by SOD1 aggregates has not been elucidated.
We have studied the reactivity of a copper(II)–OOH, proposed as an important intermediate in the SOD reaction in order to obtain the comprehensive solution for the correlation between the mutant SOD and pathogenesis of fALS. For this purpose, we have synthesized many copper(II) compounds with the ligands containing N,N-bis(2-picolylmethyl)amine moiety, as illustrated in Fig. 14 [35]. The structural features of all the copper(II) compounds are essentially similar to each other (as an example, the crystal structure of [Cu(bdpg)Cl]+ is illustrated in Fig. 15).
In the presence of hydrogen peroxide, a copper(II)-peroxide adduct formation as shown in Fig. 16 may occur. We have found that formation and reactivity of the peroxide adduct of the copper(II) compound is highly dependent on the R of the ligand system. This clearly demonstrates that formation and reactivity of the Cu(II)–OOH is controlled by the chemical interactions among copper(II)–OOH species, peripheral groups, and substrate (see Fig. 16) [15, 17, 30, 35], and in some cases the hydrogen peroxide of copper(II)–OOH species is changed to exhibit high reactivity similar to that of the singlet oxygen ( 1 Δ g ) [15, 30, 35], although hydrogen peroxide has been believed to be relatively inert and not toxic to cells.
Based on our experimental facts [15, 17, 35], we have concluded that the “gain-of-function” of the mutant SOD is due to the formation of a long-lived highly reactive copper(II)–OOH as an intermediate in the process of SOD reaction. The chemical structures around the copper(II) in the mutant SOD are slightly changed, and this gives an unexpected effect on the reactivity of a copper(II)–OOH as observed in our papers. In the mutant SOD, the C–N bond cleavage by the Cu(II)–OOH is thought to cause great changes in the surface of SOD, leading to destabilization of the dimer contact of the SOD enzyme [36], and thus, it is quite likely that formation and existence of a highly reactive Cu(II)–OOH species is an intrinsic origin for oxidative stress in the pathogenesis of fALS, which seems to be quite consistent with the recent studies on the destabilization of the dimer contact of the SOD enzyme [17, 35].
Dissociation of dimeric SOD molecule into monomers
At present it has been widely recognized that protein aggregation is a common pathological feature of many neurological disorders, including Huntington’s, Alzheimer’s, and Parkinson’s diseases and that SOD1 aggregates may be inherently toxic or cause motor neuron toxicity by sequestering chaperons and blocking proper functioning of the proteasome [17].
In 2004, Rakhit et al. reported that SOD1, normally a dimeric enzyme, dissociates to monomers prior to aggregation in both wild type and mutant proteins [36]. They used the “Dynamic Light Scattering (DLS)” method to detect the dissociation of dimeric SOD to monomers. Very recently we have reported that the capillary electrophoresis method (CE) is very suitable to investigate the conformational change of the proteins and aggregation states of the proteins in solution [17, 35].
We have observed that the drastic decrease of the peak strength due to the dimeric SOD molecule occurs when the copper(II)/ascorbic acid solution was added to the SOD molecule [37]; our experimental system was same as that reported by Rakhit et al. This clearly shows that the dissociation of the dimeric SOD molecule can be readily detected by the CE method. We also have found that the presence of excess hydrogen peroxide induces the dissociation of dimeric structure of wild-type SOD molecule, because drastic decrease of the peak height due to the dimeric structure was observed (see Fig. 17) [17, 35, 38]. Thus, it seems quite likely that the oxidant in the system, the copper(II)/ascorbic acid solution, used by Kakhit et al. should be hydrogen peroxide, and that sporadic ALS should be closely related with the presence of excess hydrogen peroxide, and the same discussion may be applied to the elucidation of sporadic prion diseases (see later).
By using antibody methods to rapidly purified SOD1 and coupling this with mass spectrometry, Sato et al. have measured the relative accumulated levels of wild-type and mutant SOD1 in erythrocytes of 29 SOD1-mutated fALS patients [39]. They observed that the patients with undetectable SOD1 mutant had the shortest disease durations. Although age at disease onset was found to be uncorrelated with the amount of mutant SOD1, the evidence convincingly shows a strong inverse correlation between disease duration and mutant accumulation. In other words, an accelerated disease course is found for mutants that are less stable. This surprising discovery implies that it is the misfolded unstable forms of SOD1 mutants that contribute to toxicity underlying disease progression, and that despite its apparent importance for progression, SOD1 mutant stability is not correlated with disease onset. Thus dissociation of the dimeric SOD1 molecule to misfolded monomers should be an essential important process for ALS pathogenesis. As it has become apparent that hydrogen peroxide plays an important role in the formation of SOD1 monomers [17, 35, 37], we should pay attention to the formation of excess hydrogen peroxide in the human body, especially due to the reaction between a dimeric iron(III) species and glutathione cycle and other related systems as described in the section “Toxicity due to the binuclear iron(III) species: 2” [15, 30].
Copper(II)–OOH in sporadic bovine spongeform encephalopathies
PrPC is a glycoprotein expressed on the surface of many cell types and its genetic code was identified only after the isolation of an abnormal isoform, PrPSc, from brains of mice that were infected with the disease scrapie. It is generally recognized that PrPC is a copper-containing protein [at most 4 copper ions are present within the octarepeat region located in the unstructured N-terminus (Fig. 1 in section “Prion diseases and prion protein”)]. Since 1996 there has been increasing evidence that PrPC increases cellular resistance to oxidative stress. Cerebelle neurons and astrocytes from PrPC knockout mice are more sensitive to superoxide toxicity, whereas cells with higher levels of PrPC expression are more resistant to oxidative stress [8]. Analysis of recombinant mouse and chicken PrPC has led to the discovery of an important “gain-of-function” following the formation of the PrPC copper complex.
The copper at the synapse is released in vesicles, and the copper released in this way appears to be taken up rapidly by the neurons, and deployed within 30 min of this process. It is unknown in what form this copper is bound; however, it is probable that the copper is chelated to some peptides or amino acids because there is little free copper found in the body [40]. It has been pointed out that the copper(II) chelates compounds which across the membrane may originate from the cleavage of the PrPC [8] and thus it seems quite likely that these copper(II) chelates react with hydrogen peroxide to yield a Cu(II)–OOH species, giving serious effects towards the PrP C such as oxygenation at methionine residue, conformational change, and degradation of protein, if hydrogen peroxide is present in the vicinity (see Scheme 1).
As described in the section “Prion diseases and prion protein,” the misfolded prions (PrPSc) ultimately kill neurons and leave the brain riddled with holes, like a sponge. In addition to PrPSc, another protease-resistant PrP of 27–30 kDa, which is called as PrP27-30, was extracted from affected brains. It should be noted here that PrP27-30 is derived from only PrPSc (not from PrPC), and no difference in amino acid sequence between PrPC and PrPSc has been identified. Based on these facts, we may assume that the chemical environment around the copper ion in the PrPSc should be different from those in the PrPC; this situation is similar to the difference observed between the copper(II) ion in the wild-type and mutant SOD enzyme. Thus, it is most likely that the “gain-of-function” in the PrPSc due to a “highly reactive” Cu(II)–OOH formation may appear as described for the mutant SOD molecule, leading to the cleavage of the peptide bonds around the copper ion (near at about 90 site), giving PrP27-30, and also the conformational change of PrPC to PrPSc (see Scheme 1).
We reported that some copper(II) complexes exhibit high catalytic activity to oxygenate the sulfur atom of methionine of amyloid beta-peptide in the presence of hydrogen peroxide [17, 35]. Oxidation of methionine residues in the prion protein by the hydrogen peroxide attracts recent interests; it has become apparent that Met 129, a residue located in a polymorphic position of human PrP and modulating risk of prion diseases, is easily oxidized as is Met 134 by hydrogen peroxide, and that H2O2-induced methionine oxidation leads to a modest increase in β-sheet structure. Several experimental facts observed for the native prion proteins [13, 41–46] seem to be consistent with our results as described above, and all these findings support our proposal [15, 17, 35] that hydrogen peroxide should be the serious origin for the oxidative stress in sporadic prion diseases.
New chelation therapy for prion diseases and several neurodegenerative disorders
Manganism in both the sporadic and infected prion diseases
Prion diseases may be divided into two groups: sporadic and infected types. We have demonstrated that pathogenesis of the sporadic prion diseases is closely related with the excess accumulation of manganese ions in the brain. Excess accumulation of manganese ion in the brain induces the abnormality in the synthesis of several neurotransmitters, and iron metabolism in the brain increases the quantity of labile plasma iron, and these iron ions cause a serious damage to the proteins, DNA, and other important substances.
Recent studies on mice experimentally infected with scrapie suggested that large increase in the levels of manganese ion occurs in blood and brain prior to the onset of symptoms of the prion disease; at the same time, elevated manganese ions in several central nervous systems and increase of labile plasma iron in certain regions of the brain were observed. The reason for the above facts observed in the infected type is not clear at present, but it is quite likely that much hydrogen peroxide is produced by the misfolded Cu-PrPSc protein through its SOD-like function, and this hydrogen peroxide may induce the increasing of the labile manganese ion in the blood and brain as described in section “Manganism and labile manganese ions“. Thus, it is reasonable to conclude that the prion diseases including both the sporadic and infected types should be elucidated by combined toxicity due to the labile plasma manganese and iron ions.
New chelation therapy for the neurodegeneration
The above discussions clearly demonstrate that the removal of the labile plasma iron and manganese ions should be one of the best ways to prevent the prion diseases of sporadic type and other neurodegenerative disorders. For this purpose, we have prepared new chelates to capture both the labile plasma iron and manganese ions effectively and to remove both the ions without toxicity in vitro. We hope that our new chelates should make notable contribution to the prevention and therapeutics for the prion disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, schizophrenia, and dementia, which is now in progress in Japan.
References
Chani AC, Ferguson NM, Donnell CA, Anderson RM (2000) Nature 406:583
Beale AJ (2001) J R Soc Med 94:207
Houston F, Foster JD, Chong A, Hunter N, Bostock CJ (2000) Lancet 356:955
Cohen FE, Prusiner SB (1998) Annu Rev Biochem 67:793
Collinge J (2001) Annu Rev Neurosci 24:519
Prusiner SB (1996) Trends Biochem Sci 21:482
Caughey B (2001) Trends Biochem Sci 25:235
Brown D (2001) Trends Neurosci 24:85
Wong BS, Chen SG, Colucci M, Xie Z, Pan T, Liu T, Li R, Gambetti P, Sy MS, Brown DR (2001) J Neurochem 78:1400
Dobson AW, Erikson KM, Aschner M (2004) Ann N Y Acad Sci 1012:115
Kaiser J (2003) Science 300:926
Hesketh S, Sassoon J, Knight R, Hopkins J, Brown DR (2007) J Anim Sci 85:1596
Fernaeus S, Reis K, Bedecs K, Land T (2005) Neurosci Lett 389:133
Fernaeus S, Halldin J, Bedecs K, Land T (2005) Mol Brain Res 133:266
Nishida Y (2004) Med Hypothesis Res 1:227–245
Nishida Y (2003) Z Naturforsch 58c:752
Nishida Y (2011) Monatsh Chem 142:375
Shiraki H, Yase Y (1991) In: Vinken PI, Bruyn GW, Klawans HL (eds) Handbook of clinical neurology, vol 15, pp 273–300
Gerlach M, Schachar DB, Riederer P, Youdim MBH (1994) J Neurochem 63:793
Youdim MBH, Riederer P (1997) Sci Am 1997:52
Heilig EA, Thonpson KJ, Molina RM, Ivanov AR, Brain JD, Resnick MW (2006) Am J Physiol Lung Cell Mol Physiol 290:L1247
Abe K, Chiba Y, Nishida Y (2008) Z Naturforsch 63c:154
Nishida Y, Ito Y, Satoh T (2007) Z Naturforsch 62c:608
Sutoh Y, Nishino S, Nishida Y (2005) Chem Lett 34:140
Abragam A, Bleaney B (1970) Electron paramagnetic resonance of transition ions. Clarendon, London
Okuno T, Nishida Y (1996) Polyhedron 15:1509–1515
Que L Jr, Ho RYN (1996) Chem Rev 96:2607
Sutoh Y, Nishida Y (2005) Synth React Inorg Metal-org Nano-metal Chem 35:575
Harrison PM, Arosio P (1996) Biochem Biophys Acta 1275:161
Nishida Y (2009) TCIMAIL 141:2. http://www.tciamerica.com/tcimail/backnumber/article/141drE.pdf
Nishida Y, Takeuchi M (1987) Z Naturforsch 42b:52
Nishida Y, Nasu M, Akamatu T (1992) J Chem Soc Chem Commun 1992:94
Yamanaka K, Cleveland DW (2005) Neurology 65:1859
Alessandra G, Hider RC (2005) Br J Pharm 146:1041
Nishida Y (2007) TCIMail 135:2. http://www.tciamerica.com/tcimail/backnumber/135drE.pdf
Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A (2004) J Biol Chem 279:15499
Abe K, Nishida Y (2008) Z Naturforsch 63c:151
Chiba Y, Sutoh Y, Nishida Y (2006) Z Naturforsch 61c:273
Sato T, Nakanishi T, Yamamoto Y, Andersen PM, Ogawa Y, Fukada K, Zhou Z, Aoike F, Sugai F, Nagano S, Hirata S, Ogawa M, Nakano R, Ohi T, Kato T, Nakagawa M, Hamasaki T, Shimizu A, Sakoda S (2005) Neurology 65:1954
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV (1999) Science 284:805
MaMahon EHM, Mange A, Nishida N, Creminon C, Casanova D, Lehmann S (2001) J Biol Chem 276:2286
Requena JR, Groth D, Legname G, Sradtman ER, Prusiner SB, Revine RL (2001) Proc Natl Acad Sci USA 98:7170
Watt NT, Taylor DR, Gillott A, Thomas DA, Perera WS, Hooper NM (2005) J Biol Chem 280:35914
Tabler BJ, Turnbull S, Fullwood NJ, German M, Allsop D (2005) Biochem Soc Trans 33:548
Tabler BJ, Agnaf OMEA, Turnbull S, German MJ, Paleologou KE, Hayashi Y, Kooper LJ, Fullwood NJ, Allsop D (2005) J Biol Chem 280:35789
Watt NT, Hopper NM (2005) Biochem Soc Trans 33:1123
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Wien
About this chapter
Cite this chapter
Nishida, Y. (2012). Prion diseases and manganism. In: Linert, W., Kozlowski, H. (eds) Metal Ions in Neurological Systems. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1001-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1001-0_6
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1000-3
Online ISBN: 978-3-7091-1001-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)