Abstract
High mountains are climatically extreme environments. Short growing seasons and low temperatures are the most important factors limiting plant life at higher altitudes. In the mountains of temperate and cold climates, the period available for growth, flowering and seed production varies with relief and snow accumulation in winter (e.g. Crawford 2008; Galen and Stanton 1991; Kudo 1991, 1992; Galen and Stanton 1995; Kudo and Suzuki 1999; Inouye et al. 2002, 2003; Körner 2003; Ladinig and Wagner 2005; Molau et al.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
High mountains are climatically extreme environments. Short growing seasons and low temperatures are the most important factors limiting plant life at higher altitudes. In the mountains of temperate and cold climates, the period available for growth, flowering and seed production varies with relief and snow accumulation in winter (e.g. Crawford 2008; Galen and Stanton 1991; Kudo 1991, 1992; Galen and Stanton 1995; Kudo and Suzuki 1999; Inouye et al. 2002, 2003; Körner 2003; Ladinig and Wagner 2005; Molau et al. 2005; Kudo and Hirao 2006; Ladinig and Wagner 2007; Inouye 2008). In the European Alps, the growing season lasts 3–5 months in the alpine belt and 1–3 months in the ice-free areas of the nival belt (Larcher 1980; Larcher and Wagner 2009; Wagner et al. 2010). Not only short snow-free periods but also large temperature fluctuations and sudden cold spells with fresh snow, which can occur at any time during the growing season, are typical of mountain habitats. This produces a stop–start situation, additionally shortening the time available for growth and development. The plant species differ in how well they have adapted to such climatic extremes which increase with elevation. Accordingly, in the Alps, species richness decreases from more than 200 species in the upper alpine zone to about 30 species in the nival zone (Grabherr et al. 1995). Only a dozen specialists still occur above 4,000 m a.s.l. (Ozenda 1988; Grabherr et al. 1995; Körner 2003). To be successful in such a harsh environment, plants need to cope with temperature extremes while actively growing (Larcher and Wagner 1976; Neuner et al. 1999; Taschler and Neuner 2004; Larcher et al. 2010), to maintain metabolism over a broad temperature range (Larcher and Wagner 1976; Körner and Diemer 1987) and to complete vegetative and reproductive development within a short period of time.
Reproductive development, which is particularly susceptible to disturbances, requires the precisely coordinated timing of different processes from floral induction to seed maturation. During floral induction the shoot apex shifts from vegetative to reproductive, forming an inflorescence or a single flower. In most mountain plants, floral development is initiated 1 year prior to maturation (Billings and Mooney 1968; Mark 1970; Nakhutsrishvili 1999; Larl and Wagner 2006; Ladinig and Wagner 2009), or even earlier (Diggle 1997). Overwintering flower buds are also the rule in most arctic plants (Sørensen 1941). The earlier floral development starts and the further developed flower buds enter winter, the earlier they bloom in the following growing season (Molau et al. 2005). Thus, the timing of reproductive phases in the year of anthesis is determined by the course of floral development in the preceding year.
Anthesis is the functional phase of a flower. The length of time a flower is functional depends on a variety of factors. On the one hand, species-specific properties such as type of gender sequence (adichogamous or dichogamous) and pollination mechanisms are decisive. On the other hand environmental factors such as temperature and, in insect pollinated flowers, pollinator frequency affect the course of anthesis. Fertilization marks the onset of seed development which is comprised of histogenesis (formation of seed tissues and early embryogenesis) and maturation. With the release of mature seeds the reproductive cycle is terminated. Mature seeds of many alpine plants exhibit relative dormancy (Amen 1966; Billings and Mooney 1968; Giménez-Benavides et al. 2005; Shimono and Kudo 2005) and can persist for a variable length of time in seed banks. This persistence is pivotal in renewing populations (Stöcklin and Bäumler 1996; Erschbamer et al. 2001; Marcante et al. 2009).
Timing and dynamics of all these reproductive processes depend on both the species-specific developmental pattern and on environmental factors – in particular on temperature and photoperiod. In the literature, the time course of reproductive development in high-mountain plants is mainly documented by phenological observations at the population level (e.g. Arroyo et al. 1981; Bahn and Körner 1987; Prock 1990) or at the plot level (e.g. Kudo 1991; Theurillat and Schlüssel 2000; Kudo and Hirao 2006; Inouye 2008; Makrodimos et al. 2008). Such records show the length of different phenophases such as prefloration (time span between snowmelt and first flowering), anthesis, seed development and seed maturity for a cohort of individuals, however, they provide little information about the species-specific developmental dynamics in individual flowers.
To get a more in-depth view of reproductive processes in high-mountain plants, the reproductive timing and the development dynamics were analysed on the basis of single flowers in a multi-year study. Eleven abundant herbaceous plant species with different altitudinal distributions in the European Alps were studied (Table 10.1). Species are common either in the alpine zone (Gentianella germanica, Ranunculus alpestris, Saxifraga androsacea, S. caesia) or from the subnival to the nival zone (Androsace alpina, Cerastium uniflorum, R. glacialis, S. biflora, S. bryoides). Saxifraga moschata and S. oppositifolia cover a particularly wide altitudinal range and occur from the alpine to the nival zone. Some of the nival species have even been recorded above 4,000 m at climatically favourable microsites.
In this chapter we give an overview of the species-specific patterns and strategies of reproductive development. Special focus is given to developmental dynamics and to the influence of the environmental factors, temperature and day length. We further address the question of whether reproductive strategies differ with respect to the altitudinal distribution and what impact prolongation of the growing season might have on the reproductive performance in the investigated species. Some of the results have already been presented in individual publications (Ladinig and Wagner 2005, 2007, 2009; Larl and Wagner 2006; Steinacher and Wagner 2010; Wagner et al. 2010; Steinacher and Wagner 2011). Here we draw general conclusions from the comparative analyses.
10.2 Study Sites and Methods
Most investigations took place between 2001 and 2008. The investigations were carried out at different elevations at four localities in the Tyrolean Alps (alpine zone: Hafelekar 2,320 m a.s.l., Northern Calcareous Alps, 47°18′N, 11°23′E; subnival zone: forelands of the Tux Ferner 2,650 m a.s.l., Zillertal Alps, 47°04′N, 11°40′E and the Schaufelferner 2,850 m a.s.l., Stubai Alps, 46°59′N, 11°07′E). At each alpine and subnival locality, early and late-thawing sites were chosen. Plant temperatures (boundary layer temperatures) were recorded at all sites at hourly intervals throughout the investigation period, using small data loggers (Tidbit, Onset, Bourne, MA, USA). To follow the developmental dynamics exactly, all investigations were conducted on individually labelled plants and flowers. Structural changes to reproductive tissues were quantitatively recorded using different microscopic methods (DIC, SEM, fluorescence microscopy) and image analysis software (Optimas 6.5, Optimas Corp., Seattle, WA, USA). For more details see e.g. Ladinig and Wagner (2007, 2009), Steinacher and Wagner (2010), and Wagner et al. (2010).
10.3 Timing of Flower Development
The majority of the investigated species extend the reproductive cycle over two growing seasons, but there were marked differences among species in the extent of flower preformation and the timing of the different reproductive phases (Fig. 10.1). Most investigated species show a two-season strategy, i.e. flower bud initiation occurs in the first year and flowering and fruiting in the second year. Only two species (C. uniflorum, S. caesia) follow the one-season strategy and develop the flower buds completely in the year of anthesis.
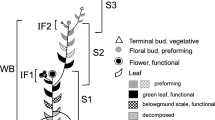
Timing of flower development in saxifrages in the investigation period 2001–2002 at climatically different sites. A alpine site; SN subnival site; Early, Mid, Late refer to the melting dates of the winter snow cover. Columns show different events during the year; narrow hatched: winter snow cover; wide hatched: temporary snow cover; white: periods without active flower development; grey: period of flower development in the first year; black: period of flower development in the year of anthesis (corresponds to the prefloration period); round symbol: anthesis
In S. oppositifolia, single terminal flowers develop on short-stem shoots. The preformed flower buds overwinter in a nearly fully differentiated pre-meiotic state. Meiosis is passed immediately after snowmelt; often female gametogenesis is still ongoing during anthesis (Wagner and Tengg 1993; Ladinig 2005; Larl and Wagner 2006). Anthesis starts about 1 week after snowmelt regardless of the date of snowmelt. This differs from flower bud initiation. In our investigations we found flower bud initials only in June and July. This means when anthesis occurs in May – which is the case in earlier melting sites in the alpine zone – flower bud formation starts about 1 month later. At later melting sites in the subnival zone, anthesis and flower bud formation started at the same time. This has led to the assumption that flower initiation of S. oppositifolia is day-length dependent and occurs under long-day conditions only (Larl and Wagner 2006). For arctic ecotypes it is possible that the long-day requirement for floral induction is particularly marked, as the plants experience 24-h days during the period of active growth.
S. biflora shows a similar developmental pattern to the closely related S. oppositifolia (Hörandl and Gutermann 1994; Gugerli 1997), however development proceeds faster. Unlike S. oppositifolia, S. biflora inflorescences bear 1–12 flowers. As S. biflora colonizes late melting sites in the subnival and nival zone, reproductive and vegetative development starts under the thinning snow cover, a phenomenon regularly observed when radiation reaches the ground (Kimball and Salisbury 1974; Salisbury 1985). The terminal flower starts anthesis about 1 week after snowmelt, lateral flowers open at intervals over the following week. During anthesis of the current year, flower buds for the following year show all flower whorls in a primordial state (Larl 2007). Flower preformation is terminated at the time of fruit maturity of the current year flowers, which occurs about 8 weeks after the plants have become snow-free. Terminal flowers reach a well-differentiated pre-meiotic state, lateral flowers lag somewhat behind.
S. moschata flowers about 1 month after snowmelt, which in our study on an alpine population was at the end of June in earlier melting sites and at the end of July for later sites. Irrespective of the flowering date, flower development did not start before late August. As a consequence, flower buds entered winter in an early primordial state and had to pass through most floral development stages in the year of anthesis (Larl 2007).
Similarly, flowers of S. bryoides develop largely or even completely in the year of anthesis (Ladinig and Wagner 2009). New floral apices appear as day-length decreases from August on. Flower buds attain only primordial stages before winter and form three cohorts of flowers in the second year. The most developed buds immediately resume floral development after winter and bloom about 7 weeks later. A second cohort of buds flowers about 10 weeks after snowmelt, whereas a third cohort does not develop beyond a middle stage. At the end of the growing season, flower buds of different stages are present, but only primordial stages survive winter.
S. caesia follows the one-season strategy (Larl 2007). The transition from the vegetative to reproductive apex (Fig. 10.2) possibly occurs during snowmelt in spring. In individuals becoming snow-free in early May it took 3 weeks until the floral apex of the terminal flower bud became visible. Bolting began 6 weeks and anthesis about 2 months after snowmelt. In late melting individuals (at the end of June–early July), development was clearly accelerated: early stages of flower development were passed within 1 week and anthesis set in within 7 weeks. At the latest melting site (mid-July) floral development was markedly retarded again and flower buds did not enter anthesis before winter.
Stages of floral development in Saxifraga caesia. (a) Stage 0: vegetative shoot apex (VA) with alternately arranged leaf primordia (L). (b) Stage 1: floral apex (FA) of the terminal flower forms; bract (B) with lateral flower bud (LB) visible. (c) Stage 2: sepal primordia (S) arise, stamen primordia (St) weakly visible. (d) Stage 3: stamen primordia clearly visible, petal primordia (P) appear. (e) Stage 4: carpels (C) emerge. (f) Early stage 5: stamen primordia differentiate into filaments and anthers, carpels begin to elongate. During the remaining course of stage 5 floral organs further elongate and differentiate (not shown). Carpels become cone-shaped and ovule primordia emerge. Meiosis occurs shortly before anthesis
Among the non-saxifrages, the Ranunculus species show the most advanced flower preformation (Widmann and Wagner, unpublished). In R. glacialis, inflorescences develop at the end of lateral branches of the below-ground sympodial rhizome system. In plants emerging from the winter snow, the shoot apex is already floral when it appears, which suggests that the transition from the vegetative to reproductive apex occurs at the end of the previous growing season. Flower bud preformation goes on below ground during flowering and fruiting of the current year and stops when the above-ground parts of the plants senesce. By this time sepals fully cover the flower bud, petals are still short, stamens begin to differentiate into filaments and anthers, and in the still poorly developed carpels ovule primordia emerge. After winter, flower buds need 2–3 more weeks before entering anthesis. During this period stamens and carpels further differentiate and sporogenesis and gametogenesis take place.
Taking all species together, the length of the prefloration period was negatively correlated with the degree of flower bud preformation at snowmelt in spring (Fig. 10.3). This signifies that the timing of flower development and the state of flower bud preformation in the first year had a clear impact on the length of the prefloration period (snowmelt to first flowering) in the second year. Or in other words, the differences in flowering phenology among different species at the same site to a large extent reflect the species-specific pattern of flower preformation. This is in accordance with what Molau et al. (2005) report for tundra plants in northern Swedish Lapland, when relating prefloration periods to the winter bud stages documented by Sørensen (1941) in northeast Greenland.
Correlation between the stage of flower preformation at snowmelt in spring and the length of the prefloration period (r = 0.94, Pearson, p < 0.001). For each species, the median of the maximum stage of flower development in n = 10 individuals emerging from the snow was determined and plotted against the mean prefloration period of individuals with the same melting date at the same site; error bars indicate the minimum and maximum of the first individual flowering; for staging see Fig. 10.2. For abbreviations of species names see Table 10.1. Data: S. Widmann, unpublished
Among the species investigated in our study, S. oppositifolia had the shortest prefloration period (6–10 days, Larl and Wagner 2006) which is in the range reported for arctic and alpine genotypes (Bliss 1971; Stenström and Molau 1992; Stenström et al. 1997). The prefloration time in S. biflora is similarly short. In order of increasing length, it is followed by R. alpestris (7–14 days), S. androsacea (9–20 days), R. glacialis (14–21 days, up to 30 days in the nival zone, Wagner et al. 2010) and A. alpina (21–28 days). S. moschata needed about 1 month (Ladinig and Wagner 2005). The longest prefloration periods were observed in C. uniflorum (6 weeks), S. bryoides (6–7 weeks, Ladinig and Wagner 2009) and S. caesia (8 weeks), in which flower preformation in the year before was limited (S. bryoides) or completely absent (C. uniflorum, S. caesia).
The total time taken from flower bud initiation to anthesis was longest in S. oppositifolia, S. biflora, R. glacialis, R. alpestris (about 1 year) and was shortest in C. uniflorum and S. caesia (6 and 8 weeks, respectively). Active flower development (i.e. the time between flower initiation and anthesis excluding the periods of winter dormancy and summer snow cover, which are not effectively used for development), however, did not differ much among species and generally amounted to 6–8 weeks. This signifies that there is little difference in the time needed to construct reproductive tissues among species, and it is the species-specific timing which causes developmental diversity.
10.4 Anthesis – The Functional Phase of the Flower
At corolla opening, the flower enters the functional phase, which in hermaphroditic flowers comprises the male phase (pollen dissemination) and the female phase (pollen deposition on the stigma and fertilization). The length of time a flower is functional may be an important determinant of male and female reproductive success (Evanhoe and Galloway 2002). Floral longevity is basically species-specific and depends on heritable traits such as gender sequence, breeding system and flower morphology (Primack 1985). However, flower longevity can be optimized by natural selection in response to the pollination environment (Ashman and Schoen 1994). Several studies have shown that flower longevity generally increases with altitude (Arroyo et al. 1981; Primack 1985; Bingham and Orthner 1998; Blionis and Vokou 2002), which is seen as compensation for the variability in pollinator visitation rates in the stochastic high mountain climate (Primack 1978; Arroyo et al. 1985; Muñoz and Arroyo 2006). Within a plant species, flower longevity is plastic and not a fixed trait. It may be extended or shortened in response to short-term environmental variations (Evanhoe and Galloway 2002; Clark and Husband 2007; Lundemo and Totland 2007). For 26 species tested in the European Alps, a mean flower longevity of 8.7 days was found (Fabbro and Körner 2004; Steinacher and Wagner 2010). However, there is a high variation among species, and within a species among different investigation periods, ranging from a few days (e.g. G. germanica, R. alpestris, C. uniflorum) to more than 2 weeks (R. glacialis, saxifrages); (Table 10.2). For the species listed in Table 10.2 we further tested the potential flower longevity, i.e. the capacity to prolong flower functions (corolla life-time; duration of stigma, style and ovule receptivity) in the case when pollinators are absent or rare (Steinacher and Wagner 2010). Unpollinated flowers generally increased longevity, but the plasticity of single floral functions was quite different. Among the female functions, stigma receptivity could be maintained longest (maximum stigma life-times were 29 days in R. glacialis and 24 days in G. germanica). Ovule receptivity, however, ceased between 16 and 20 days after onset of anthesis in most species. In some species, corolla life-time was even less plastic. Thus, the maximum longevities of individual flowers with fresh corolla and receptive pistils were around 20 days in saxifrages but only 8 days in R. alpestris and C. uniflorum.
As soon as compatible pollen is deposited on the stigma, the progamic phase, i.e. the period between pollination and fertilization, starts. Pollen germination and pollen tube growth are strongly temperature-dependent. Mountain plants show a wide optimum temperature range for progamic processes (Steinacher and Wagner 2011), which is consistent with the high temporal variability as a result of large diurnal variations in site temperatures (see Larcher, chap. 3, this book; Neuner and Buchner, chap. 6, this book). In the studied species (listed in Table 10.2), most progamic processes were still functioning at near freezing temperatures, which can be seen as an adaptation to the generally low night temperatures in high mountains (about 5°C in the alpine zone, 3–5°C in the subnival zone and ≤0–3°C in the nival zone; Larcher and Wagner 2009). At the other extreme, sexual functions were still intact at 25–30°C, which corresponds to the flower temperatures on clear summer days (Luzar and Gottsberger 2001; Steinacher and Wagner 2011). The length of the progamic phase strongly depends on the speed of pollen tube growth and on the species-specific lengths of stigma and style, which is the distance the pollen tubes have to cover. Highest speeds were attained at 30°C (mean growth rates 3,100 μm h−1 in G. germanica, 1,550 μm h−1 in C. uniflorum, 418 μm h−1 in R. glacialis, and 250 μm h−1 in R. alpestris) and at 25°C (saxifrages, mean maximal growth rate about 600 μm h−1). These speeds are within the range reported for lowland plants: e.g. Lilium longiflorum 2,400 μm h−1 at 17–20°C (Janson et al. 1994), Prunus avium 300 μm h−1 at 25°C and Primula obconica 290 μm h−1 at 30°C (Lewis 1942). Due to efficient pollen tube growth in most mountain species, the progamic phase lasted only a few hours at 20–30°C and between 12 and 30 h at 5°C (Steinacher and Wagner 2011). In comparison, lowland plants need 12–72 h at 20–30°C (Dafni et al. 2005) but mostly show a drastically reduced pollen performance below 10°C (e.g. Lewis 1942; Pasonen et al. 2000).
The full period from onset of anthesis until fertilization (Figs. 10.4 and 10.5) primarily depends on whether pistils are receptive from the very beginning (adichogamous and protogynous flowers), or whether the female phase follows the male phase (protrandrous flowers).
Phase lengths during seed development in different Saxifraga species at alpine and subnival sites. Columns give the mean duration ±SD for n sites (s) and years (y). Data based on about 100 investigated flowers and 300 seeds per site and year. Phases: (fert) time-span between onset of anthesis and fertilization, (hist) histogenesis, (mat) maturation phase, (dev) seed development from fertilization to seed maturity, and (total) total phase from onset of anthesis until seed maturity
Phase lengths during seed development in the nival plant species A. alpina, C. uniflorum and R. glacialis. Columns give the mean duration ±SD for n sites (s) and years (y). Data based on about 100 investigated flowers and 300 seeds per site and year. For phases see Fig. 10.4
Additionally, weather conditions (temperature, precipitation) and related pollinator frequency, and the speed of pollen tube growth (see above) affect this time span. Fertilization occurs fastest in the adichogamous species G. germanica, whose stigma is already receptive on the first day of anthesis (Steinacher and Wagner 2010). Seed development starts only a few hours after pollination (Steinacher and Wagner 2011). In the adichogamous to weakly protandrous species R. glacialis, stigmas lie close together at the onset of anthesis, however stigma tips are already papillous and receptive (Steinacher and Wagner 2010). Nevertheless, first fertilizations were observable only 2–3 days after onset of anthesis (DAA). This is because only about 60% of ovules contain mature embryo sacs when flowers open. In the remainder of ovules, gametogenesis was still going on. Similar holds true for the protogynous saxifrages S. androsacea, S. oppositifolia and S. biflora. Fertilizations occurred from two DAA in S. androsacea and after four to five DAA in S. oppositifolia and S. biflora. In the two latter species most ovules were still in an early stage of gametogenesis at the beginning of flowering (Wagner and Tengg 1993; Ladinig 2005). Thus pistils were ready for pollination but not for fertilization which can be seen as a mechanism to make self-fertilization more difficult. The moderately protrandrous flowers of C. uniflorum and A. alpina were fertilized four to six DAA, whereas the markedly protrandrous flowers of S. caesia, S. moschata and S. bryoides needed up to 10 days for fertilization to take place (Ladinig 2005; Ladinig and Wagner 2005, 2007).
10.5 Dynamics of Seed Development
Fertilization marks the onset of seed development which, depending on the species, started 2–10 days after onset of anthesis (DAA) (cf. Figs. 10.4 and 10.5). Two main phases of seed development can be distinguished: (1) histogenesis during which the seed coat, the nutrient tissue (endosperm or perisperm) and a globular embryo form and (2) seed maturation which comprises seed filling (reserve deposition), further embryo growth and maturation drying to acquire desiccation tolerance (Fig. 10.6a).
Seed development in Saxifraga androsacea. (a) Dynamics of seed development expressed as the increase in the length of the entire seed, the endosperm, and the embryo. Values are means ±SD of 100 seeds and 30 embryos on average. (b) Duration of histogenesis (open symbols), maturation period (grey symbols) and total period for seed development (black symbols) plotted against thermal time. Trend lines: linear regression for each period
The length of time taken to complete histogenesis differed markedly among species, even within the same genus. Within the saxifrages (Fig. 10.4), the mean time taken to form the seed tissues was relatively short in S. androsacea (15 days), S. bryoides (15 days) and S. caesia (16 days). The longest time taken to complete histogenesis was observed in the subnival population of S. oppositifolia (32 days), whereas the alpine population completed this phase in only 26 days. The length of histogenesis in saxifrages appears to be linked to seed size, as there is a positive correlation (Pearson r = 0.93, p = 0.007) between seed size and the length of histogenesis (Fig. 10.7). R. glacialis, C. uniflorum and A. alpina are exceptions with histogenesis taking only around 20 days despite seeds being comparatively large. The mode of resource allocation and the developmental pattern in these species might play a role. C. uniflorum and A. alpina partition most of their dry matter in above-ground tissues, and obviously invest carbon in offspring rather than in filling large below-ground reserve pools (Körner and Renhardt 1987). Furthermore, in the seeds of C. uniflorum, perisperm, instead of endosperm, evolves rather quickly from the existing nucellus tissue. In R. glacialis, the above-/below-ground dry matter ratio is comparatively small (Körner and Renhardt 1987; Prock and Körner 1996). However, the comparatively large leaves already show a highly positive leaf carbon balance 3–4 weeks after snowmelt (Diemer and Körner 1996), at a time when anthesis is largely over and young seeds start to develop. Thus, an abundant supply of carbohydrates by the leaves can be assumed.
Relationship between seed size and duration of histogenesis, the period of seed growth. There is a good correlation (Pearson r = 0.93, p = 0.007) between seed size and the length of histogenesis in saxifrages, but not for the remainder of species. For abbreviations of species names see Table 10.1
In most species investigated here, the zygote divides in an early stage of nuclear endosperm development. Firstly, a proembryo consisting of suspensor cells and an apical cell is formed. From the apical cell the embryo proper arises, which as a rule attains the globular stage at the end of histogenesis (Akhalkatsi and Wagner 1997; Ladinig 2005; Wagner et al. 2010). During seed maturation, the embryo further enlarges within the nutrient tissue. The final stage of embryo development depends both on the species and on the climatic site conditions. In a long growing season under favourable weather conditions, embryos of saxifrages mostly reach the early to late torpedo stage. In cool and short seasons, however, embryo growth often does not go beyond the heart stage, and seeds with underdeveloped embryos are shed (Ladinig and Wagner 2005, 2007). Mature seeds of R. glacialis generally contain a morphologically undifferentiated embryo in the late globular or early heart stage with only one poorly developed cotyledon (Wagner et al. 2010). By contrast, embryos of C. uniflorum are usually highly developed: the hypocotyl and cotyledons elongate markedly and due to spatial restrictions within the seed become curved (Wagner and Tengg 1993). During maturation, seeds of most investigated species become dormant. The mechanisms range from cold-stratification requirements (C. uniflorum, G. germanica) to complex still unknown dormancy mechanisms (R. glacialis, most saxifrages).
Depending on the plant species, the maturation phase lasted from 1 week (C. uniflorum, R. glacialis) to 1 month (A. alpina). No relationships between the length of the maturation phase on the one hand and the length of histogenesis, seed size and embryo size on the other hand could be found. The maturation process obviously follows a species-specific autonomous programme and moreover seems to be scarcely affected by temperature (Wagner and Reichegger 1997; Wagner and Mitterhofer 1998).
The period for seed development (histogenesis plus maturation) in a single flower is shortest in R. glacialis and C. uniflorum (28 days on average; Fig. 10.5). S. androsacea, S. bryoides und S. moschata require about 31 days (Fig. 10.4). Seed development lasts longest in S. oppositifolia (subnival site 46 days, alpine site 58 days), and A. alpina (52 days) which represents a doubling of time compared with the fastest group of species. Adding the time for seed development to the time between onset of anthesis and fertilization results in a total postfloration period of about 1 month for an individual flower in the fastest group of species (Fig. 10.8a).
(a) Species order according to the average length of the postfloration period (i.e. the time-span between onset of anthesis and seed maturity) of individual flowers presented by the total length of each column. Within the column the time spans from onset of anthesis until fertilization (grey filling), for histogenesis (hatched) and for maturation (dark grey) are indicated. (b) Species order after adding the prefloration period (i.e. the time span between snowmelt and first flowering; white filling) to the columns in A. The total column length gives the time from snowmelt to first seed maturity
Within the saxifrages development is only as fast in S. androsacea, mainly because flowers are protogynous and histogenesis is particularly short. Most saxifrages need between 40 and 50 days. However, the alpine population of S. oppositifolia needs more than 60 days and the postfloration period in A. alpina is similarly long (57 days on average). Remarkably, seeds of S. oppositifolia mature more rapidly in the subnival than in the alpine population. It is possible that subnival genotypes – which have adapted to the shorter growing season by enhancing development including floral development and leaf turnover (Larl and Wagner 2006) – have evolved. Evidence for adaptive variation within S. oppositifolia comes from early and late flowering genotypes in the high Arctic which differ markedly in morphology, growth speed, and various ecophysiological characteristics (Crawford et al. 1995; Brysting et al. 1996; Kume et al. 1999).
10.6 Time Lapse from Snowmelt to Seed Maturity
The species-specific period required for reproductive development within a growing season comprises the prefloration period (snowmelt until onset of anthesis) and the postfloration period (onset of anthesis until seed maturity); (Fig. 10.8b). The minimum total period to produce at least some mature seeds is shortest in R. alpestris, R. glacialis and S. androsacea (40–44 days), somewhat longer in S. biflora (55 days), followed by A. alpina, C. uniflorum, S. oppositifolia and S. moschata (about 70 days). The longest reproductive periods were found in S. bryoides (90 days) and S. caesia (110 days). This period can be seen as decisive for the colonization potential of a species in high mountain habitats. Only species that regularly produce mature seeds have the chance to establish at a site. To prevent seed production from being reduced to a level below that necessary for recruitment, at least some individuals within a population have to produce mature seeds. To achieve this, more time than the minimal periods indicated above is necessary. For the investigated species, the period needed to complete reproductive development in most individuals at a site extends by a further 2–3 weeks (Fig. 10.9).
Time span between snowmelt and first seed maturity (dark bar) and seed maturity in most individuals (right end of the white bar) compared with the lengths of the growing seasons 2003–2009 at early, mid and late-melting alpine and subnival sites (open circles). In the alpine zone only the late flowering species S. caesia was at risk of not maturing seeds. In the subnival zone, R. glacialis was never at risk, whereas the remainder of species, depending on the site, failed to mature seeds in 1–4 years. Mean melting dates at early/mid/late sites were day numbers 125/140/150 for the alpine zone and day numbers 155/170/180 for the subnival zone
Comparing the time required from snowmelt to first seed maturity with the length of the growing season shows that in the alpine zone only later flowering individuals such as S. caesia are at risk of not maturing seeds in time, particularly at mid and late melting sites. The situation is different in the subnival zone. During the investigation period 2003–2009 the seed crop of R. glacialis was never at risk. Only in 1 year did S. biflora and S. oppositifolia fail to produce any mature seeds and then only at a later melting site. C. uniflorum failed to produce mature seeds in the same year at both a mid and late melting site. For A. alpina and S. bryoides reproductive success was ensured only at early melting sites, but failed at mid and late melting sites in 3 and 4 years out of 7, respectively.
10.7 Reproductive Development and Temperature
Temperature generally influences the length of reproductive phases as reported for a number of arctic and alpine plant species (Sandvik and Totland 2000; Inouye et al. 2002; Molau et al. 2005 and citations therein; Huelber et al. 2006). This in principle also applies to the species investigated here, but our analyses have shown that the temperature effect is not linear over the full temperature range. We tested the hypothesis that variation in the speed of development was affected by site temperatures, by calculating cumulative degree-days (thermal time) as a measure for total heat accumulation during different developmental phases. Contrary to our expectations, there was frequently no relationship or even a positive one between the heat sum and the length of the different developmental phases, e.g. in S. moschata (Ladinig and Wagner 2005) and S. oppositifolia (Larl and Wagner 2006). A further example is shown in Fig. 10.6b for the phases of seed development in S. androsacea. In different periods of investigation, histogenesis remained constant at around 13 days, while the temperature sum varied between 30 and 200 degree days. Maturation and the whole period for seed development turned out to be even longer the higher the temperature sum was. A missing or a positive relationship between developmental time and thermal time indicates that temperature was not generally limiting reproductive development, on the contrary, there were warmer periods during which tissue differentiation could not be further accelerated. However, in some cases the heat sum did matter. So, the length of seed development and its sub-phases were negatively correlated with thermal time in S. bryoides (Ladinig and Wagner 2007) and G. germanica (Wagner and Mitterhofer 1998). In both cases seed development occurred relatively late in the growing season (August and September–October, respectively), when a heat surplus is less likely. Thus, thermal time is useful only to a degree for explaining development times in mountain plants and mostly fails during warmer periods in high summer and at thermally favoured, sun-lit sites where plants can be up to 20 K warmer than the free air temperature (Larcher and Wagner 2009; Larcher, chap. 3, this book; Neuner and Buchner, chap. 6, this book). The relationships between the length of a developmental phase and the frequency of hours which can not be used for growth was more consistent (Ladinig and Wagner 2007; Wagner et al. 2010). For cold-adapted plant species the thermal limit for growth usually lies around 2–3°C (Körner 2003, 2006). Thus in mountain plants, the length of a developmental phase is not necessarily a function of the sum of warm hours but to a large extent depends on the frequency of hours with low temperatures when development slows down or even stops.
10.8 Reproductive Development and Day Length
Photoperiod can affect both flower initiation (primary induction) and flowering (secondary induction; Heide 1994). Particularly in cold habitats with marked seasonal variations in climate, photoperiod control plays a crucial role in the correct timing of developmental processes. But this also means that the time available for certain developmental processes to be initiated and completed is minimized. Most knowledge about the effect of photoperiod on primary and secondary induction stems from laboratory investigations on cold-adapted plants of northern origin (e.g. Heide 1989, 1992a, b; Heide et al. 1990). In phytotron experiments most species show a short day (SD) requirement for flower induction over a wide temperature range, which is remarkable, as plants usually do not experience SD in their natural environment at high latitudes during the growing season. However, most species also have an alternative long day (LD) pathway for floral initiation at low temperatures (12°C and lower) and possibly initiate floral primordia in the late arctic summer in response to low temperatures (Heide et al. 1990). Flowering occurs under LD conditions only, whereby a 24 h LD (i.e. continuous light) is most promoting. European ecotypes of Oxyria digyna from different latitudes (45–78°N) show a short-long-day response as well. However, the critical day lengths vary among plants of different origins, which clearly indicates adaptation to the respective environment (Heide 2005). The SD response for flower initiation was greatest in provenances from Central Europe and decreased with increasing latitude; conversely, the critical day length for LD secondary induction of flowering increased from the southern to the northern populations. Keller and Körner (2003) investigated the role of photoperiodism and temperature on flowering (second induction) in 23 high-elevation species of the European Central Alps in the laboratory. About half of the species were found to be sensitive to photoperiod and flowered under LD conditions only; the rest were either insensitive to photoperiod or needed decreasing day length for flowering (three species). Most species were insensitive to temperature under LD-conditions, whereas an increase in temperature enhanced flowering under SD conditions. Interestingly, typical high-elevation species such as C. uniflorum, Elyna myosuroides, R. glacialis, S. oppositifolia and S. seguieri were insensitive to both photoperiod and temperature.
In our in situ study, S. oppositifolia was found to initiate flower buds (primary induction) only under long-day conditions (above 15 h) in June and July (Larl and Wagner 2006). Individuals thawing in early May did not develop floral shoot apices until the beginning of June. By contrast, individuals that became snow-free in July started floral development immediately after thawing. Similarly, in S. biflora early floral stages were observed immediately after thawing in mid-July. Flower development quickly proceeded during August, but at this time no more flowers were initiated (Larl 2007). Thus, S. oppositifolia and S. biflora follow the strategy of long-day plants combined with a low temperature requirement during winter as a precondition for flowering in the following growing season. Proleptic flowers in S. oppositifolia without winter vernalization were not functional, showing malformed anthers and pistils. Unlike S. oppositifolia and S. biflora, S. moschata did not set flowers before the end of August (day length 12–13 h), irrespective of the date of thawing in spring (cf. Fig. 10.1). This species obviously needs a decreasing day length to shift from the vegetative to the reproductive state and thus can be classified as short-day plant with a vernalization requirement for flowering in the next growing season. S. caesia is different from the other saxifrages as this species sets flowers in the year of anthesis, irrespectively of the day-length. When cultivated under lowland conditions, floral initiation occurs already in March (day length 11–12 h) and flowering starts in mid-May (A. Seiwald and J. Wagner, unpublished). At the alpine sites, floral initiation can be observed soon after snowmelt from late May onwards.
Thus, day length seems to affect flower initiation in at least three of the investigated saxifrages, however the date of anthesis is not affected. First flowering was primarily dependent on the date of snowmelt and set in after a species-specific prefloration period which is needed to complete floral development after winter dormancy (cf. Fig. 10.3). However, it has to be added that a possible LD requirement for flowering would have remained undetected in our field studies, as most of the investigated species had already experienced photoperiods of 15 h (passed in mid-May in Central Europe) and longer when thawing. According to Keller and Körner (2003) the critical photoperiod is 15 h, below which photoperiod-sensitive species show a response. In this context the observation that S. biflora individuals, transplanted to the alpine site, did not start flowering before mid-June though plants had become snow-free 2–3 weeks earlier is noteworthy. This would point to a distinct LD requirement for flowering, which is met at the later melting sites where this species usually occurs (J. Wagner, pers. obs.).
10.9 Differences in Reproductive Strategies Between Alpine and Nival Plant Species
One of our objectives was to find out whether plant species colonizing the nival zone employ special reproductive strategies enabling quick and effective seed production. Our investigations have shown that there is more than one reproductive strategy which is suited to the particular climatic requirements in the nival zone. There are species restricted to the alpine zone that reproduce quickly and effectively (e.g. R. alpestris, S. androsacea) and typical nival plant species that require a surprisingly long period for reproduction (S. bryoides, A. alpina, C. uniflorum).
R. glacialis appears best adapted for a life at high altitudes combining a short developmental period with a high phenological plasticity, and a relatively high reproductive success even at nival sites (Wagner et al. 2010). S. biflora, though requiring about 55 days for seed production, can use even shorter snow-free periods by maturing seeds below the snow (Ladinig 2005). In contrast to S. oppositifolia, S. biflora does not show reduced reproductive fitness when thawing late and flowering and fruiting in August. The sexual reproduction of S. bryoides is amazingly vulnerable to climatic extremes. Summer snow fall and frost from −2°C and lower regularly injure a large number of flower buds, flowers and young fruits (Ladinig et al. in prep.). In addition, the species is a typical seed-risker in the sense of Molau (1993), failing to mature seeds when winter conditions set in too early. But a high seed number in a single matured capsule might compensate for the regular losses of reproductive structures (Ladinig and Wagner 2007). Equally, C. uniflorum and A. alpina are at high risk of losing the seed crop when the growing season is too short. Accordingly, fruit set varies between 0% and 100% (C. uniflorum) and between 0% and 60% (A. alpina) among sites and years (G. Steinacher, S. Erler unpublished). These results show that quick and efficient reproduction, though advantageous, does not seem to be a prerequisite to colonize the nival zone. More important than a yearly seed crop might be the individual lifetime-reproductive success. Compact alpine cushion plants can live for several decades (Morris and Doak 1998) and contribute to seed banks in climatically favourable growing seasons (Molau and Larsson 2000).
Germination and seedling establishment is the most critical phase in the life cycle of a plant. Though climatic and mechanistic constraints increase with altitude, recent studies did not show significant relationships between establishment and altitude (Venn and Morgan 2009; Cavieres et al. 2007). Rather, the combination of various microsite factors such as shelter, soil moisture, substrate type, and extreme low and high substrate temperatures seems to be more decisive for seedling survival (Giménez-Benavides et al. 2007a; Wenk and Dawson 2007; Venn and Morgan 2009). Seed size may play a positive role in seedling emergence, particularly at higher altitudes. When compared to related lowland species, alpine species tend to have larger seeds as was shown for 29 species pairs in the Swiss Alps (Pluess et al. 2005). As seedlings of large-seeded species have higher survivorship than those of small-seeded species (Westoby et al. 1997), there might be a selection pressure for species with heavier seeds at higher altitude (Pluess et al. 2005). The species investigated in our study largely match this thesis (cf. Fig. 10.7). Within the saxifrages, the pure alpine species S. androsacea and S. caesia form the smallest seeds, S. oppositifolia the largest at both sites. When comparing all species, the typical nival species A. alpina, R. glacialis, and C. uniflorum are characterised by particularly large seeds.
10.10 Reproduction in a Changing Climate
The extraordinarily warm year of 2003 can serve as a model for how climate warming, together with a longer growing season, could affect the reproductive performance of high mountain plants. The growing season started about 1 month earlier at all sites and warm and dry weather conditions prevailed until the beginning of September. During the growing season, mean boundary layer temperatures were about 3 K higher at all altitudinal levels than in climatically normal years, and precipitation was 30–40% less than usual (Ladinig and Wagner 2005, 2007, 2009). Under these climatic conditions reproductive development was expected to be enhanced, the reproductive success to be particularly high and more shoot apices were expected to shift from vegetative to reproductive, increasing the flower frequency in the following year. As already stated earlier, above a certain temperature threshold, more warmth could not accelerate reproductive development. However, the long growing season was beneficial for later flowering species in that fruits became ripe long before onset of winter conditions. Nevertheless, reproductive success was not increased in all species. For S. moschata, S. bryoides and S. caesia the period of seed development coincided with the warmest but also driest months June and July. Enduring drought led to substantial losses during seed development (Ladinig and Wagner 2005, 2007). On the other hand, species with quick, early seed development such as R. glacialis and R. alpestris were not affected by summer drought, because they were supplied with sufficient water from snowmelt during their active phase. In these species, seed set was significantly higher than in a standard year. A check of the flowering frequency in the 2004 growing season did not show a significant increase in flowering shoots. On the contrary, S. oppositifolia sets even less flowers than in the preceding year, and in S. moschata a high percentage of shoots had died off.
These examples show that the effect of a change in site climate on different plant species would strongly depend on the seasonal timing of their development. Climate warming and a longer growing season might be beneficial for middle to late flowering species that need longer for reproductive development, but would increase the risk of heat and drought damage (Buchner and Neuner 2003; Giménez-Benavides et al. 2007b). When the growing season starts earlier, early flowering species are more at risk of damage from late spring frost events. An increase in the frequency of frost damage because of earlier snowmelt has been reported for both high altitudes (Bannister et al. 2005; Inouye 2008) and high latitudes (Molau 1996, 1997). Hence, the impacts of a changing climate differ among species according to the phenological response. The persistence of each species will essentially depend on how often and to what extent sensitive developmental phases are impaired by changing climatic forces.
References
Akhalkatsi M, Wagner J (1997) Comparative embryology of three Gentianaceae species from the central Caucasus and the European Alps. Plant Syst Evol 204:39–48
Amen RD (1966) The extent and role of seed dormancy in alpine plants. Quart Rev Biol 4:271–281
Anchisi E (1985) Quatrieme contribution à l´étude de la flore valaisanne. Bull Murithienne 102:115–126
Arroyo MTK, Armesto JJ, Villagran C (1981) Plant phenological patterns in the high Andean Cordillera of central Chile. J Ecol 69:205–223
Arroyo MTK, Armesto J, Primack R (1985) Community studies in pollination ecology in the high temperate Andes of central Chile. II. Effect of temperature on visitation rates and pollination possibilities. Plant Syst Evol 149:187–203
Ashman TL, Schoen DJ (1994) How long should flowers live? Nature 371:788–791
Bahn M, Körner C (1987) Vegetation und Phänologie der hochalpinen Gipfelflur des Glungezer in Tirol. Ber Nat med Verein Innsbruck 74:61–80
Bannister P, Maegli T, Dickinson KJM, Halloy STP, Knight A, Lord JM, Mark AF, Spencer KL (2005) Will loss of snow cover during climatic warming expose New Zealand alpine plants to increased frost damage? Oecologia 144:245–256
Billings WD, Mooney HA (1968) The ecology of arctic and alpine plants. Biol Rev 43:481–529
Bingham RA, Orthner AR (1998) Efficient pollination of alpine plants. Nature 391:238–239
Blionis GJ, Vokou D (2002) Structural and functional divergence of Campanula spatula subspecies on Mt Olympos (Greece). Plant Syst Evol 232:89–105
Bliss LC (1971) Arctic and alpine plant life cycles. Annu Rev Ecol Syst 2:405–438
Brysting AK, Gabrielsen TM, Sørlibråten O, Ytrehorn O, Brochmann C (1996) The purple saxifrage, Saxifraga oppositifolia, in Svalbard: two taxa or one? Polar Res 15:93–105
Buchner O, Neuner G (2003) Variability of heat tolerance in alpine plant species measured at different altitudes. Arct Antarct Alp Res 35:411–420
Cavieres L, Badano EI, Sierra-Almeida A, Molina-Montenegro MA (2007) Microclimatic modifications of cushion plants and their consequences for seedling survival of native and non-native herbaceous species in the high Andes of central Chile. Arct Antarct Alp Res 39:229–236
Clark MJ, Husband BC (2007) Plasticity and timing of flower closure in response to pollination in Chamerion angustifolium (Onagraceae). Int J Plant Sci 168:619–625
Crawford RMM (2008) Plants at the margin. Ecological limits and climate change. Cambridge University Press, Cambridge/New York/Melbourne
Crawford RMM, Chapman HM, Smith LC (1995) Adaptation to variation in growing season length in arctic populations of Saxifraga oppositifolia L. Bot J Scotland 41:177–192
Dafni A, Kevan PG, Husband BC (2005) Practical pollination biology. Enviroquest Ltd., Cambridge
Diemer M, Körner C (1996) Lifetime leaf carbon balances of herbaceous perennial plants from low and high altitudes in the central Alps. Funct Ecol 10:33–43
Diggle PK (1997) Extreme preformation in alpine Polygonum viviparum: an architectural and developmental analysis. Am J Bot 84:154–169
Erschbamer B, Kneringer E, Niederfriniger-Schlag R (2001) Seed rain, soil seed bank, seedling recruitment, and survival of seedlings on a glacier foreland in the central Alps. Flora 196:304–312
Evanhoe L, Galloway LF (2002) Floral longevity in Campanula americana (Campanulaceae): a comparison of morphological and functional gender phases. Am J Bot 89:587–591
Fabbro T, Körner C (2004) Altitudinal differences in flower traits and reproductive allocation. Flora 199:70–81
Galen C, Stanton M (1991) Consequences of emergence phenology for reproductive success in Ranunculus adoneus (Ranunculaceae). Am J Bot 78:978–988
Galen C, Stanton M (1995) Responses of snowbed plant species to changes in growing-season length. Ecology 76:1546–1557
Giménez-Benavides L, Escudero A, Pérez-García F (2005) Seed germination of high mountain mediterranean species: altitudinal, interpopulation and interannual variability. Ecol Res 20:433–444
Giménez-Benavides L, Escudero A, Iriondo JM (2007a) Local adaption enhances seedling recruitment along an altitudinal gradient in a high mountain mediterranean plant. Ann Bot 99:723–734
Giménez-Benavides L, Escudero A, Iriondo JM (2007b) Reproductive limits of a late-flowering high-mountain mediterranean plant along an elevational climate gradient. New Phytol 173:367–382
Grabherr G, Gottfried M, Gruber A, Pauli H (1995) Patterns and current changes in alpine plant diversity. In: Chapin FS III, Körner C (eds) Arctic and alpine biodiversity, Ecological studies 113. Springer, Berlin, pp 167–181
Grabherr G, Nagy L, Thompson DBA (2003) An outline of Europe’s alpine areas. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe, Ecological studies 167. Springer, Berlin/Heidelberg, pp 3–12
Gugerli F (1997) Hybridization of Saxifraga oppositifolia and S. biflora (Saxifragaceae) in a mixed alpine population. Plant Syst Evol 207:255–272
Hegi G (1975) Illustrierte Flora von Mitteleuropa, vol V/3. Paul Parey, Berlin
Heide OM (1989) Environmental control of flowering and viviparous proliferation in seminiferous and viviparous Arctic populations of two Poa species. Arct Alp Res 21:305–315
Heide OM (1992a) Flowering strategies of the high-arctic and high-alpine snow bed grass species Phippsia algida. Physiol Plant 85:606–610
Heide OM (1992b) Experimental control of flowering in Carex bigelowii. Oikos 65:371–376
Heide OM (1994) Control of flowering and reproduction in temperate grasses. New Phytol 128:347–362
Heide OM (2005) Ecotypic variation among European arctic and alpine populations of Oxyria digyna. Arct Antarc Alp Res 37:233–238
Heide OM, Pedersen K, Dahl E (1990) Environmental control of flowering and morphology in the high-arctic Cerastium regelii, and the taxonomic status of C. jenisejense. Nord J Bot 10:141–147
Hörandl E, Gutermann W (1994) Populationsstudien an Sippen von Saxifraga sect. Porphyrion (Saxifragaceae) in den Alpen: I. Hybriden von S. biflora und S. oppositifolia. Phyton (Horn) 34:143–167
Huelber K, Gottfried M, Pauli H, Reiter K, Winkler M, Grabherr G (2006) Phenological responses of snowbed species to snow removal dates in the central Alps: implications for climate warming. Arct Antarc Alp Res 38:99–103
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362
Inouye D, Morales MA, Dodge GJ (2002) Variation in timing and abundance of flowering by Delphinium barbeyi Huth (Ranunculaceae): the roles of snowpack, frost, and La Niña, in the context of climate change. Oecologia 130:543–550
Inouye DW, Saavedra F, Lee-Yang W (2003) Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae). Am J Bot 90:905–910
Janson J, Reinders MC, Valkering AGM, Vantuyl JM, Keijzer CJ (1994) Pistil exudate production and pollen-tube growth in Lilium longiflorum Thunb. Ann Bot 73:437–446
Kaplan K (1995) Saxifragaceae. In: Weber HE (ed) Gustav Hegi – Illustrierte Flora von Mitteleuropa, vol 4/2A. Blackwell, Berlin, pp 130–229
Keller F, Körner C (2003) The role of photoperiodism in alpine plant development. Arct Antarc Alp Res 35:361–368
Kimball SL, Salisbury FB (1974) Plant development under snow. Bot Gaz 135:147–149
Körner C (2003) Alpine plant life, 2nd edn. Springer, Berlin
Körner C (2006) Significance of temperature in plant life. In: Morison JIL, Morecroft MD (eds) Plant growth and climate change. Blackwell, Oxford, pp 48–69
Körner C (2011) Coldest places on earth with angiosperm plant life. Alp Botany 121:11–22
Körner C, Diemer M (1987) In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct Ecol 1:179–194
Körner C, Renhardt U (1987) Dry matter partitioning and root length/leaf area rations in herbaceous perennial plants with diverse altitudinal distribution. Oecologia 74:411–418
Kudo G (1991) Effects of snow-free period on the phenology of alpine plants inhabiting snow patches. Arct Alp Res 23:436–443
Kudo G (1992) Performance and phenology of alpine herbs along a snow-melting gradient. Ecol Res 7:297–304
Kudo G, Hirao AS (2006) Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul Ecol 48:49–58
Kudo G, Suzuki S (1999) Flowering phenology of alpine plant communities along a gradient of snowmelt timing. Polar Biosci 12:100–113
Kume A, Nakatsubo T, Bekku Y, Masuzawa T (1999) Ecological significance of different growth forms of purple saxifrage, Saxifraga oppositifolia L., in the High Arctic, Ny-Ålesund, Svalbard. Arct Antarct Alp Res 31:27–33
Ladinig U (2005) Reproductive development of Saxifraga-species in a high mountain climate. Doctoral thesis, University Innsbruck
Ladinig U, Wagner J (2005) Sexual reproduction of the high mountain plant Saxifraga moschata Wulfen at varying lengths of the growing season. Flora 200:502–515
Ladinig U, Wagner J (2007) Timing of sexual reproduction and reproductive success in the high-mountain plant Saxifraga bryoides L. Plant Biol 9:683–693
Ladinig U, Wagner J (2009) Dynamics of flower development and vegetative shoot growth in the high mountain plant Saxifraga bryoides L. Flora 204:63–73
Landolt E (1992) Unsere Alpenflora. Fischer, Stuttgart
Larcher W (1980) Klimastreß im Gebirge – Adaptationstraining und Selektionsfilter für Pflanzen. Rheinisch-Westfälische Akad Wiss 291:49–88
Larcher W, Wagner J (1976) Temperaturgrenzen der CO2-Aufnahme und Temperaturresistenz der Blätter von Gebirgspflanzen im vegetationsaktiven Zustand. Oecol Plant 11:361–374
Larcher W, Wagner J (2009) High mountain bioclimate: temperatures near the ground recorded from the timberline to the nival zone in the Central Alps. Contrib Nat Hist Berne 12:765–782
Larcher W, Kainmüller C, Wagner J (2010) Survival types of high mountain plants under extreme temperatures. Flora 205:3–18
Larl I (2007) Flower development in high mountain Saxifrages. Doctoral thesis, University Innsbruck
Larl I, Wagner J (2006) Timing of reproductive and vegetative development in Saxifraga oppositifolia in an alpine and a subnival climate. Plant Biol 8:155–166
Lewis D (1942) The physiology of incompatibility in plants I. The effect of temperature. Proc R Soc Lond B Biol Sci 131:13–26
Lundemo S, Totland Ø (2007) Within-population spatial variation in pollinator visitation rates, pollen limitation on seed set, and flower longevity in an alpine species. Acta Oecol 32:262–268
Luzar N, Gottsberger G (2001) Flower heliotropism and floral heating of five alpine plant species and the effect on flower visiting in Ranunculus montanus in the Austrian Alps. Arct Antarct Alp Res 33:93–99
Makrodimos N, Blionis GJ, Krigas N, Vokou D (2008) Flower morphology, phenology and visitor patterns in an alpine community on Mt Olympos, Greece. Flora 203:449–468
Marcante S, Schwienbacher E, Erschbamer B (2009) Genesis of a soil seed bank on a primary succession in the central Alps (Ötztal, Austria). Flora 204:434–444
Mark AF (1970) Floral initiation and development in New Zealand alpine plants. N Z J Bot 8:67–75
Molau U (1993) Relationships between flowering phenology and life history strategies in tundra plants. Arct Alp Res 25:391–402
Molau U (1996) Climatic impacts on flowering, growth and vigour in an arctic–alpine cushion plant, Diapensia lapponica, under different snow cover regimes. Ecol Bull 45:210–219
Molau U (1997) Phenology and reproductive success in arctic plants: susceptibility to climate change. In: Oechel WC, Callaghan T, Gilmanov T, Holten JI, Maxwell B, Molau U, Sveinbjörnsson B (eds) Global change and arctic terrestrial ecosystems, Ecological studies 124. Springer, Berlin/Heidelberg, pp 153–170
Molau U, Larsson E-L (2000) Seed rain and seed bank along an alpine altitudinal gradient in Swedish Lapland. Can J Bot 78:728–747
Molau U, Nordenhäll U, Eriksen B (2005) Onset of flowering and climate variability in an alpine landscape: a 10-year study from Swedish Lapland. Am J Bot 92:422–431
Morris WF, Doak DF (1998) Life history of the long-lived gynodioecious cushion plant Silene acaulis (Caryophyllaceae), inferred from size-based population projection matrices. Am J Bot 85:784–793
Muñoz A, Arroyo MTK (2006) Pollen limitation and spatial variation of reproductive success in the insect-pollinated shrub Chuquiraga oppositifolia (Asteraceae) in the Chilean Andes. Arct Antarct Alp Res 38:608–613
Nakhutsrishvili G (1999) The vegetation of Georgia (Caucasus). Braun Blanquetia 15:5–74
Neuner G, Braun V, Buchner O, Taschler D (1999) Leaf rosette closure in the alpine rock species Saxifraga paniculata Mill.: significance for survival of drought and heat under high irradiation. Plant Cell Environ 22:1539–1548
Ozenda P (1988) Die Vegetation der Alpen. Elsevier, München
Pasonen HL, Kapyla M, Pulkkinen P (2000) Effects of temperature and pollination site on pollen performance in Betula pendula Roth – evidence for genotype-environment interactions. Theor Appl Genet 100:1108–1112
Pauli H, Gottfried M, Grabherr G (1999) Vascular plant distribution patterns at the low-temperature limits of plant life – the alpine-nival ecotone of Mount Schrankogel (Tyrol, Austria). Phytocoenologia 29:297–325
Pluess A, Schütz W, Stöcklin J (2005) Seed weight increases with altitude in the Swiss Alps between related species but not among populations of individual species. Oecologia 144:55–61
Primack R (1978) Variability in New Zealand montane and alpine pollinator assemblages. N Z J Ecol 1:66–73
Primack RB (1985) Longevity of individual flowers. Annu Rev Ecol Syst 16:15–37
Prock S (1990) Symphänologie der Pflanzen eines kalkalpinen Rasens mit besonderer Berücksichtigung der Wachstumsdynamik und Reservestoffspeicherung charakteristischer Arten. Ber Nat-med Verein Innsbruck 77:31–56
Prock S, Körner C (1996) A cross-continental comparison of phenology, leaf dynamics and dry matter allocation in arctic and temperate zone herbaceous plants from contrasting altitudes. Ecol Bull 45:93–103
Salisbury FB (1985) Plant adaptations to the light environment. In: Kaurin A, Junttila O, Nilsen J (eds) Plant production in the north. Norwegian University Press, Tromsø, pp 43–61
Sandvik S, Totland O (2000) Short-term effects of simulated environmental changes on phenology, reproduction, and growth in the late-flowering snowbed herb Saxifraga stellaris L. Ecoscience 7:201–213
Shimono Y, Kudo G (2005) Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol Res 20:189–197
Sørensen T (1941) Temperature relations and phenology of the northeast Greenland flowering plants. Medd Grønland 125:1–307
Steinacher G, Wagner J (2010) Flower longevity and duration of pistil receptivity in high mountain plants. Flora 205:376–387
Steinacher G, Wagner J (2011) Effect of temperature on the progamic phase in high-mountain plants. Plant Biology, in press
Stenström M, Molau U (1992) Reproductive ecology of Saxifraga oppositifolia: phenology, mating system, and reproductive success. Arct Alp Res 24:337–343
Stenström M, Gugerli F, Henry GHR (1997) Response of Saxifraga oppositifolia L. to simulated climate change at three contrasting latitudes. Global Change Biol 3:44–54
Stöcklin J, Bäumler E (1996) Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. J Veg Sci 7:45–56
Taschler D, Neuner G (2004) Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ 27:737–746
Theurillat J-P, Schlüssel P (2000) Phenology and distribution strategy of key plant species within the subalpine-alpine ecocline in the Valaisan Alps (Switzerland). Phytocoenologia 30:439–456
Venn SE, Morgan JW (2009) Patterns in alpine seedling emergence and establishment across a stress gradient of mountain summits in south-eastern Australia. Plant Ecol Div 1:5–16
Wagner J, Mitterhofer E (1998) Phenology, seed development, and reproductive success of an alpine population of Gentianella germanica in climatically varying years. Bot Acta 111:159–166
Wagner J, Reichegger B (1997) Phenology and seed development of the alpine sedges Carex curvula and Carex firma in response to contrasting topoclimates. Arct Alp Res 29:291–299
Wagner J, Tengg G (1993) Phänoembryologie der Hochgebirgspflanzen Saxifraga oppositifolia und Cerastium uniflorum. Flora 188:203–212
Wagner J, Steinacher G, Ladinig U (2010) Ranunculus glacialis L.: successful reproduction at the altitudinal limits of higher plant life. Protoplasma 243:117–128
Wenk EH, Dawson TE (2007) Interspecific differences in seed germination, establishment, and early growth in relation to preferred soil type in an alpine community. Arct Antarct Alp Res 39:165–176
Westoby M, Leishman M, Lord J (1997) Comparative ecology of seed size and dispersal. In: Silvertown J, Franco M, Harper JL (eds) Plant life histories – ecology, phylogeny and evolution. Cambridge University Press, Cambridge, pp 143–162
Zimmermann W (1975) Ranunculaceae. In: Rechinger KH, Damboldt J (eds) Gustav Hegi – Illustrierte Flora von Mitteleuropa, vol 3/3. Paul Parey, Berlin, pp 53–341
Acknowledgements
This work was supported by funding from the Austrian Science Foundation (FWF-projects P15595-B3 “Diversity of sexual reproduction in high-mountain plants” and P18398-BO3 “Pollen tube growth and pistil receptivity of high-mountain plants under extreme climatic conditions”) to J. Wagner. We thank S. Erler and S. Widmann for providing data, and W. Sakai for SEM preparation. We further thank the Patscherkofelbahn and the Stubai Gletscherbahn for free transportation by cable-car.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/Wien
About this chapter
Cite this chapter
Wagner, J., Ladinig, U., Steinacher, G., Larl, I. (2012). From the Flower Bud to the Mature Seed: Timing and Dynamics of Flower and Seed Development in High-Mountain Plants. In: Lütz, C. (eds) Plants in Alpine Regions. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0136-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0136-0_10
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0135-3
Online ISBN: 978-3-7091-0136-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)