Abstract
Perovskite solar cells (PSCs) have attracted more attention recently due to their high efficiency, low cost, and long charge carrier diffusion length. Generally, PSCs consist of three layers: the electron transport layer (ETL), the absorber layer (perovskite), and the hole transfer layer (HTL). To improve the device efficiency and enhance the cell stability of PSCs, great efforts toward developing novel and efficient electron and hole-transporting materials are needed. Due to its unique properties such as high efficiency, simple process and low-cost organic-inorganic halide perovskite solar cells (PSCs) provide great potential for the photovoltaics industry. Particularly, efficient interfacial layers are very important to enhance PSCs, as charge carriers need to have smooth transport pathway, and this can be achieved by manipulating the interfacial layers and by choosing suitable interfacial layers.
Author Contribution
The first draft was written by SA and AMM. The final draft was reviewed and edited by FA and MA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
8.1 Introduction
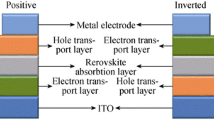
Perovskite solar cells (PSCs) were regarded as the most promising photovoltaic technology due to its low production cost, easy fabrication, and high power conversion efficiency (PCE). The PCE leaping from 3.8% to a certified 22.7% in just a few years and the stability of the devices from seconds to thousand hours have been improved [1,2,3,4,5,6]. The typical device structure of PSCs consists of transparent conductive electrode (fluorine-doped tin oxide (FTO) or indium tin oxide (ITO))/electron transport layer (ETL)/perovskite absorption layer/hole-transport layer (HTL)/metal electrode. For the mesoporous structure, the ETL includes the condense layer and mesoporous layer. To remove the organic material in titania (TiO2) paste and enhance TiO2 crystallinity in TiO2 mesoporous layer, high-temperature sintering is required. The mesoporous part can be removed in the planar structure of PSCs and only the condense layer was needed as ETL. Furthermore, all the layers in the planar structure of PSCs can be processed at low temperature. Planar structure is further divided into an n-i-p (regular) structure and p-i-n (inverted) structure based on the bottom charge transport layer near the conductive layer [7].
ETL and HTL should meet the following criteria in order to obtain efficient PSCs: (1) suitable energy-level alignment for efficient charge transfer and hole blocking, (2) high electron mobility to ensure fast electron transport within the ETL, (3) high transmittance to minimize the optical energy loss, (4) have high stability, and (5) easy processing and low cost [8,9,10,11]. In general, the interfacial layer materials can be classified as organic and inorganic ETL/HTLs. Graphene, fullerene, and their derivatives have been widely used as ETLs in PSCs [12]. Organic ETLs have some advantages such as solution process can be done easily and the devices display good performance, but at the same time have some drawbacks such as their environmental, thermal, and photostability is a great concern for the long-term stability of the devices. The most widely used inorganic metal oxide materials as ETL is TiO2. When the device with TiO2 was illuminated by ultraviolet light, the oxygen could be absorbed and perovskite material could be decomposed [13]. In addition, the bulk electron mobility (<1 cm2 V−1 s−1) is low, which makes the mobility of the TiO2 film pretty low (10−5 cm2 V−1 s−1) [14]. For achieving efficient charge transport layer and developing suitable and reliable ETL, many attempts have been made to replace TiO2 in ETLs. Many other low-temperature processed semiconductor metal oxides have been investigated as potential candidates to replace TiO2 such as zinc oxide (ZnO) [7, 15]; ternary metal oxides (Zn2SO4 [16, 17], BaSnO3 [18, 19], SrTiO4 [20]); metal sulfide (CdS) [21]; and metal selenide (CdSe) [22].
In this chapter, we have critically summarized about the materials used so far in ETL and HTL for PSCs applications. Methods for enhancing ETL performance such as deposition methods and metal-doping are also discussed in detail. This review aims to give insights into the current state-of-the-art of ETLs and HTLs in perovskite solar cells applications.
8.2 Electron Transport Layer (ETLs) in Perovskite Solar Cells
One of the most important components in the PSCs for highly efficient performance which plays an essential role in extracting and transporting photogenerated electrons is the electron-transport layer (ETL). Electron transport layers serve as a hole-blocking layer to suppress carrier recombination. Figure 8.1 shows the schematic illustration of ETL roles in PSCs. The major characteristics which determine the device behavior and photovoltaic performance of PSCs are carrier mobility, energy band alignment, morphology, trap states, and related interfacial properties [4, 23]. To efficiently transport and collect electrons, relatively high electron mobility materials are needed, which increase short-circuit current density (Isc) and fill factor (FF) [24,25,26]. Moreover, trap states in the ETLs play important roles in charge transport; hence, improving interface contact between ETLs and the perovskite layer is an efficient method to increase device performance and enhance charge transport [27].
In order to achieve better device performance, morphology of the ETL is modified to enhance its contact with the perovskite layer. Most frequently TiO2 is used as ETLs in PSCs. The rate of electron injection between the TiO2 ETLs and perovskite absorber is very fast, at the same time there is a probability of electron recombination due to the low electron mobility and transport properties [28]. In addition, to get high-quality mesoscopic TiO2 layer, a high-temperature process is required [4, 29, 30]. Therefore, to develop low-cost perovskite solar cells on various flexible substrates, the material characteristics may act as impediments to improve device performance and their application [31, 32]. In addition, a different type of zinc oxide (ZnO) morphologies has been found such as thin film, single-crystal, nanowire, and nanorod at low-temperature solution processes [33]. Additionally, ZnO has a similar energy level as TiO2 but better electron mobility (bulk mobility: 200–300 cm2/V.s [33,34,35]) than TiO2, which makes it an ideal candidate for a low-temperature processed electron-selective contact for transparent electrodes, organic solar cell, thin-film transistors, light-emitting diodes, and perovskite solar cell. Liu and Kelly (2014) first used low-temperature processed thin compact ZnO layer to fabricate efficient planar PSC at room temperature and achieve efficiency up to 15.7% [15]. However, it was observed that the ZnO could easily react with perovskite if thermal annealing (>100 °C) is carried out, which leads to the poor longtime stability of the devices [36]. To improve the device stability ZnO was combined with phenyl-C61-butric acid methyl ester (PCBM) to form PCBM/ZnO bilayer ETL, in which PCBM layer acts in charge extraction and the ZnO layer acts as a protecting layer to exclude oxygen and moisture, and also avoiding the metal electrode from diffusion [37]. To avoid a chemical reaction, zinc stannate (Zn2SnO4) with a good bulk electron mobility of 10–30 cm2 V−1 s−1 was investigated. Shin et al. used a quite tedious hydrothermal solution process (<100 °C) to synthesize the ternary Zn2SnO4 as ETL for methylammonium iodide (MAPbI3) perovskite solar cells (PSC), yielding a power conversion efficiency (PCE) of 15.3% [17]. Dai and coworkers first used lanthanum (La)-doped barium stannate (BaSnO3, LBSO) as ETL in PSCs and got device PCE of 15.1% [18]. Seok and coworkers prepared LBSO electrode below 300 °C by using a superoxide colloidal solution route, combined with MAPbI3 as perovskite layer, the device showed a stable output PCE of 21.2% and showed good long-time full-sun illumination stability [19]. In addition to metal oxide, several sulfides/selenides were also regarded as a replacement for TiO2. Mitzi and coworkers used chemical bath deposition (CBD) to deposit cadmium sulfide (CdS) and 15% stabilized efficiency were achieved. Somehow, the devices using this sulfide suffer from current losses in the ultraviolet (UV) range due to parasitic absorption caused by the rather low band gap of CdS film (Eg = 2.4 eV) [21, 22]. Cadmium sulfide has been used as ETL because of its high bulk electron mobility of 450–900 cm2 V−1 s−1 and it is easily obtained in low temperature. Using low-temperature solution processed CdSe nanocrystals have been prepared and a PCE of 12% have been achieved [38]. But the concern is the high toxicity of cadmium, which makes it necessary and critical to handle the liquid waste of cadmium-containing solutions. Although huge signs of progress have been obtained by replacing titania (TiO2), as mentioned above, there are still some problems such as low device efficiency, poor chemical stability, or high-temperature processing. Recently, tin oxide (SnO2) has attracted great attention as ETL for PSCs, and it is regarded as the most promising alternative to TiO2 due to the following reasons: (a) SnO2 owns good energy level and deep conduction band [39]. The excellent band energy enhances electron extraction and hole blocking at ETL/perovskite interface. (b) Potentially it can improve the electron transport efficiency and reduce the recombination loss due to its high bulk electron mobility of up to 240 cm2 V−1 s−1 and high conductivity. (c) Due to its wide optical band gap (3.6–4.0 eV) and high transmittance over the whole visible spectra, SnO2 will guarantee that most of light can come through and can be absorbed by the perovskite layer. (d) It is easily processed by low-temperature methods (<200 °C), which is compatible with flexible solar cells, tandem solar cells, and large-scale commercialization. (e) In comparison with TiO2 or other ETLs, SnO2 shows excellent chemical stability, ultraviolet (UV) resistance properties, and less photocatalytic activity which is helpful for overall device stability [40, 41].
8.2.1 Titanium Oxide (TiO2) as ETLs
Titanium oxide is considered as the most used electron transport layer in perovskite solar cell as it owns proper electron mobility, high transparency, long electron lifetime, and favorable energy [42,43,44]. Generally, wide band gap of TiO2 (> 3.0 eV) can provide sufficient solar energy passing through it and maximize the absorption ability of perovskite active layer to light. The TiO2 band gap associated with that of the perovskite layer should be compatible in order to produce superior electron transfer effect and hole blocking effect [45,46,47,48,49]. However, most of the TiO2 ETLs are formed via high-temperature sintering (> 450 °C) with high crystallinity, which hampers the commercialization of flexible PSCs [50, 51]. This makes a number of researchers to focus more on the low-temperature PSCs that possess a simple device structure and low fabrication costs such as atomic layer deposition, electrodeposition, and chemical bath deposition method (CBD) [52,53,54,55,56,57,58].
Generally, it is observed that the band gap and trap states of metal oxide could be modified by doping with suitable metal elements into the lattice. Previous studies on TiO2 doping as ETLs in PSCs are summarized in Table 8.1. Several elements have been inserted into TiO2 such as magnesium, ruthenium, niobium, tantalum, yttrium, lithium, lanthanum, iron (III), zinc, and indium in order to enhance the efficiency and stability of the corresponding devices [8, 59,60,61,62,63,64,65,66,67]. These results show that the semiconductive properties of TiO2 modifying the TiO2 conduction band minimum (CBM) and valence band maximum (VBM) level to increase the short-circuit current density (Isc) or the open-circuit voltage (Voc) of PSCs can be improved by elemental doping [61, 63,64,65, 68, 69]. However, the doping methods have some drawbacks including the complexity of the preparation procedure, high-temperature manufacturing, limited control of large-area spin-coating film quality, and expensive Ti/dopant organometal precursor; hence, investigating an efficient low-temperature method to obtain metal-doped TiO2 layer is imminent. Introducing zinc ions into the compact TiO2 lattice can result in better interfacial carrier extraction between ETL and perovskite. The experimental result shows that Zn doping can enhance carrier concentration of TiO2 layer and passivate trap-state sites within TiO2, resulting in less carrier recombination and in turn improve the carrier transportation [70].
8.2.2 Zinc Oxide (ZnO) as ETLs
Zinc oxide (ZnO) is a II–VI semiconductor material with a band gap of 3.37 eV and a 60 meV exciton binding energy at room temperature [71, 72]. In comparison with TiO2, ZnO-based PSCs have some attractive advantages. For instance, ZnO is a wide band gap semiconductor; its band gap is similar to that of TiO2. Additionally, ZnO electron mobility is higher than TiO2, which makes it an ideal choice to replace TiO2 [73, 74]. Furthermore, high crystalline ZnO can be achieved without sintering processes, which means that the ZnO thin films could give sufficient conductivity without a higher temperature sintering process [12, 75].
Studies found that deposition methods and morphological properties of ETLs are important factors in manipulating the performance of PSCs. Planar heterojunction devices have been fabricated by two-step deposition procedures, and the highest PCE of 15.7% was attained on rigid substrates [76]. In 2013, Kumar et al. used ZnO as ETL of PSCs; PCE of 8.90% were attained on rigid substrates [77]. In the same year, Bi et al. applied well-aligned ZnO nanorod arrays as ETLs; their results showed that the ZnO nanorod-based PSCs had good long-term stability of PSCs [78]. It was found that the ZnO nanorod length has a certain effect on electron transport time and lifetime. At the end of 2013, Liu and Kelly used thin compact ZnO ETL prepared by a co-precipitation method without any sintering steps to fabricate a highly efficient planar perovskite solar cell with a champion efficiency of 15.7% and an average efficiency of 13.7%, and the flexible ones yielded 10.2% [15]. Besides, solution-based co-precipitation ZnO ETLs prepared by Kim et al. in 2014 reported a sol-gel solution process to synthesize ZnO ETLs [79]. Moreover, for high-efficiency, PSCs’ ZnO ETL has been prepared using vacuum process such as an atomic layer deposition (ALD) [80] or sputtering method [81].
On the other hand, the electrical characteristics of ZnO can be improved by extrinsically doping a small amount of aluminum [82, 83]. In the lattice point, aluminum (Al3+) can replace zinc (Zn2+) which acts as a donor and increase the conductivity of ZnO [84, 85]; Al-doped ZnO thin film inhibits charge recombination at the ZnO/perovskite interface, resulting in better efficiency than pure ZnO devices [86]. Magnesium also can be used to suppress the charge recombination of ZnO-based device which refers to the conduction band offset at the interface between ZnO ETL and perovskite layer [87]. In addition, the electron extraction by the ETL in a perovskite cell depends on the work function of the ETL. An energy barrier mismatch (a Schottky barrier) between the work function of the ETL and the lowest unoccupied molecular orbital (LUMO) of perovskite absorber could lead to inefficient electron extraction, so matching the work function of ETL with the absorber could reduce a Schottky barrier or form an Ohmic contact to facilitate the electron injection or collection [88,89,90]. Another way to modify the ZnO ETL is the use of 3-aminopropanol acid [C3H9NO] monolayer which can enhance the interfacial energy level alignment by forming permanent dipole moments, which decrease the work function of ZnO from 4.17 to 3.52 eV, and hence obtain highly crystalline perovskite layer with reduced pin-hole and trap-state density [91]. The presence of oxygen vacancy of the lattice has a certain effect on the surface conductivity of ZnO. Zinc oxide film with more oxygen vacancies has higher surface conductivity. Furthermore, ZnO using pure Ar deposition has a lower work function of 4.33 eV than that using argon/oxygen (Ar/O2) mixed gas deposition of 4.48 eV. Therefore, ZnO using pure argon (Ar) deposition lower conduction band level shift down than that using Ar/O2 mixed gas deposition to increase the driven force of electron injection from ZnO/perovskite interface and lower valance edge can block the hole more efficiently [92]. In 2015, Yang et al. observed the thermal instability of PSCs prepared using ZnO as the ETLs [36]. Moreover, they showed that the basic nature of the ZnO surface leads to proton-transfer reactions at the zinc oxide/methylammonium lead halide (ZnO/CH3NH3PbI3) interface, which results in decomposition of the perovskite film. Cheng et al. introduced a buffer layer between the zinc oxide nanoparticles (ZnO-NPs) and perovskite layers to minimize the decomposition. They found that [6, 6]-phenyl-C61-butyric acid methyl ester can reduce it but not avoid completely. Furthermore, a polymeric buffer layer such as poly ethylenimine can effectively use to separate the ZnO-NPs and perovskite, which allows larger crystal formation with thermal annealing [93].
8.2.3 Tin Oxide (SnO2) as ETLs
Recently, SnO2 has attracted great attention as ETL for PSCs, and it is considered as the most promising alternative to TiO2 due to following reasons: (1) SnO2 owns deep conduction band and good energy level [39]. The excellent band energy at ETL/perovskite interface will enhance electron extraction and hole blocking. (2) SnO2 owns high bulk electron mobility of up to 240 cm2.V−1.s−1 and high conductivity, which can potentially improve the electron transport efficiency and reduce the recombination loss. (3) SnO2 has wide optical band gap (3.6–4.0 eV) and high transmittance over the whole visible spectra, which will guarantee that most of light can come through and can be absorbed by the perovskite layer. (4) SnO2 is easily processed by low-temperature methods (<200 °C), which is compatible with flexible solar cells, tandem solar cells, and large-scale commercialization. (5) SnO2 showed excellent chemical stability, UV-resistance properties, and less photocatalytic activity in comparison with TiO2 or other ETLs, which is helpful for overall device stability [40, 41]. In 2015, several groups apart from each other applied SnO2 in PSCs. Dai et al. using mesoporous SnO2 nanoparticle films as ETL got an efficiency of 10.18% [94]. Ma et al. used SnO2 combined with TiO2 mesoporous layer as ETL and attained PCE of 7.43% [95]. Then, Kuang et al. prepared SnO2 nanocolloidal film treated with titanium chloride (TiCl4) as ETL and a PCE of 14.69% was achieved [96]. Although these considerable signs of progress in SnO2-based perovskite solar cells have been achieved, the performance of the device is still lower than the devices using TiO2 as ETL. This could be referred to the existence of a large number of charge traps or recombination centers such as oxygen vacancies in the SnO2 layer at high-temperature annealing. Low-temperature processed SnO2 has been developed in order to prevent the defects induced by high-temperature processing. Tian et al. used SnO2 thin films annealed at 200 °C and a PCE of 13% was achieved [97]. Subsequently, Hagfeldt et al. prepared SnO2 ETL using atomic layer deposition (ALD) process and a PCE of more than 18% was achieved [98]. The device’s performance is still falling behind TiO2 counterpart. You et al. reported high-quality SnO2 nanocrystal colloidal as ETL, which annealed under moderate temperature and a PCE of 19.9% have been attained [14]. At the same time, Hagfeldt et al. using the chemical bath deposition (CBD) method prepared a double layer SnO2 and a PCE of 20.7% was achieved [99].
8.3 Hole Transport Layers (HTLs) in Perovskite Solar Cells
The perovskite cell absorber layer is sandwiched between electron and hole transport layers (ETL and HTL). When the device is illuminated by the sun, the light is excited in the absorber layer when the excitons are extracted by the electron/hole transporting carrier layer, and finally, the charges are collected at the appropriate electrodes [100]. The basic function of the HTL is to transfer an electron from the HTL to the oxidized perovskite, but the holes should quickly move away from the interface to prevent the recombination of electrons from the metal oxide layer. The highest occupied molecular orbital (HOMO) of the HTL must lie above the highest occupied molecular orbital (HOMO) of the perovskite to provide a driving force for hole injection. Furthermore, the HTL must have good p-type conductivity and be compatible with the perovskite [101]. In order to achieve high performance in perovskite cells, HTLs have to possess: (1) suitable highest occupied molecular orbital (HOMO) energy levels for matching with the valence band energy (VBE) of perovskite materials, together with ensuring holes injection and transporting at each interfaces; (2) high hole mobility and photochemical stability; (3) suitable solubility in organic solvents and good film-forming ability for processing and device fabrication; (4) suitable light absorption in visible and near-IR region of the solar spectrum for high photocurrent [101, 102].
The stability and performance depend on the transporting layers used in the device architecture as they serve the various aspects in perovskite cells such as the following: (i) acting as a physical/energetic barrier between ETL and perovskite layer by blocking the electron transfer; (ii) improving the hole transporting efficiency due to its high hole mobility and its matching energy level with those of ETL/HTLs and electrode, (iii) avoiding the degradation and corrosion which can take place in the absence of an HTL at the metal-perovskite interface; (iv) suppressing charge recombination by fully separating the top contact from the bottom transport or contact layer leading to improved performance. Though we have a wide variety of device architectures available for achieving both stability and performance, the light-to-electricity conversion in PSCs can be further improved by using suitable HTMs with well-established device architecture [100, 103].
Insertion of suitable HTLs between perovskite layer and metal electrode can promote the separation of electrons and holes in the functional layer interface, and thus reduce charge recombination and improve the performance of solar cells. The HTLs with stable thermodynamic and optical properties would help to improve the stability of PSCs as well. Perovskite crystal can be easily decomposed in humid environments. Therefore, the quality of perovskite films can be mostly decided by the hydrophobicity of the hole-transporting layer. Those hydrophobic hole-transporting layers can protect perovskite materials from moisture and thus ensure perovskite materials to sustain crystals with larger grain size and fewer grain boundaries [102]. In general, HTLs can be classified as inorganic, organic, and carbonaceous HTLs.
8.3.1 Organic HTLs
Organic HTLs include long polymer-based HTLs and small molecule-based HTLs. The prominent ones used are 2,2′, 7,7′-Tetrakis (N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene (spiro-OMeTAD, C73H52N4), poly(3,4-ethylenedioxythiophene):polystyrene sulfonate [(C6H4O2S)n:(C8H8O3S)n] (PEDOT:PSS), polytri-arylamine (PTAA, [C6H4N(C6H2(CH3)3)C6H4]n), poly(3-hexylthiophene-2,5-diyl) (P3HT, [(C10H14S)n]), crosslinked polymers, and other derivatives of smaller molecules like pyrene [C16H10] derivatives, thiophene [C4H4S] derivatives, porphyrin [C34H34N4O] derivatives, and carbazole [C12H9N] derivatives. Figure 8.2 shows the molecular structures of organic HTLs. Till now a large number of reports on various organic HTLs such as spiro-OMeTAD, PEDOT:PSS, PTAA, and P3HT are available which have been used to provide higher open circuit voltage and achieve higher efficiencies, yet the industrial development and market potential of PSCs is restricted due to their high cost and instability in water, heat, and light [100,101,102,103,104,105].
8.3.2 Inorganic HTLs
Inorganic HTLs commonly used in perovskite solar cell can be classified into three namely: Nickel (Ni) and copper (Cu) based, a [positive] p-typed semiconductor based, and transition metal oxide based. Nickel oxide (NiO), copper iodide (CuI), cupric oxide (CuO), cuprous oxide (Cu2O), copper chromium oxide (CuCrO2), and copper gallium oxide (CuGaO2) are the examples of nickel- and copper-based HTLs used in perovskite, while copper(II) phthalocyanine (CuPc) and copper(I) thiocyanate (CuSCN) are the examples of p-typed semiconductor used. Likewise, molybdenum trioxide (MoO3), vanadium(x) oxide (VOx), and tungsten(x) oxide (WOx) are the examples of transition metal oxide-based HTLs used in perovskite solar cell. To overcome the drawbacks of organic HTLs, alternate HTLs must be identified which are inexpensive and stable under all ambient conditions. This has led to the identification of effective inorganic hole transporters such as an interlayer which are inexpensive, abundant, nontoxic, and energy nonintensive. Many researchers have reported about the efficiency and stability of PSCs fabricated using various metal oxide HTLs. They have given an overview of both organic and inorganic HTLs and concluded that inorganic HTLs are better market competitors than organic HTLs [100, 101, 103, 105, 106]. Table 8.2 summarizes the examples of inorganic HTLs used in PSCs and its PCE.
8.3.2.1 Nickel Oxide
Nickel oxide (NiO) is one of the most promising HTLs with expected stability as it has good optical transparency, prevents electron leakage, and has appropriate energy levels. Nickel oxide (NiO) layer has a wide band gap and acts as a [positive] p-type transporting layer and acts as an electron blocking layer suppressing charge recombination and enhances the hole extraction in solar cells since the performance of the solid-state PSC device mainly depends on the charge transport. Nickel oxide (NiO) as HTL also has the favorable deep-lying HOMO (valence band edge value) with work function from 5 eV to 5.6 eV. Nickel oxide (NiO) acts as a potential HTL with methylammonium lead iodide perovskite and exhibits good hole transfer characteristics and this leads to improved device performance. The inertness of NiO makes it noncorrosive toward ITO substrates, and its work function makes NiO a good HTL for PSCs. NiO is marked as a promising material for p-type or inverted PSCs. When Snaith’s group used NiO layer as HTL, they found a photoconversion efficiency of <0.1%, due to poor adhesion between perovskite and the NiO layer [100, 103, 108]. Docampo and his coworkers first reported a thin NiO layer as HTL using methyl ammonium lead iodide doped chloride [CH3NH3PbI3−xClx] PSCs and noticed that a low coverage of perovskite surface leads to direct contact between the HTL (NiO) and the ETL (PCBM) resulting in low device performance PCE~0.1%. However, when mesoporous NiO is used as HTL in mesoscopic architecture compact layered-nickel oxide/nanoparticulate nickel oxide/methylammonium lead triiodate/phenyl-C61-butyric acid methyl ester/aluminum [CL-NiO/NP-NiO/CH3NH3PbI3/PCBM/Al], they got a significant increase in PCE (~1.5%) by controlling the thickness of the bilayer nickel oxide (BL-NiO) and nanoparticulate nickel oxide (NP-NiO) to be 80 and 120 nm, respectively [103, 108, 114]. Similarly, Anand S. Subbiah et al. reported planar, inverse CH3NH3PbI3 − xClx-based cells with inorganic hole conductors, using electrodeposited NiO as hole conductor, in which they are able to achieve a power conversion efficiency of 7.3% [107]. Likewise, Jong H. Kim et al. fabricated high-efficiency planar heterojunction perovskite solar cells based on solution-processed copper (Cu)-doped NiOx(Cu:NiOx) with impressive PCEs up to 15.40% and decent environmental stability; the doping significantly improved the performance [109].
8.3.2.2 Copper Iodide
Copper iodide (CuI) is a highly efficient, inexpensive, stable, wide band gap semiconductor with high conductivity and good material to use as an HTL in PSCs [108]. The first inorganic hole transport material which has been used in PSCs is CuI that resulted in a moderate PCE~6% with photovoltaic (PV) parameters ([saturation current density] JSC ~17.8 mA cm−2, [open circuit voltage] VOC~0.55 V, [form factor] FF~0.62%) comparable to a spiro-OMeTAD based PSC (JSC~16.1 mA cm−2, VOC~0.79 V, FF~0.61% yielding a PCE~7.9%). However, a lowering of ~300 mV in VOC in CuI based PSCs was attributed to the high recombination rate in CuI due to free iodine that could act as valence band trap and work as a recombination channel, as reported for solid state dye sensitized solar cells (s-DSSC) [110, 115]. High hole mobility of CuI which is five orders of magnitude greater than that of Spiro-OMeTAD still encourages its use in mesoscopic PSCs. Similarly, Chen et al. demonstrated that CuI as HTL in inverted planar PSCs showed PCE~13.6%, VOC 1.04 V owing to its high transmittance and deep valance band (~5.1 eV), and JSC 21.06 mA cm−2 [111].
8.3.2.3 Copper Oxide and Cuprous Oxide
Cuprous oxide and cupric oxide are a typical p-type semiconductors with low valence band energy that can match very well with the perovskite (CH3NH3PbI3), as well as they have high hole mobility of about 100 cm2.v-1.s-1 and 0.129 cm2.v-1.s-1, respectively and hence the good possibility for HTLs in PSCs [108]. CuOx-based solar cells were previously studied by creating a [positive-negative] p–n junction device structure. Cuprous oxide, with a band gap of 2.2–2.8 eV, is a promising and environmental friendly HTL for p–n junction solar. Moreover, very large hole mobility and charge diffusion length are reported for Cu2O [113]. Considering the broad absorption of Cu2O, as well as its close energy level alignment with the perovskite material, it could be an appropriate HTL candidate to boost the efficiency by extracting generated holes from the perovskite layer, and consequently reducing the production cost of perovskite solar cells with respect to cells employing Spiro-OMeTAD. It was shown through simulation that Cu2O may be a strong HTL candidate in perovskite solar cells possibly reaching up to 13% efficiency conversion; however, the experimental fabrication of the proposed architecture was difficult owing to a large number of limitations related to the processing conditions [113].
Bahram Abdollahi Nejand et al. proposed a successful technique for the physical deposition for Cu2O as a novel HTLs in perovskite solar cells. In the study, they introduced inorganic sandwiched perovskite solar cells with PCE values of up to 8.93%. Owing to the high sensitivity of Cu2O to perovskite precursor solutions, reactive magnetron sputtering was used to deposit the Cu2O on to the perovskite layer. The technique was used to achieve good coverage of the underlying perovskite layer and provide an effective junction between the perovskite and Cu2O [113].
Similarly, there is a facile low-temperature method to prepare Cu2O and CuO films and use them as HTMs in perovskite solar cells in which Cu2O film was prepared via in situ conversion of CuI film in aqueous NaOH solution. CuO film was made by heating Cu2O film in air. Compared with the cells using PEDOT:PSS, perovskite solar cells using Cu2O and CuO as HTMs show significantly enhanced open-circuit voltage (Voc), short-circuit current (Jsc), and PCE. PCEs of 13.35% and 12.16% were achieved for Cu2O and CuO cells, respectively, which are close to the highest PCE of perovskite solar cells using inorganic HTMs. Furthermore, Cu2O cells present improved stability.
Yan Wang et al. performed a device modeling study on planar perovskite solar cells with cuprous oxide (Cu2O) hole transporting layers (HTLs) by using a solar cell simulation program, wxAMPS. The performance of a Cu2O/perovskite solar cell was correlated to the material properties of the Cu2O HTL, such as thickness, carrier mobility, mid-gap defect, and doping concentrations. They also investigated the effect of interfacial defect densities on the solar cell performance. The simulation indicates that, with an optimized Cu2O HTL, high-performance perovskite solar cells with efficiencies above 13% could be achieved, which shows the potential of using Cu2O as an alternative HTL over other inorganic materials, such as NiOx and MoOx. Their study provides a theoretical breakthrough for developing perovskite solar cells with an inorganic hole transporting materials via a printing process [112].
8.3.3 Carbonaceous HTLs
Carbonaceous based HTLs involved hole-transport-free (carbon) based HTLs, graphene oxide, and reduced graphene oxide. Since efficiency and stability are both essential in photovoltaics, thus, carbon has also been identified as a potential HTL in terms of efficiency and long-term stability to some extent [100, 101, 103, 105, 106].
8.4 Conclusions and Future Directions
The major characteristics which determine the device behavior and photovoltaic performance of PSCs are carrier mobility, energy band alignment, morphology, trap states, and related interfacial properties. To efficiently transport and collect electrons, a relatively high electron mobility materials are needed, which increase short-circuit current density (Isc) and fill factor (FF). Both ETLs and HTLs that are able to extract electrons and holes from the perovskite absorbing layers, respectively, are needed for the high-efficiency PSCs. Many other low-temperature processed semiconductor metal oxides have been investigated as potential candidates to substitute TiO2, such as ZnO, BaSnO3, SrTiO4, etc. Organic HTLs are found to be advantageous in terms of efficiency; however, it fails in ensuring long-term stability, while inorganic HTLs are found to be better when considering stability factor and relatively good efficiency. Copper and cuprous oxides can be said to be a better candidate than most of the inorganic HTLs, similarly copper iodide. Doping can significantly affect the efficiency of almost all the inorganic HTLs as can be seen in nickel oxide in which the efficiency was able to reach up to 15.40%. However, the high price of most dopant and the complexity of the doping process make it less economical. Thus, manipulating the properties of ETLs and HTLs such as varying the thickness of the interfacial layers in order to decrease the band gap of the respective material is seen as a good alternative for high performance and stable perovskite solar cells.
References
A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131(17), 6050–6051 (2009)
J.H. Im, C.R. Lee, J.W. Lee, S.W. Park, N.G. Park, 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3(10), 4088–4093 (2011)
H.S. Kim, C.R. Lee, J.H. Im, K.B. Lee, T. Moehl, A. Marchioro, M. Grätzel, Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012)
W.S. Yang, J.H. Noh, N.J. Jeon, Y.C. Kim, S. Ryu, J. Seo, S.I. Seok, High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348(6240), 1234–1237 (2015)
W.S. Yang, B.W. Park, E.H. Jung, N.J. Jeon, Y.C. Kim, D.U. Lee, S.I. Seok, Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 356(6345), 1376–1379 (2017)
NREL Best Research-Cell Efficiencies, https://www.nrel.gov/pv/assets/images/efficiency-chart.png. Accessed Apr 2018
J. You, L. Meng, T.B. Song, T.F. Guo, Y.M. Yang, W.H. Chang, Y. Liu, Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 11(1), 75 (2016)
H. Zhou, Q. Chen, G. Li, S. Luo, T.B. Song, H.S. Duan, Y. Yang, Interface engineering of highly efficient perovskite solar cells. Science 345(6196), 542–546 (2014)
S.K. Pathak, A. Abate, P. Ruckdeschel, B. Roose, K.C. Gödel, Y. Vaynzof, A. Sepe, Performance and stability enhancement of dye-sensitized and perovskite solar cells by Al doping of TiO2. Adv. Funct. Mater. 24(38), 6046–6055 (2014)
H. Sun, J. Deng, L. Qiu, X. Fang, H. Peng, Recent progress in solar cells based on one-dimensional nanomaterials. Energy Environ. Sci. 8(4), 1139–1159 (2015)
C.C. Chueh, C.Z. Li, A.K.Y. Jen, Recent progress and perspective in solution-processed interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 8(4), 1160–1189 (2015)
J. You, Z. Hong, Y. Yang, Q. Chen, M. Cai, T.B. Song, Y. Yang, Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility. ACS Nano 8(2), 1674–1680 (2014)
T. Leijtens, G.E. Eperon, S. Pathak, A. Abate, M.M. Lee, H.J. Snaith, Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 4, 2885 (2013)
Q. Jiang, L. Zhang, H. Wang, X. Yang, J. Meng, H. Liu, J. You, Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2(1), 16177 (2017)
D. Liu, T.L. Kelly, Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 8(2), 133 (2014)
S.S. Mali, C.S. Shim, C.K. Hong, Highly porous Zinc Stannate (Zn2SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci. Rep. 5, 11424 (2015)
S.S. Shin, W.S. Yang, J.H. Noh, J.H. Suk, N.J. Jeon, J.H. Park, S.I. Seok, High-performance flexible perovskite solar cells exploiting Zn2SnO4 prepared in solution below 100 C. Nat. Commun. 6, 7410 (2015)
L. Zhu, J. Ye, X. Zhang, H. Zheng, G. Liu, X. Pan, S. Dai, Performance enhancement of perovskite solar cells using a La-doped BaSnO3 electron transport layer. J. Mater. Chem. A 5(7), 3675–3682 (2017)
S.S. Shin, E.J. Yeom, W.S. Yang, S. Hur, M.G. Kim, J. Im, S.I. Seok, Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 356(6334), 167–171 (2017)
A. Bera, K. Wu, A. Sheikh, E. Alarousu, O.F. Mohammed, T. Wu, Perovskite oxide SrTiO3 as an efficient electron transporter for hybrid perovskite solar cells. J. Phys. Chem. C 118(49), 28494–28501 (2014)
J. Liu, C. Gao, L. Luo, Q. Ye, X. He, L. Ouyang, W. Lau, Low-temperature, solution processed metal sulfide as an electron transport layer for efficient planar perovskite solar cells. J. Mater. Chem. A 3(22), 11750–11755 (2015)
W.A. Dunlap-Shohl, R. Younts, B. Gautam, K. Gundogdu, D.B. Mitzi, Effects of Cd diffusion and doping in high-performance perovskite solar cells using CdS as electron transport layer. J. Phys. Chem. C 120(30), 16437–16445 (2016)
G. Yang, H. Tao, P. Qin, W. Ke, G. Fang, Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 4(11), 3970–3990 (2016)
S. Ryu, J.H. Noh, N.J. Jeon, Y.C. Kim, W.S. Yang, J. Seo, S.I. Seok, Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 7(8), 2614–2618 (2014)
A.R.B.M. Yusoff, M.K. Nazeeruddin, Organohalide lead perovskites for photovoltaic applications. J. Phys. Chem. Lett. 7(5), 851–866 (2016)
Y.F. Chiang, J.Y. Jeng, M.H. Lee, S.R. Peng, P. Chen, T.F. Guo, C.M. Hsu, High voltage and efficient bilayer heterojunction solar cells based on an organic–inorganic hybrid perovskite absorber with a low-cost flexible substrate. Phys. Chem. Chem. Phys. 16(13), 6033–6040 (2014)
G.E. Eperon, V.M. Burlakov, P. Docampo, A. Goriely, H.J. Snaith, Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 24(1), 151–157 (2014)
S. Gubbala, V. Chakrapani, V. Kumar, M.K. Sunkara, Band-edge engineered hybrid structures for dye-sensitized solar cells based on SnO2 nanowires. Adv. Funct. Mater. 18(16), 2411–2418 (2008)
N.J. Jeon, J.H. Noh, W.S. Yang, Y.C. Kim, S. Ryu, J. Seo, S.I. Seok, Compositional engineering of perovskite materials for high performance solar cells. Nature 517(7535), 476 (2015)
D. Bi, S.J. Moon, L. Häggman, G. Boschloo, L. Yang, E.M. Johansson, A. Hagfeldt, Using a two-step deposition technique to prepare perovskite (CH3NH3PbI3) for thin film solar cells based on ZrO2 and TiO2 mesostructures. RSC Adv. 3(41), 18762–18766 (2013)
Z.M. Beiley, M.D. McGehee, Modeling low cost hybrid tandem photovoltaics with the potential for efficiencies exceeding 20%. Energy Environ. Sci. 5(11), 9173–9179 (2012)
C. Roldán-Carmona, O. Malinkiewicz, A. Soriano, G.M. Espallargas, A. Garcia, P. Reinecke, H.J. Bolink, Flexible high efficiency perovskite solar cells. Energy Environ. Sci. 7(3), 994–997 (2014)
Ü. Özgür, Y.I. Alivov, C. Liu, A. Teke, M. Reshchikov, S. Doğan, H. Morkoç, A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98(4), 11 (2005)
H. Tang, K. Prasad, R. Sanjines, P.E. Schmid, F. Levy, Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 75(4), 2042–2047 (1994)
H.S. Bae, M.H. Yoon, J.H. Kim, S. Im, Photodetecting properties of ZnO-based thin-film transistors. Appl. Phys. Lett. 83(25), 5313–5315 (2003)
J. Yang, B.D. Siempelkamp, E. Mosconi, F. De Angelis, T.L. Kelly, Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 27(12), 4229–4236 (2015)
L.Q. Zhang, X.W. Zhang, Z.G. Yin, Q. Jiang, X. Liu, J.H. Meng, H.L. Wang, Highly efficient and stable planar heterojunction perovskite solar cells via a low temperature solution process. J. Mater. Chem. A 3(23), 12133–12138 (2015)
L. Wang, W. Fu, Z. Gu, C. Fan, X. Yang, H. Li, H. Chen, Low temperature solution processed planar heterojunction perovskite solar cells with a CdSe nanocrystal as an electron transport/extraction layer. J. Mater. Chem. C 2(43), 9087–9090 (2014)
A.J. Nozik, R. Memming, Physical chemistry of semiconductor− liquid interfaces. J. Phys. Chem. 100(31), 13061–13078 (1996)
B. Bob, T.B. Song, C.C. Chen, Z. Xu, Y. Yang, Nanoscale dispersions of gelled Sno2: material properties and device applications. Chem. Mater. 25(23), 4725–4730 (2013)
H.J. Snaith, C. Ducati, SnO2-based dye-sensitized hybrid solar cells exhibiting near unity absorbed photon-to-electron conversion efficiency. Nano Lett. 10(4), 1259–1265 (2010)
C. Liang, P. Li, Y. Zhang, H. Gu, Q. Cai, X. Liu, G. Shao, Mild solution-processed metal-doped TiO2 compact layers for hysteresis-less and performance-enhanced perovskite solar cells. J. Power Sources 372, 235–244 (2017)
P. Li, C. Liang, B. Bao, Y. Li, X. Hu, Y. Wang, Y. Song, Inkjet manipulated homogeneous large size perovskite grains for efficient and large-area perovskite solar cells. Nano Energy 46, 203–211 (2018)
C. Liang, P. Li, H. Gu, Y. Zhang, F. Li, Y. Song, G. Xing, One-step inkjet printed perovskite in air for efficient light harvesting. Solar RRL 2(2), 1700217 (2018)
C. Liang, Z. Wu, P. Li, J. Fan, Y. Zhang, G. Shao, Chemical bath deposited rutile TiO2 compact layer toward efficient planar heterojunction perovskite solar cells. Appl. Surf. Sci. 391, 337–344 (2017)
L. Wang, Z. Yuan, T.A. Egerton, Comparison of nano-particulate TiO2 prepared from titanium tetrachloride and titanium tetraisopropoxide. Mater. Chem. Phys. 133(1), 304–310 (2012)
J.H. Heo, M.S. You, M.H. Chang, W. Yin, T.K. Ahn, S.J. Lee, S.H. Im, Hysteresis-less mesoscopic CH3NH3PbI3 perovskite hybrid solar cells by introduction of Li-treated TiO2 electrode. Nano Energy 15, 530–539 (2015)
D.O. Scanlon, C.W. Dunnill, J. Buckeridge, S.A. Shevlin, A.J. Logsdail, S.M. Woodley, G.W. Watson, Band alignment of rutile and anatase TiO2. Nat. Mater. 12(9), 798 (2013)
H. Tao, Z. Ma, G. Yang, H. Wang, H. Long, H. Zhao, G. Fang, Room-temperature processed tin oxide thin film as effective hole blocking layer for planar perovskite solar cells. Appl. Surf. Sci. 434, 1336–1343 (2018)
Q. Yue, J. Duan, L. Zhu, K. Zhang, J. Zhang, H. Wang, Effect of HCl etching on TiO2 nanorod-based perovskite solar cells. J. Mater. Sci. 53(21), 15257–15270 (2018)
D. Bi, C. Yi, J. Luo, J.D. Décoppet, F. Zhang, S.M. Zakeeruddin, M. Grätzel, Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 1(10), 16142 (2016)
M. Xiao, F. Huang, W. Huang, Y. Dkhissi, Y. Zhu, J. Etheridge, L. Spiccia, A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 53(37), 9898–9903 (2014)
Z. Xiao, C. Bi, Y. Shao, Q. Dong, Q. Wang, Y. Yuan, J. Huang, Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 7(8), 2619–2623 (2014)
Q. Cai, Y. Zhang, C. Liang, P. Li, H. Gu, X. Liu, G. Shao, Enhancing efficiency of planar structure perovskite solar cells using Sn-doped TiO2 as electron transport layer at low temperature. Electrochim. Acta 261, 227–235 (2018)
Y. Wu, F. Xie, H. Chen, X. Yang, H. Su, M. Cai, L. Han, Thermally stable MAPbI3 perovskite solar cells with efficiency of 19.19% and area over 1 cm2 achieved by additive engineering. Adv. Mater. 29(28), 1701073 (2017)
F. Di Giacomo, V. Zardetto, A. D'Epifanio, S. Pescetelli, F. Matteocci, S. Razza, T.M. Brown, Flexible perovskite photovoltaic modules and solar cells based on atomic layer deposited compact layers and UV-irradiated TiO2 scaffolds on plastic substrates. Adv. Energy Mater. 5(8), 1401808 (2015)
D. Yang, R. Yang, J. Zhang, Z. Yang, S.F. Liu, C. Li, High efficiency flexible perovskite solar cells using superior low temperature TiO2. Energy Environ. Sci. 8(11), 3208–3214 (2015)
K. Wang, Y. Shi, Q. Dong, Y. Li, S. Wang, X. Yu, T. Ma, Low-temperature and solution-processed amorphous WO X as electron-selective layer for perovskite solar cells. J. Phys. Chem. Lett. 6(5), 755–759 (2015)
J. Wang, M. Qin, H. Tao, W. Ke, Z. Chen, J. Wan, G. Fang, Performance enhancement of perovskite solar cells with Mg-doped TiO2 compact film as the hole-blocking layer. Appl. Phys. Lett. 106(12), 121104 (2015)
Z. Xu, X. Yin, Y. Guo, Y. Pu, M. He, Ru-doping in TiO2 electron transport layers of planar heterojunction perovskite solar cells for enhanced performance. J. Mater. Chem. C 6(17), 4746–4752 (2018)
B.X. Chen, H.S. Rao, W.G. Li, Y.F. Xu, H.Y. Chen, D.B. Kuang, C.Y. Su, Achieving high-performance planar perovskite solar cell with Nb-doped TiO2 compact layer by enhanced electron injection and efficient charge extraction. J. Mater. Chem. A 4(15), 5647–5653 (2016)
R. Ranjan, A. Prakash, A. Singh, A. Singh, A. Garg, R.K. Gupta, Effect of tantalum doping in a TiO2 compact layer on the performance of planar spiro-OMeTAD free perovskite solar cells. J. Mater. Chem. A 6(3), 1037–1047 (2018)
D. Liu, S. Li, P. Zhang, Y. Wang, R. Zhang, H. Sarvari, Z.D. Chen, Efficient planar heterojunction perovskite solar cells with Li-doped compact TiO2 layer. Nano Energy 31, 462–468 (2017)
J. Peng, T. Duong, X. Zhou, H. Shen, Y. Wu, H.K. Mulmudi, K.J. Weber, Efficient indium-doped TiOx electron transport layers for high-performance perovskite solar cells and perovskite-silicon tandems. Adv. Energy Mater. 7(4), 1601768 (2017)
X.X. Gao, Q.Q. Ge, D.J. Xue, J. Ding, J.Y. Ma, Y.X. Chen, J.S. Hu, Tuning the fermi-level of TiO2 mesoporous layer by lanthanum doping towards efficient perovskite solar cells. Nanoscale 8(38), 16881–16885 (2016)
X. Gu, Y. Wang, T. Zhang, D. Liu, R. Zhang, P. Zhang, S. Li, Enhanced electronic transport in Fe3+-doped TiO2 for high efficiency perovskite solar cells. J. Mater. Chem. C 5(41), 10754–10760 (2017)
M. Lv, W. Lv, X. Fang, P. Sun, B. Lin, S. Zhang, N. Yuan, Performance enhancement of perovskite solar cells with a modified TiO2 electron transport layer using Zn-based additives. RSC Adv. 6(41), 35044–35050 (2016)
K. Wojciechowski, M. Saliba, T. Leijtens, A. Abate, H.J. Snaith, Sub-150 C processed meso-superstructured perovskite solar cells with enhanced efficiency. Energy Environ. Sci. 7(3), 1142–1147 (2014)
J.T.W. Wang, J.M. Ball, E.M. Barea, A. Abate, J.A. Alexander-Webber, J. Huang, R.J. Nicholas, Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 14(2), 724–730 (2013)
M. Thambidurai, J.Y. Kim, Y. Ko, H.J. Song, H. Shin, J. Song, C. Lee, High-efficiency inverted organic solar cells with polyethylene oxide-modified Zn-doped TiO2 as an interfacial electron transport layer. Nanoscale 6(15), 8585–8589 (2014)
C.X. Xu, X.W. Sun, Z.L. Dong, G.P. Zhu, Y.P. Cui, Zinc oxide hexagram whiskers. Appl. Phys. Lett. 88(9), 093101 (2006)
L. Wu, Y. Wu, X. Pan, F. Kong, Synthesis of ZnO nanorod and the annealing effect on its photoluminescence property. Opt. Mater. 28(4), 418–422 (2006)
W. Ke, G. Fang, Q. Liu, L. Xiong, P. Qin, H. Tao, G. Yang, Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 137(21), 6730–6733 (2015)
O. Malinkiewicz, A. Yella, Y.H. Lee, G.M. Espallargas, M. Graetzel, M.K. Nazeeruddin, H.J. Bolink, Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 8(2), 128 (2014)
Q. Wang, Y. Shao, Q. Dong, Z. Xiao, Y. Yuan, J. Huang, Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process. Energy Environ. Sci. 7(7), 2359–2365 (2014)
J. Burschka, N. Pellet, S.J. Moon, R. Humphry-Baker, P. Gao, M.K. Nazeeruddin, M. Grätzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499(7458), 316 (2013)
M.H. Kumar, N. Yantara, S. Dharani, M. Graetzel, S. Mhaisalkar, P.P. Boix, N. Mathews, Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Commun. 49(94), 11089–11091 (2013)
D. Bi, G. Boschloo, S. Schwarzmüller, L. Yang, E.M. Johansson, A. Hagfeldt, Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells. Nanoscale 5(23), 11686–11691 (2013)
J. Kim, G. Kim, T.K. Kim, S. Kwon, H. Back, J. Lee, K. Lee, Efficient planar-heterojunction perovskite solar cells achieved via interfacial modification of a sol–gel ZnO electron collection layer. J. Mater. Chem. A 2(41), 17291–17296 (2014)
X. Dong, H. Hu, B. Lin, J. Ding, N. Yuan, The effect of ALD-Zno layers on the formation of CH3NH3PbI3 with different perovskite precursors and sintering temperatures. Chem. Commun. 50(92), 14405–14408 (2014)
L. Liang, Z. Huang, L. Cai, W. Chen, B. Wang, K. Chen, B. Fan, Magnetron sputtered zinc oxide nanorods as thickness-insensitive cathode interlayer for perovskite planar-heterojunction solar cells. ACS Appl. Mater. Interfaces 6(23), 20585–20589 (2014)
K. Ellmer, Magnetron sputtering of transparent conductive zinc oxide: relation between the sputtering parameters and the electronic properties. J. Phys. D. Appl. Phys. 33(4), R17 (2000)
Liu, Y., Li, Y., and Zeng, H. (2013). ZnO-based transparent conductive thin films: doping, performance, and processing. J. Nanomater., 2013
J.A. Sans, J.F. Sánchez-Royo, A. Segura, G. Tobias, E. Canadell, Chemical effects on the optical band-gap of heavily doped ZnO: M III (M= Al, Ga, In): an investigation by means of photoelectron spectroscopy, optical measurements under pressure, and band structure calculations. Phys. Rev. B 79(19), 195105 (2009)
J.L. Zhao, X.W. Sun, H. Ryu, Y.B. Moon, Thermally stable transparent conducting and highly infrared reflective Ga-doped ZnO thin films by metal organic chemical vapor deposition. Opt. Mater. 33(6), 768–772 (2011)
K. Mahmood, B.S. Swain, H.S. Jung, Controlling the surface nanostructure of ZnO and Al-doped ZnO thin films using electrostatic spraying for their application in 12% efficient perovskite solar cells. Nanoscale 6(15), 9127–9138 (2014)
J. Dong, J. Shi, D. Li, Y. Luo, Q. Meng, Controlling the conduction band offset for highly efficient ZnO nanorods based perovskite solar cell. Appl. Phys. Lett. 107(7), 073507 (2015)
H. Kang, S. Hong, J. Lee, K. Lee, Electrostatically self-assembled nonconjugated polyelectrolytes as an ideal interfacial layer for inverted polymer solar cells. Adv. Mater. 24(22), 3005–3009 (2012)
J.C. Yu, D.B. Kim, G. Baek, B.R. Lee, E.D. Jung, S. Lee, M.H. Song, High-performance planar perovskite optoelectronic devices: a morphological and interfacial control by polar solvent treatment. Adv. Mater. 27(23), 3492–3500 (2015)
Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A.J. Giordano, H. Li, M. Fenoll, A universal method to produce low–work function electrodes for organic electronics. Science 336(6079), 327–332 (2012)
L. Zuo, Z. Gu, T. Ye, W. Fu, G. Wu, H. Li, H. Chen, Enhanced photovoltaic performance of CH3NH3PbI3 perovskite solar cells through interfacial engineering using self-assembling monolayer. J. Am. Chem. Soc. 137(7), 2674–2679 (2015)
Z.L. Tseng, C.H. Chiang, C.G. Wu, Surface engineering of ZnO thin film for high efficiency planar perovskite solar cells. Sci. Rep. 5, 13211 (2015)
Y. Cheng, Q.D. Yang, J. Xiao, Q. Xue, H.W. Li, Z. Guan, S.W. Tsang, Decomposition of organometal halide perovskite films on zinc oxide nanoparticles. ACS Appl. Mater. Interfaces 7(36), 19986–19993 (2015)
Y. Li, J. Zhu, Y. Huang, F. Liu, M. Lv, S. Chen, S. Dai, Mesoporous SnO2 nanoparticle films as electron-transporting material in perovskite solar cells. RSC Adv. 5(36), 28424–28429 (2015)
Q. Dong, Y. Shi, K. Wang, Y. Li, S. Wang, H. Zhang, T. Ma, Insight into perovskite solar cells based on SnO2 compact electron-selective layer. J. Phys. Chem. C 119(19), 10212–10217 (2015)
H.S. Rao, B.X. Chen, W.G. Li, Y.F. Xu, H.Y. Chen, D.B. Kuang, C.Y. Su, Improving the extraction of photogenerated electrons with SnO2 nanocolloids for efficient planar perovskite solar cells. Adv. Funct. Mater. 25(46), 7200–7207 (2015)
J. Song, E. Zheng, J. Bian, X.F. Wang, W. Tian, Y. Sanehira, T. Miyasaka, Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A 3(20), 10837–10844 (2015)
J.P.C. Baena, L. Steier, W. Tress, M. Saliba, S. Neutzner, T. Matsui, A. Petrozza, Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 8(10), 2928–2934 (2015)
E.H. Anaraki, A. Kermanpur, L. Steier, K. Domanski, T. Matsui, W. Tress, J.P. Correa-Baena, Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide. Energy Environ. Sci. 9(10), 3128–3134 (2016)
S. Pitchaiya, M. Natarajan, A review on the classification of organic /inorganic /carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem.. King Saud University (2018). https://doi.org/10.1016/j.arabjc.2018.06.006
P. Dhingra, P. Singh, J.S. Rana, A. Garg, P. Kar, Hole-transporting materials for perovskite-sensitized solar cells. Energy Technology. 4(8), 891–938 (2016). https://doi.org/10.1002/ente.201500534
X. Zhao, M. Wang, Organic hole-transporting materials for efficient perovskite solar cells. Materials today energy. 7, 208–220 (2018). https://doi.org/10.1016/j.mtener.2017.09.011. Elsevier Ltd
Z.H. Bakr, Q. Wali, A. Fakharuddin, L. Schmidt-mende, T.M. Brown, R. Jose, Nano energy advances in hole transport materials engineering for stable and efficient perovskite solar cells. Nano Energy. 34, 271–305 (2017). https://doi.org/10.1016/j.nanoen.2017.02.025. Nov 2016. Elsevier Ltd
D. Zhao, M. Sexton, H. Park, G. Baure, C.J. Nino, F. So, High-efficiency solution-processed planar perovskite solar cells with a polymer hole transport layer. Advanced Energy Materials. 5(6), 1401855 (2015). https://doi.org/10.1002/aenm.201401855
L. Cali, S. Kazim, M. Gratzel, S. Ahmad, Hole-transport materials for perovskite solar cells. Angewandte Chemie International Edition. 55(47), 14522–14545 (2016). https://doi.org/10.1002/anie.201601757
W. Yan, S. Ye, Y. Li,W. Sun, H. Rao, Z. Liu, Z. Bian, C. Hung, Hole-transporting materials in inverted planar perovskite solar cells. Advanced Energy Materials. 6(17), 1600474 (2016). https://doi.org/10.1002/aenm.201600474
A.S. Subbiah, A. Halder, S. Ghosh, N. Mahuli, G. Hodes, S.K. Sarkar, inorganic hole conducting layers for perovskite-based solar cells. J. Phys. Chem. Lett. 5(10), 1748-53 (2014).
R. Rajeswari, M. Mrinalini, S. Prasanthkumar, L. Giribabu, Emerging of inorganic hole transporting materials for perovskite solar cells, 1–20 (2017). https://doi.org/10.1002/tcr.201600117
J.H. Kim, P. Liang, S.T. Williams, N. Cho, C. Chueh, M.S. Glaz, D.S. Ginger, A.K.Y. Jen, High-performance and environmentally stable planar heterojunction perovskite solar cells based on a solution-processed copper-doped nickel oxide hole-transporting layer. Advanced materials, 27(4), 695–701 (2014). https://doi.org/10.1002/adma.201404189
V.P.S. Perera, K. Tennakone, Recombination processes in dye-sensitized solid-state solar cells with CuI as the hole collector. Solar Energy Materials and Solar Cells. 79(2), 249–255 (2003). https://doi.org/10.1016/S0927-0248(03)00103-X
W.Y. Chen, L.L. Deng, S.M. Dai, X. Wang, C.B. Tian, X.X. Zhan, S.Y. Xie, R.B. Huangb, L.S. Zheng, low-cost solution-processed copper iodide as an alternative to PEDOT: PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A., 3(38), 19353-9 (2015). https://doi.org/10.1039/C5TA05286F
Y. Wang, Z. Xia, J. Liang, X. Wang, Y. Liu, C. Liu, S. Zhang, H. Zhou, Towards printed perovskite solar cells with cuprous oxide hole transporting layers : a theoretical design. Semicond. Sci. Technol. 30(5), 54004 (2015). https://doi.org/10.1088/0268-1242/30/5/054004. IOP Publishing
B.A. Nejand, V. Ahmadi, S. Gharibzadeh, Cuprous oxide as a potential low-cost hole-transport material for stable perovskite solar cells. Chem Sus Chem. 9(3), 302–313 (2016). https://doi.org/10.1002/cssc.201501273
P. Docampo, J.M. Ball, M. Darwich, G.E. Eperon, H.J. Snaith, Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 4, 1–6 (2013). https://doi.org/10.1038/ncomms3761. Nature Publishing Group
J.A. Christians, R.C. Fung, P.V. Kamat, An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 136(2), 758–64 (2013)https://doi.org/10.1021/ja411014k
Acknowledgments
The authors wish to thank the Ministry of Education (MOE) Malaysia [FRGS: R. J130000.7851.5F007] and Universiti Teknologi Malaysia (UTM) [GUP: Q.J130000.2513.20H63 and Q. J130000.2546.18H39] for funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
Mhamad, S.A., Mohammed, A.M., Aziz, M., Aziz, F. (2019). Impact of Electron Transport Layers (ETLs) and Hole Transport Layer (HTLs) on Perovskite Solar Cells Performance. In: Atesin, T.A., Bashir, S., Liu, J.L. (eds) Nanostructured Materials for Next-Generation Energy Storage and Conversion. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-59594-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-662-59594-7_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-59592-3
Online ISBN: 978-3-662-59594-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)