Abstract
The progress of transdermal therapeutic systems is based on the development of chemical and physical penetration enhancers. Especially, physical enhancers transiently disrupt the stratum corneum’s barrier function using electrical methods (iontophoresis and electroporation), other energy sources such as ultrasound (sonophoresis), and mechanical force (microneedles). Iontophoresis (a direct current ≤ 0.5 mA/cm2) can enhance and control the skin penetration flux of low molecular weight drugs (≤300 Da), while the flux of high molecular weight drugs (≥ 7 kDa) is difficult to increase. The other enhancers are able to improve the flux of macromolecules (i.e., proteins and nucleic acid) by creating and expanding pores in the stratum corneum by high-voltage pulses (electroporation), changing the stratum corneum structure by cavitation (sonophoresis), and creating microchannels through the stratum corneum (microneedles). This chapter provides a review about the combined use of iontophoresis and other physical enhancement methods, and the advantages and disadvantages of the combination will be discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Combination
- Physical enhancer
- Iontophoresis
- Electroporation
- Sonophoresis
- Microneedle
- Transdermal
- Synergistic enhancement
1 Introduction
The skin protects the internal environment in our body and has two unique barriers. The outermost layer of the skin, the stratum corneum, is a lipophilic, physical barrier, and the second barrier is biochemical—the viable epidermis and dermis underneath the stratum corneum (viable skin). The stratum corneum’s function as a barrier by both diffusion and partition makes the transdermal delivery of drugs difficult. Thus, the first phase in the past development of transdermal therapeutic systems was focused on identifying potent drugs that would be well absorbed in the skin. However, the transdermal route allowed the transport of only a limited amount of drugs (Wester and Maibach 1983), and it was difficult to increase the drug absorption because of a limiting size of transdermal patches (≤ approximately 40 cm2) (Pfister 1997). Thus, the focus of the second phase was to increase the amount of drug that penetrated transdermally. Researchers created chemical penetration enhancers (Williams and Barry 2004) against the stratum corneum’s barrier function, and this succeeded for low molecular weight drugs. The third phase was to enhance and control the skin penetration flux by physical enhancement methods (Brown et al. 2006). The physical enhancers also brought a pulsed control of the flux. The more recent progress in transdermal drug delivery has been achieved based on not only medicine and pharmacology but also engineering science and bioinformatics.
In this chapter, we summarize the fundamental knowledge about skin penetration and physical or active enhancing methods, in particular iontophoresis, electroporation, sonophoresis (ultrasound), and microneedle methods. We discuss the advantages and disadvantages of the combined use of physical enhancers, which is the next stage of transdermal drug delivery.
2 General Principles of Skin Penetration
Skin penetration flux follows Fick’s second law of diffusion. Because the skin is a bilayered structure, the following diffusion equations are given for each layer:
Stratum corneum
Viable skin
where C is the drug concentration, D is the diffusion coefficient, h is the thickness of the stratum corneum, H is the thickness of the skin, t is time, and k is the first-order enzymatic reaction rate. The subscripts sc and vs stand for stratum corneum and viable skin, respectively. We can solve the diffusion phenomena through skin using Eqs. (22.1) and (22.2) to obtain the following initial and boundary conditions:
Initial condition
Boundary conditions
where C d is the concentration in the drug formulation, K sc is the partition coefficient between the skin surface and the drug formulation, and K sc/vs is the partition coefficient between the stratum corneum and the viable skin.
We simplify the skin penetration as a single-layered skin model because the skin penetration flux of drugs is controlled by the stratum corneum’s barrier function. Under the conditions of steady-state penetration with perfect sink condition, Eq. (22.1) can be replaced by the following equation:
where Q is the cumulative amount of drug and ∆C is the concentration gradient between both ends of the skin.
3 Physical Enhancement
In the past two decades, the progress in biotechnology and bioinformatics has led to novel and potent drugs (i.e., peptides, antibodies, oligonucleotides, and small interfering RNAs (siRNAs)) and the possibility to establish safer and more effective drug delivery strategies. Transdermal delivery would be advantageous for these new drugs because they are metabolized easily in the gastrointestinal tract by oral delivery. However, these drugs can hardly penetrate through the skin because of their high molecular weight and hydrophilic properties. Because the skin penetration flux is simplified as Eq. (22.3), the flux is increased by the improvement of D sc, ∆C, K sc, and h individually and at the same time. Three effective enhancement methods are (1) to increase the diffusion coefficient in the stratum corneum, (2) to increase the concentration in the drug formulation and in the stratum corneum, and (3) to decrease the thickness of the stratum corneum or to create new pathways across the stratum corneum. The physical enhancements are achieved by the use of external energy to add a driving force for the skin penetration and mechanical force to reduce the barrier function of the stratum corneum.
3.1 Iontophoresis
Iontophoresis using a low current density (the limitation is ≤ 0.5 mA/cm2 for human skin) is a superior method for the percutaneous absorption of drugs with a molecular weight between 200 and 300 Da (Yoshida and Roberts 1992). This enhancement method is based on electrorepulsion and/or electroosmosis. Electroosmosis is a convective flow that goes across the stratum corneum from the anode to the cathode when the pH value of the skin surface is more than 4.0 (Kim et al. 1993). Thus, the flux of nonionized drugs is also increased by electroosmosis. The diffusion equation with no enzymatic reaction across the stratum corneum under iontophoresis becomes the following:
where z is the charge number of the ionized drug, F is the Faraday constant, E is the electric field, R is the gas constant, T is the absolute temperature, and u is the velocity of the convective flow.
Because the electrorepulsion and electroosmosis occur only under an electric field and are affected by the current density, it becomes possible to achieve a continuous, pulsed, and reversed delivery of drugs, to terminate the application immediately, and to reduce inter- and intraindividual variation. The advantages of iontophoresis bring possibilities for transdermal therapeutic systems. Iontophoresis has been studied for systematic and topical delivery, and some devices using electric fields such as the E-TRANS® (Alza/Ortho-McNeil Pharmaceutical Co.) for fentanyl (Gupta et al. 1999), the LidoSite® (Vyteris Inc.) for lidocaine (Pasero 2006), the IontoPatch® (Travanti Pharma Inc.) for dexamethasone (Chaturvedula et al. 2005), and the GlucoWatch® (Cygnus Inc.) as a glucose monitor (Garg et al. 1999) have actually come onto the market.
It must be noted that there are several reports in which iontophoresis caused irreversible damages to the skin (Burnette and Ongpipattanakul 1988; Inada et al. 1994; Wang et al. 1993). Miyagi et al. (2006) reported about the effect of molecular weight of drugs on the iontophoretic enhancement of flux. The flux of vitamin B12 (MW = 1355) increased appreciably in parallel with the constant current density (~0.6 mA/cm2) and reached a plateau after the current was turned off (Fig. 22.1). In contrast, the flux of fluorescein isothiocyanate (FITC)-dextran (FD-4, average MW = 4400; FD-10, average MW = 11,000; FD-20, average MW = 19,000) increased continuously after a lag time (0.5 h for FD-4 and 1.5 h for FD-10 and FD-20) (Fig. 22.2). Kanikkannan (2002) also reported that iontophoresis might not be a suitable method for the transdermal delivery of peptides (>7000 Da). These results indicate that iontophoresis might be able to enhance the flux of drugs with high molecular weight (≥5 kDa), but it is impossible to control the flux.
Effect of current density on the skin penetration of vitamin B12. The electric field was applied for 1 h after 1 h of passive transport. Closed circles, 0.6 mA/cm2; closed squares, 0.3 mA/cm2; closed triangles, 0.15 mA/cm2; open circles, control experiment (0 mA/cm2). IP iontophoresis (Reproduced with permission from The Society of Chemical Engineers, Japan)
3.2 Electroporation
Electroporation is also electro-assisted enhancement methods as well as iontophoresis and was originally used as a transfection method entering deoxyribonucleic acid (DNA) into the cell; high-voltage pulse applications for very short durations of time make transient pores in the cell membrane (Zerbib et al. 1985). Prausnitz et al. (1993) first reported the use of electroporation in transdermal delivery research. They achieved the enhancement of the transdermal flux of calcein (MW = 623, −4 charge) at in vitro and in vivo experiments. After this report, the skin penetration enhancement for macromolecules has been reported (Lombry et al. 2000; Riviere et al. 1995; Vanbever et al. 1998; Zhang et al. 2002; Zhao et al. 2006). Electrical studies have shown that the enhancement mechanism of electroporation and the factors of voltage, pulse length, and pulse rate affect the drug penetration flux (Banga et al. 1999; Denet et al. 2004; Sharma et al. 2000; Vanbever et al. 1996). Skin resistance dramatically decreases on a time scale of milliseconds by high-voltage pulses (Prausnitz 1996). The fast decrease of skin resistance causes the creation and expansion of pores in the stratum corneum (Pliquett et al. 1995), and the slow decrease may involve the change of the stratum corneum structure by thermal effects (Pliquett and Gusbeth 2000). The pathways created by electrical pulse immediately close after cutting off the pulse, and, however, the skin resistance does not completely recover when electrical stimulus was too strong (Pliquett et al. 1995). Riviere et al. (1995) observed skin irritation after application of electroporation (a single exponential voltage pulse for 5 ms, ≤ 1000 V). An electroporation pulse had a transient erythema and no adverse irritation. Therefore, the advantages of electroporation are: (1) to cause insignificant skin damage, (2) to show the enhancement effect quickly, and (3) to increase the skin flux of macromolecules with a molecular weight greater than 7000 Da which limit for iontophoresis (Denet et al. 2004; Kanikkannan 2002).
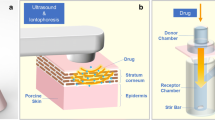
3.3 Ultrasound
The enhancement of drug penetration is determined by ultrasound parameters (i.e., frequency, intensity, duty cycle, and duration of application) (Shirouzu et al. 2008). This is because physicochemical phenomena, rising temperature, acoustic streaming, the generation of convective flow, and the cavitation (Barnett et al. 1994; Liu et al. 1998; Mitragotri et al. 2000) caused by sonophoresis are influenced by: each ultrasound parameter, skin models, physicochemical properties of drugs, and experimental conditions. The flux of lidocaine hydrochloride (MW = 270, Fig. 22.3a) and that of vitamin B12 (Fig. 22.3b) were influenced by the ultrasound frequency (2 MHz and 300 kHz, 410 J/cm2). The vitamin B12 flux was also affected by the energy flux (intensity × treatment time × duty cycle) (Fig. 22.4) (Shirouzu et al. 2008). The enhancement mechanism involves mechanical, thermal, and physiological changes of the skin, in particular the imploding cavitation bubbles that disrupt the structure of the lipid bilayers in the stratum corneum (Tezel and Mitragotri 2003).
Enhancement of the skin penetration of lidocaine hydrochloride (LID, (a)) and vitamin B12 (VB, (b)) by sonophoresis (SP) for 30 min. The energy flux of ultrasound was controlled at 410 J/cm2. Closed circles, low-frequency ultrasound (300 kHz); closed squares, therapeutic-frequency ultrasound (2 MHz); open circles, control experiment without sonophoresis
Sonophoresis is divided into three categories based on frequency: low-frequency (~kHz), therapeutic-frequency (1–3 MHz), and high-frequency (3–16 MHz) ultrasound. Bommannan et al. (1992) reported that high-frequency ultrasound increased the flux of salicylic acid and, at the same time, resulted in the structural alteration of skin tissue by the application of heat for 20 min. Therapeutic-frequency ultrasound is widely used in treatment, diagnosis, and physiotherapy. Therapeutic-frequency ultrasound at the intensity within 0–2 W/cm2 can induce reversible changes in the skin barrier and can enhance the flux of low molecular weight drugs (Mitragotri et al. 1995; Yamashita et al. 1996). However, it hardly improves the flux of high molecular weight drugs. The sonic waves of low-frequency ultrasound can deeply penetrate into the skin tissue, and, moreover, low-frequency ultrasound generates cavitation bubbles at lower intensity than therapeutic-frequency ultrasound. Low-frequency sonophoresis (20 kHz, 7 W/cm2, 50 % duty cycle) is used for the enhancement of high molecular weight drugs, insulin (MW = 5805 Da), heparin (12 k–15 kDa), and interferon-gamma (IFN-γ) (15 k–25 kDa) (Mitragotri et al. 1996; Mitragotri and Kost 2001). The US Food and Drug Administration (FDA) recently approved the SonoPrep® system (55 kHz, 15 W/cm2, Santra Medical Co.) as a transdermal delivery system for lidocaine. This system, using low-frequency ultrasound, is expected to be used as a needle-free blood glucose monitor.
Sonophoresis is an excellent method for transdermal drug delivery, but some researchers have reported skin tissue damage caused by sonophoresis under the higher intensity. Low-frequency ultrasound (20 kHz) at intensities lower than 2.5 W/cm2 did not affect skin tissues, whereas intensities of 5.2 W/cm2 caused irreversible changes in skin tissue (Boucaud et al. 2001). The skin is a water-rich tissue, and, thus, enzyme deactivation may certainly occur in the skin tissue by cavitation. The effect of the intensity and duration of ultrasound application (1 MHz, 4.3 W/cm2) on the bioconversion of an ester drug was investigated using a hairless mouse skin in vitro (Fig. 22.5) (Hikima et al. 1998). Enzyme deactivation may be partly responsible for free radicals generated in the reservoir solution and tissue fluid during an ultrasound pretreatment of the skin. More research is needed to identify the interactions among ultrasound parameters and to establish the safe use of drugs for transdermal therapeutic systems.
3.4 Microneedles
Microfabrication technology for drug delivery has been used in oral delivery (Verma et al. 2000), dermal delivery (Prausnitz et al. 2003), and implantable delivery (LaVan et al. 2003; Staples et al. 2006). Microneedles have been fabricated using microelectromechanical systems (MEMS) and have been developed to enhance the flux without the pain of piercing, as microneedles do not reach the nerve endings at the upper dermis. The drug application by microneedles was classified according to the material and design: (1) solid microneedles made from silicon, metal, and polymer that pierce the stratum corneum before drug application (Martanto et al. 2004; Hikima et al. 2012), (2) solid microneedles from metal and polymer coated with drug (Gill and Prausnitz 2007), (3) solid microneedles from biodegradable polymer coated with drug and contained the drug (Park et al. 2006), and (4) hollow microneedles from metal and polymer for drug solutions (Häfeli et al. 2009).
The Macroflux® transdermal microprojection delivery system with titanium microneedles coated with drug and the microstructured transdermal system (MTS) microneedle patch were developed by ALZA Co. and 3 M, respectively. Intracutaneous immunization with Macroflux® had similar immunoglobulin G (IgG) titers compared to intramuscular, subcutaneous, and intradermal injections (Matriano et al. 2002). Low-dose influenza vaccines with NanoPass® comprised of hollow silicon microneedles resulted in immunogenic reactions similar to the full-dose intramuscular vaccination (Damme et al. 2009). Microneedles may be suitable for the intracutaneous delivery of high molecular weight drugs, but there have been some reports that the insertion depth of a microneedle into the stratum corneum is influenced by the shape of the microneedle (Bal et al. 2010), the force of insertion (Davis et al. 2004), and the skin compaction during microneedle insertion (Martanto et al. 2006). Therefore, further detailed investigations into the mechanisms of drug transport by microneedles through the stratum corneum and the mechanical properties of the stratum corneum are necessary.
4 Combination of Iontophoresis with Other Physical Enhancement Methods
Chemical and physical enhancers have been studied for their use with transdermal therapeutic systems, to achieve active transport and to control the penetration flux of drugs. However, researchers reported that the strength of an undesirable stimulus, dermatitis, and irreversible skin damage increases in proportion to the enhancement effect of enhancers on the penetration flux of drugs (Boucaud et al. 2001; Ledger 1992). Thus, the application of enhancers in humans is limited due to their undesirable side effects. Protein and peptide drugs with high molecular weights do not penetrate across the skin easily (Kanikkannan 2002), and the penetration flux continuously increases after the electric current is removed (Miyagi et al. 2006). Therefore, researchers have been investigating the combined use of enhancers for reasons of safety, economy, and efficacy (Fang et al. 2002; Mitragotri 2000; Wang et al. 2005). Moreover, the combination of enhancers leads to the synergistic enhancement of transdermal drug delivery. There are many reports about the synergistic enhancement by the combined use of enhancers, such as the combinations of iontophoresis with chemical enhancers (Pillai et al. 2004; Rastogi and Singh 2005), iontophoresis with electroporation (Chang et al. 2000), iontophoresis with sonophoresis (Fang et al. 2002; Le et al. 2000; Shirouzu et al. 2008), iontophoresis with microneedles (Katikaneni et al. 2009; Lin et al. 2001), as well as sonophoresis with chemical enhancers (Johnson et al. 1996; Lavon et al. 2005), sonophoresis with electroporation (Kost et al. 1996), laser radiation with microdermabrasion (Fang et al. 2004), and others.
However, some researchers reported also a lower penetration flux when a combination of enhancers was used compared to the use of a single enhancement method. Denet et al. (2003) indicated that the electroosmotic flux during iontophoresis (0.25 mA/cm2 for 3 h or 0.5 mA/cm2 for 9 h) was decreased by the accumulation of a positively charged drug, timolol maleate, in the stratum corneum by electroporation pretreatment (400 V, 10 msec, 10 pulses). Singh and Jayaswal (2008) reported that the chemical enhancer Azone® inhibited the effect of an electric current (0.45 mA/cm2, 6 h) on 5-FU transport because it interacted with the components of the stratum corneum. X-ray, attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), and differential scanning calorimetry (DSC) studies revealed that the pretreatment with the chemical enhancer hexadecyltrimethylammonium bromide changed the electrical and structural properties of the stratum corneum, with the result that the skin penetration flux of propranolol hydrochloride decreased (Chesnoy et al. 1999). Therefore, the mechanisms underlying the penetration enhancement by each enhancer must be further investigated so that the appropriate combination of enhancers can be identified.
4.1 Combination of Iontophoresis and Electroporation
While iontophoresis directly acts on the drug molecule and the movement of water through the stratum corneum, electroporation causes the change of stratum corneum structure. These methods have a different penetration mechanism, thereby the combined use of iontophoresis and electroporation could synergistically enhance the skin penetration flux of drug. Examples of the combined use of iontophoresis and electroporation are summarized in Table 22.1. Although electrical pulses of lower voltage less than 100 V followed by iontophoresis did not increase the flux of salmon calcitonin (MW = 3600), the flux was enhanced synergistically by the combined use of iontophoresis and electric pulse of 120 V (Chang et al. 2000). Ching et al. (2012) studied whether three different molecular weight biomarkers, urea (MW = 60 Da), osteopontin (MW = 33 kDa), and prostate-specific antigen (MW = 34 kDa), can be extracted from the skin by the combination of iontophoresis and electroporation in vitro. This technique is well known as reverse iontophoresis that is used as a diagnostic method by extracting molecules through the skin (Garg et al. 1999; Mize et al. 1997). Ching et al. (2012) concluded that transdermal extraction of prostate-specific antigen and osteopontin was possible only when applying reverse iontophoresis in combination with a high-voltage (≥ 296 V/cm) electroporation.
Iontophoresis can control the skin penetration flux of drugs by switching current on/off, and, on the other hand, electroporation can enhance the flux of macromolecules. The combined use of iontophoresis and electroporation may be possible to control the flux of macromolecules; however, it may be difficult for resealing pathways created by electrical pulse immediately. Therefore, further detailed investigations are needed for the practical application of the combined use of iontophoresis and electroporation.
4.2 Combination of Iontophoresis and Sonophoresis
The combined use of iontophoresis and sonophoresis is a practical approach to enhance the flux synergistically, because the electric properties of skin tissue are not affected by ultrasound treatment at all. Table 22.2 summarizes the literature regarding the combined use of iontophoresis and sonophoresis. The frequency of the ultrasound was an important factor for the synergistic enhancement. The skin was pretreated by ultrasound (300 kHz and 2 MHz) for 30 min, and then electric field (0.35 mA/cm2, 1 h) was applied to the skin (Fig. 22.6). The combination of therapeutic-frequency ultrasound and electric field did not cause the synergistic enhancement of vitamin B12. Hikima et al. (2009) reported the effect of the application time of sonophoresis (Fig. 22.7). The flux of vitamin B12 increased to 48 times compared to the control flux when 30 min ultrasound and 1 h iontophoresis were applied simultaneously. On the other hand, a 177-fold synergistic enhancement of the drug flux was achieved when the electric field applied after the ultrasound pretreatment. These results may indicate that sonophoresis changes the stratum corneum structure by cavitation and iontophoresis produced the additional forces of electroosmosis.
Effect of ultrasound frequency on the synergistic enhancement of skin penetration of vitamin B12. The skin was pretreated by ultrasound for 30 min before starting the experiment. Closed circles, low frequency (300 kHz); closed squares, therapeutic frequency (2 MHz); open triangles, iontophoresis without ultrasound pretreatment; open circles, control experiment
Differences in the enhancement effects of vitamin B12 according to treatment time by sonophoresis and iontophoresis. Closed circles, pretreatment with sonophoresis for 30 min and then iontophoresis applied for 1 h; closed squares, sonophoresis for 30 min and iontophoresis for 1 h simultaneously applied; open circles, control experiment
Skin anesthesia using a topical anesthetic (lidocaine hydrochloride) usually requires 30–60 min. Iontophoresis (0.79 mA/cm2) enhanced the skin penetration of lidocaine, but it required at least 10 min. The combination of ultrasound pretreatment and 0.20 mA/cm2 electrocurrent for 2 min gave the same anesthetic effect as 10 min iontophoresis (0.79 mA/cm2) (Spierings et al. 2008). Ultrasound pretreatment can shorten the required intensity and duration of current intensity of electric field. For example, Shirouzu et al. (2008) reported that a combination of sonophoresis and iontophoresis synergistically increased and temporally controlled the penetration flux of vitamin B12 in vitro (Fig. 22.8). The ratio of the drug flux compared to the control flux was synergistically increased to 217 times by the combined use of iontophoresis and sonophoresis. The flux of heparin (average MW = 10,000) (Le et al. 2000) and that of sodium nonivamide acetate (MW = 375) (Fang et al. 2002) were synergistically enhanced by the application of iontophoresis and sonophoresis. Hikima et al. (2009) investigated the mechanism of the synergistic effects of sonophoresis and iontophoresis on skin penetration. They performed in vitro skin penetration experiments using seven model chemicals with different electric charges and molecular weights. The synergistic effects were observed in nonionized and high molecular weight (approximately 1500 Da) drugs (Fig. 22.9a–d). Hikima et al. (2009) concluded that the electroosmotic flow was the key factor in the synergistic penetration enhancement of drugs by the combined use of iontophoresis and sonophoresis.
Synergistic enhancement of vitamin B12 by the combined use of sonophoresis and iontophoresis. The skin was pretreated by ultrasound (300 kHz) before starting the experiment, and the electric field was applied four times during the experiment. Closed circles, combination of sonophoresis and iontophoresis; open triangles, iontophoresis; open squares, sonophoresis; open circles, control experiment (Reproduced with permission from The Society of Chemical Engineers, Japan)
Penetration enhancement of lidocaine hydrochloride (LH, a positive charged molecule, (a)), benzoic acid (BA, a negative charged molecule, (b)), hydrocortisone (HC, a nonionized and low molecular weight molecule, (c)), and vancomycin hydrochrolide (VH, a positive charged and high molecular weight molecule, (d)). Closed circles, combination of sonophoresis and iontophoresis; open triangles, iontophoresis; open squares, sonophoresis; open circles, control experiment (Reproduced with permission from Pharmaceutical Society of Japan)
4.3 Combination of Iontophoresis and Microneedles
Table 22.3 summarizes the literature about the combined uses of iontophoresis and microneedles. Although there are various types of microneedles, regarding the used material, shape, length, and density of needles, their combination with iontophoresis provided synergistic enhancement of the drug flux (Table 22.3). The mechanism of skin penetration enhancement by microneedles is to create new transport pathways across the stratum corneum, with the result that high molecular weight and highly hydrophilic drugs are able to transport across the stratum corneum. The combined use of iontophoresis and microneedles can be expected to provide a synergistic enhancement of the flux because a microneedle makes only pores in the stratum corneum mechanically, while iontophoresis improves the movement of drugs in these pores. Thus, there are many reports that iontophoresis was applied to the skin pretreated by microneedles. For example, Chen et al. (2009) reported that insulin using insulin-loaded nanovesicles with various charge and size was delivered into the skin by a combination of microneedles and iontophoresis. Positively charged nanovesicles with the average diameter of 107 nm were withdrawn on the skin pretreated by microneedles and then iontophoresis (0.2 mA/cm2, on/off ratio of 1:1, and frequency of 100 Hz) applied continuously for 3 h, and the result decreased the blood glucose levels comparable to the levels achieved by subcutaneous injection. On the other hand, Garland et al. (2012) indicated that a synergistic effect on the increase of FITC-bovine serum albumin (BSA) flux was produced by the simultaneous application of iontophoresis and microneedle. They succeeded biodegradable polymeric microneedles and iontophoresis in a one-step application.
As aforementioned in this chapter by Eq. (22.4), the enhancement mechanism of iontophoresis involves electrorepulsion and electroosmosis. Katikaneni et al. (2009) studied the effect of pretreatment with microneedles on the skin penetration of acetaminophen, as a marker of electroosmosis in vitro. Iontophoresis enhanced the penetration flux of acetaminophen by seven times across the pretreated skin, suggesting that electroosmotic flow through microchannels made by the microneedles persisted. They also reported that the flux of a large molecular weight drug, daniplestim (MW = 12.76 kDa, pI = 6.2), was increased by electroosmosis under the combination of iontophoresis and microneedles. Using a mathematical simulation, Tojo (2005) discussed the importance of electroosmotic flow caused by an electric field for the synergistic effect of iontophoresis and microneedle combination. The blood concentration of human growth hormone (MW = 22 kDa, pI = 5.0) in hairless guinea pig is shown in Fig. 22.10. The lines and plots in Fig. 22.10 express the simulation data and experimental data from Cormier and Daddona (2003), respectively. Cormier and Daddona (2003) applied human growth hormone to the skin pretreated by Macroflux® (Zosano Pharm™, Inc.) and turned electric current off at 1 h (Fig. 22.10a) and at 4 h (Fig. 22.10b). Although the concentration of human growth hormone was under detection level in passive and iontophoresis-only application (data not shown), turning the current on/off under the combination of iontophoresis and microneedles controlled the time course of the plasma concentration. Each dashed and solid line in Fig. 22.10 in the study performed by Tojo (2005) was calculated on using a bilayer skin model assuming that the thickness of the stratum corneum by microneedles was reduced. The solid lines assumed that the electroosmotic flow across the skin continued for 30 min after iontophoresis was shut off. The solid lines approximately satisfied the transient profiles following the shutoff of the current after 1 h (Fig. 22.10a) and 4 h (Fig. 22.10b). This finding suggested that the electroosmotic flow caused the synergistic enhancement, and it did not stop immediately when the iontophoresis was terminated. Therefore, the electroosmotic flow is an important factor of the combined use of iontophoresis and microneedle for the synergistic penetration enhancement of macromolecules.
Transdermal delivery of human growth hormone by the combination of iontophoresis and microneedles. Iontophoresis was applied for 1 h (a) and 4 h (b) to the skin pretreated by Macroflux®. Closed plot, in vivo experimental data from (Cormier and Daddona 2003); solid line, electroosmotic flow remained active for 30 min; dashed line, the electroosmotic flow vanished immediately after the current was turned off
Conclusions
This chapter has overviewed the combined use of iontophoresis and other physical enhancers, electroporation, sonophoresis, and microneedle. While each physical enhancer has the advantage to increase the skin penetration flux of macromolecules, the application of physical enhancer is limited due to the undesirable and irreversible skin damages. However, the combination of physical enhancers brings us the possibility to enhance and control the skin penetration flux of proteins (i.e., peptides, antibodies, and interferons) and nucleic acids (i.e., oligonucleotides, micro-RNAs (miRNAs), and siRNAs). When the combined use of enhancers is investigated, we must pay attention to the enhancement mechanism of each physical enhancer and identify the appropriate combination. A practical approach to enhance the flux synergistically with iontophoresis is to use an enhancer that increases the drug flux without changing the electric properties of the skin tissue. Further detailed investigations are needed for controlling the flux of macromolecules by combining iontophoresis with other physical enhancers.
References
Badkar AV, Banga AK (2002) Electrically enhanced transdermal delivery of a macromolecule. J Pharm Pharmacol 54(7):907–912
Bal SM, Kruithof AC, Zwier R, Dietz E, Bouwstra JA, Lademann J et al (2010) Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo. J Control Release 147(2):218–224
Banga AK, Bose S, Ghosh TK (1999) Iontophoresis and electroporation: comparisons and contrasts. Int J Pharm 179(1):1–19
Barnett SB, Ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J (1994) Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol 20(3):205–218
Bommannan D, Menon GK, Okuyama H, Elias PM, Guy RH (1992) Sonophoresis. II. Examination of the mechanism(s) of ultrasound-enhanced transdermal drug delivery. Pharm Res 9(8):1043–1047
Bommannan DB, Tamada J, Leung L, Potts RO (1994) Effect of electroporation on transdermal iontophoretic delivery of luteinizing hormone releasing hormone (LHRH) in vitro. Pharm Res 11(12):1809–1814
Bose S, Ravis WR, Lin YJ, Zhang L, Hofmann GA, Banga AK (2001) Electrically-assisted transdermal delivery of buprenorphine. J Control Release 73(2–3):197–203
Boucaud A, Montharu J, Machet L, Arbeille B, Machet MC, Patat F et al (2001) Clinical, histologic, and electron microscopy study of skin exposed to low-frequency ultrasound. Anat Rec 264(1):114–119
Brown MB, Martin GP, Jones SA, Akmeah FK (2006) Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13(3):175–187
Burnette RR, Ongpipattanakul B (1988) Characterization of the pore transport properties and tissue alteration of excised human skin during electrophoresis. J Pharm Sci 77(2):132–137
Chang SL, Hofmann GA, Zhang L, Deftos LJ, Banga AK (2000) The effect of electroporation on iontophoretic transdermal delivery of calcium regulating hormones. J Control Release 66(2–3):127–133
Chaturvedula A, Joshia DP, Anderson C, Morris RL, Sembrowich WL, Banga AK (2005) In vivo iontophoretic delivery and pharmacokinetics of salmon calcitonin. Int J Pharm 297(1–2):190–196
Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J et al (2009) Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release 139(1):63–72
Chesnoy S, Durand D, Doucet J, Couarraze G (1999) Structural parameters involved in the permeation of propranolol HCl by iontophoresis and enhancers. J Control Release 58(2):163–175
Ching CT, Fu LS, Sun TP, Hsu TH, Chang KM (2012) Use of electroporation and reverse iontophoresis for extraction of transdermal multibiomarkers. Int J Nanomedicine 7:885–894
Cormier M, Daddona PE (2003) Macroflux technology for transdermal delivery of therapeutic proteins and vaccines. In: Rathbone MJ, Hadgraft J, Roberts MS (eds) Modified-release drug delivery technology. Marcel Decker, New York, pp 589–598
Damme PV, Oosterhuis-Kafej F, Van der Wielen M, Almagor Y, Sharon O, Levin Y (2009) Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27(3):454–459
Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR (2004) Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech 37(8):1155–1163
Denet AR, Ucakar B, Préat V (2003) Transdermal delivery of timolol and atenolol using electroporation and iontophoresis in combination: a mechanistic approach. Pharm Res 20(12):1946–1951
Denet AR, Vanbever R, Préat V (2004) Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev 56(5):659–674
Fang JY, Hwang TL, Huang YB, Tsai YH (2002) Transdermal iontophoresis of sodium nonivamide acetate V. Combined effect of physical enhancement methods. Int J Pharm 235(1–2):95–105
Fang JY, Lee WR, Shen SC, Fang YP, Hu CH (2004) Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol 151(1):132–140
Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP (1999) Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care 22(10):1708–1718
Garland MJ, Caffarel–Salvador E, Migalska K, Woolfson AD, Donnelly RF (2012) Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J Control Release 159(1):52–59
Gill HS, Prausnitz MR (2007) Coating formulations for microneedles. Pharm Res 24(7):1369–1380
Gupta SK, Sathyan G, Philipps B, Klausner M, Southam M (1999) Reproducible fentanyl doses delivered intermittently at different time intervals from an electrotransport system. J Pharm Sci 88(8):835–841
Häfeli UO, Mokhtari A, Liepmann D, Stoeber B (2009) In vivo evaluation of a microneedle-based miniature syringe for intradermal drug delivery. Biomed Microdevices 11(5):943–950
Hikima T, Hirai Y, Tojo K (1998) Effect of ultrasound application on the skin metabolism of prednisolone 21-acetate. Pharm Res 15(11):1680–1683
Hikima T, Ohsumi S, Shirouzu K, Tojo K (2009) Mechanisms of synergistic skin penetration by sonophoresis and iontophoresis. Biol Pharm Bull 32(5):905–909
Hikima T, Yoshida T, Okabe H, Kawahara K, Itoh T, Tojo K (2012) Prediction of skin penetration enhancement by microneedles. Drug Deliv Syst Suppl:P2–P18. Japanese
Inada H, Ghanem AH, Higuchi WI (1994) Studies on the effect of applied voltage and duration on human epidermal membrane alteration/recovery and the resultant effects upon iontophoresis. Pharm Res 11(5):687–697
Johnson ME, Mitragotri S, Patel A, Blankschteinx D, Langer R (1996) Synergistic effects of chemical enhancers and therapeutic ultrasound on transdermal drug delivery. J Pharm Sci 85(7):670–679
Kanikkannan N (2002) Iontophoresis-based transdermal delivery systems. BioDrugs 16(5):339–347
Katikaneni S, Badkar A, Nema S, Banga AK (2009) Molecular charge mediated transport of a 13 kD protein across microporated skin. Int J Pharm 378(1–2):93–100
Kim A, Green PG, Rao G, Guy RH (1993) Convective solvent flow across the skin during iontophoresis. Pharm Res 10(9):1315–1320
Kost J, Pliquett U, Mitragotri S, Yamamoto A, Langer R, Weaver J (1996) Synergistic effect of electric field and ultrasound on transdermal transport. Pharm Res 13(4):633–638
LaVan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21(10):1184–1190
Lavon I, Grossman N, Kost J (2005) The nature of ultrasound–SLS synergism during enhanced transdermal transport. J Control Release 107(3):484–494
Le L, Kost J, Mitragotri S (2000) Combined effect of low-frequency ultrasound and iontophoresis: applications for transdermal heparin delivery. Pharm Res 17(9):1151–1154
Ledger PW (1992) Skin biological issues in electrically enhanced transdermal delivery. Adv Drug Deliv Rev 9(2–3):289–307
Lin WQ, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL et al (2001) Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux®) technology. Pharm Res 18(12):1789–1793
Liu J, Lewis TN, Prausnitz MR (1998) Non-invasive assessment and control of ultrasound-mediated membrane permeabilization. Pharm Res 15(6):918–924
Lombry C, Dujardin N, Préat V (2000) Transdermal delivery of macromolecules using skin electroporation. Pharm Res 17(1):32–37
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR (2004) Transdermal delivery of insulin using microneedles in vivo. Pharm Res 21(6):947–952
Martanto W, Moore JS, Couse T, Prausnitz MR (2006) Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release 112(3):357–361
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K et al (2002) Macroflux® microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res 19(1):63–70
Medi BM, Singh J (2003) Electronically facilitated transdermal delivery of human parathyroid hormone (1–34). Int J Pharm 263(1–2):25–33
Mitragotri S (2000) Synergistic effect of enhancers for transdermal drug delivery. Pharm Res 17(11):1354–1359
Mitragotri S, Blankschtein D, Langer R (1996) Ultrasound-mediated transdermal protein delivery. Science 269(5225):850–853
Mitragotri S, Edwards DA, Blankschtein D, Langer R (1995) A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J Pharm Sci 84(6):697–706
Mitragotri S, Farrell J, Tang H, Terahara T, Kost J, Langer R (2000) Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J Control Release 63(1–2):41–52
Mitragotri S, Kost J (2001) Transdermal delivery of heparin and low-molecular weight heparin using low-frequency ultrasound. Pharm Res 18(8):1151–1156
Miyagi T, Hikima T, Tojo K (2006) Effect of molecular weight of penetrates on iontophoretic transdermal delivery in vitro. J Chem Eng 39(3):360–365
Mize NK, Buttery M, Daddona P, Morales C, Cormier M (1997) Reverse iontophoresis: monitoring prostaglandin E2 associated with cutaneous inflammation in vivo. Exp Dermatol 6(6):298–302
Park JH, Allem MG, Prausnitz MR (2006) Polymer microneedles for controlled-release drug delivery. Pharm Res 23(5):1008–1012
Pasero C (2006) Lidocaine iontophoresis for dermal procedure analgesia. J Perianesth Nurs 21(1):48–52
Pfister WR (1997) Transdermal and dermal therapeutic systems: current status. In: Ghosh TK, Pfister WR, Yum SI (eds) Transdermal and topical drug delivery systems. Interpharm Press, Inc, Buffalo Grove, pp 33–112
Pillai O, Nair V, Panchagnula R (2004) Transdermal iontophoresis of insulin: IV. Influence of chemical enhancers. Int J Pharm 269(1):109–120
Pliquett UF, Gusbeth CA (2000) Perturbation of human skin due to application of high voltage. Bioelectrochemistry 51(1):41–51
Pliquett U, Langer R, Weaver JC (1995) Changes in the passive electrical properties of human stratum corneum due to electroporation. Biochim Biophys Acta 1239(2):111–121
Prausnitz MR (1996) Do high-voltage pulses cause changes in skin structure? J Control Release 40(3):321–326
Prausnitz MR, Ackley DE, Gyory JR (2003) Microfabricated microneedles for transdermal drug delivery. In: Rathbone MJ, Hadgraft J, Roberts MS (eds) Modified release drug delivery technology. Marcel Dekker, New York, pp 513–522
Prausnitz MR, Bose VG, Langer R, Weaver JC (1993) Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci U S A 90(22):10504–10508
Rastogi SK, Singh J (2005) Effect of chemical penetration enhancer and iontophoresis on the in vitro percutaneous absorption enhancement of insulin through porcine epidermis. Pharm Dev Technol 10(1):97–104
Riviere JE, Monterio-Riviere NA, Rogers RA, Bommannan D, Tamada JA, Potts RO (1995) Pulsatile transdermal delivery of LHRH using electroporation: Drug delivery and skin toxicology. J Control Release 36(3):229–233
Sharma A, Kara M, Smith FR, Krishnan TR (2000) Transdermal drug delivery using electroporation. I. Factors influencing in vitro delivery of terazosin hydrochloride in hairless rats. J Pharm Sci 89(4):528–535
Shirouzu K, Nishiyama T, Hikima T, Tojo K (2008) Synergistic effect of sonophoresis and iontophoresis in transdermal drug delivery. J Chem Eng Jpn 41(4):300–305
Singh BN, Jayaswal SB (2008) Iontophoretic delivery of 5-fluorouracil through excised human stratum corneum. Drug Discov Ther 2(2):128–135
Spierings ELH, Brevard JA, Katz NP (2008) Two-minute skin anesthesia through ultrasound pretreatment and iontophoresis delivery of a topical anesthetic: a feasibility study. Pain Med 9(1):55–59
Staples M, Danie K, Cima MJ, Langer R (2006) Application of micro- and nano-electromechanical devices to drug delivery. Pharm Res 23(5):847–863
Tezel A, Mitragotri S (2003) Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J 85(6):3502–3512
Tojo K (2005) Mathematical models of transdermal and topical drug delivery, 2nd edn. Biocom Systems, Fukuoka
Tokumoto S, Higo N, Sugibayashi K (2006) Effect of electroporation and pH on the iontophoretic transdermal delivery of human insulin. Int J Pharm 326(1–2):13–19
Vaka SR, Shivakumar HN, Murthy SN (2011) Constant voltage ‘Iron’tophoresis. Pharm Dev Technol 16(5):483–488
Vanbever R, Langers G, Montmayeur S, Préat V (1998) Transdermal delivery of fentanyl: rapid onset of analgesia using skin electroporation. J Control Release 50(1–3):225–235
Vanbever R, LeBoulengé E, Préat V (1996) Transdermal delivery of fentanyl by electroporation. I. Influence of electrical factors. Pharm Res 13(4):559–565
Vemulapalli V, Bai Y, Kalluri H, Herwadkar A, Kim H, Davis SP et al (2012) In vivo iontophoretic delivery of salmon calcitonin across microporated skin. J Pharm Sci 101(8):2861–2869
Verma RK, Mishra B, Garg S (2000) Osmotically controlled oral drug delivery. Drug Dev Ind Pharm 26(7):695–708
Wang Y, Allen LV Jr, Li LC, Tu YH (1993) Iontophoresis of hydrocortisone across hairless mouse skin: investigation of skin alteration. J Pharm Sci 82(11):1140–1144
Wang Y, Thakur R, Fan Q, Michnia B (2005) Transdermal iontophoresis: combination strategies to improve transdermal iontophoretic drug delivery. Eur J Pharm Biopharm 60(2):179–191
Wester RC, Maibach HI (1983) Cutaneous pharmacokinetics: 10 steps to percutaneous absorption. Drug Metab Rev 14(2):169–205
Williams AC, Barry BW (2004) Penetration enhancers. Adv Drug Deliv Rev 56(5):603–618
Wu XM, Todo H, Sugibayashi K (2007) Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J Control Release 118(2):189–195
Yamashita A, Hirai Y, Tojo K (1996) Effect of ultrasound on rate of drug absorption through skin. J Chem Eng Jpn 29(5):812–816
Yoshida NH, Roberts MS (1992) Structure-transport relationship in transdermal iontophoresis. Adv Drug Deliv Rev 9(2–3):239–264
Zerbib D, Amalric F, Teissié J (1985) Electric field mediated transformation: isolation and characterization of a TK+ subclone. Biochem Biophys Res Commun 129(3):611–618
Zhang L, Nolan E, Kreitschitz S, Rabussay DP (2002) Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta 1572(1):1–9
Zhao YL, Murthy SN, Manjili MH, Guan LJ, Sen A, Hui SW (2006) Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine 24(9):1282–1290
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hikima, T., Tojo, K. (2017). Combined Use of Iontophoresis and Other Physical Methods. In: Dragicevic, N., I. Maibach, H. (eds) Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53273-7_22
Download citation
DOI: https://doi.org/10.1007/978-3-662-53273-7_22
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53271-3
Online ISBN: 978-3-662-53273-7
eBook Packages: MedicineMedicine (R0)