Abstract
Delivery of macromolecules is primarily achieved by the use of hypodermic needles, which have several disadvantages including accidental needlesticks, pain, and needle phobia. These limitations have led to extensive research and development of alternative methods for drug and vaccine delivery across the skin, without the use of needles. Jet injectors are one such class of needle-free devices, which have been used to deliver both liquid and solid drug and vaccine formulations. In spite of their availability for research and clinical use for the past several decades, these devices have had limited acceptance. The mechanism of operation of these devices, the enhanced skin penetration of drugs, device design parameters, applications, and safety concerns for these two types of injection devices are described. Current developments in the field have also been discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Transdermal drug delivery devices encompass a variety of active and passive devices employed for delivering drugs and vaccines across the skin barrier (Barry 2001; Prausnitz et al. 2004; Schuetz et al. 2005). The classification of active vs. passive technologies depends on the technology employed for skin permeation enhancement (Arora et al. 2008). Active technologies typically increase drug transport across the skin by physically disrupting the stratum corneum barrier and via supplying an added driving force for drug transport across this disrupted barrier (Brown et al. 2006). This is especially advantageous when passive diffusion of drugs even across the disrupted skin barrier is not sufficient for reaching therapeutic levels, such as for macromolecules. In addition, active methods also offer more control over delivery profile, as the skin barrier is physically perturbed resulting in shorter delay between application and drug reaching systemic circulation compared to passive methods. Also, the device and application parameters can be adjusted to better match individual’s skin properties. Based on these classification criteria, jet injectors form a class of active drug delivery devices. Jet injectors deliver drugs or vaccines via a high-velocity jet of formulation containing the active pharmaceutical ingredient (API). The API-containing formulation can be liquid or solid/powder and is delivered using a liquid jet injector or solid/projectile jet injector, respectively.
Similar to other active delivery technologies such as iontophoresis, microneedles, and ultrasound, the challenges associated with developing jet injectors go beyond merely creating a high-velocity jet capable of penetrating the skin (Arora et al. 2008). Jet injectors can have additional requirements including customizable power source, dosage control, and separation of disposable parts from reusable components. It is this complexity of implementation of active delivery technologies into devices that makes this task challenging. In addition to the complexity of device fabrication and integration, issues related to maximizing delivery efficiency, while minimizing undesirable reactions such as pain and bruising at the site of injection, require significant research and development efforts.
Over the last decade, great progress on this front has been made with the advent of more sophisticated jet injectors, which offer greater control over delivery profile and injection parameters. Dose resolution in microliter or nanoliter volume range has been achieved (Arora et al. 2007). At the same time, the newer devices and skin breaches caused by them are small enough that they appear to be safe, well tolerated by patients, and allow rapid skin recovery post-administration.
We discuss their mechanisms of permeation enhancement, the current devices being used for injecting liquid and powder formulations, health effects, and future directions for device development.
2 Liquid Jet Injectors
Liquid jet injectors produce a high-velocity jet to puncture the skin and deliver drugs without the use of a needle. The origin of jet injector may be traced back to a device called “aquapuncture,” which was reported in literature by Béclard on behalf of H. Galante (Needle-free jet injection bibliography, device and manufacturer roster and patent list 2013).
Earliest documented research on jet injectors began when a mechanical engineer named Arnold Sutermeister noticed oil deposits underneath the skin of workers, when small leaks occurred in high-pressure oil pipelines (Sutermesiter 1954). Since then, liquid jet injectors have evolved into two separate classes of devices for single use and multiple use, i.e., disposable-cartridge jet injectors (DCJIs) and multi-use nozzle jet injectors (MUNJIs), respectively (Mitragotri 2006). MUNJIs were heavily used for mass immunization programs for diseases including measles, smallpox, cholera, hepatitis B, influenza, and polio. Their use was later discontinued due to possible involvement in the spread of hepatitis B in the 1980s (Canter et al. 1990). The outbreak was reportedly due to splash back of interstitial fluid on nozzle, leading to cross-contamination. Since then, designs of DCJIs have also evolved to separate disposable and reusable components for eliminating cross-contamination risks.
2.1 Injector Design and Operation
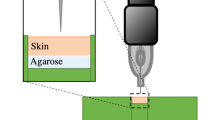
The basic components of a liquid jet injector consist of a compartment or cartridge for holding drug formulation, a piston assembly, a power source such as coil spring, and an actuation mechanism (Fig. 14.1). The drug formulation compartment has a fixed-sized orifice typically ranging between 76 and 360 μm on one end (Mitragotri 2006), through which the liquid can be forced out for jet creation, while the other side would be closed by the piston. Both prefilled disposable cartridges and cartridges supplied with a filling system for end user have been designed and used. For operation of a spring-powered jet injector, the spring is compressed by the end user and the filled drug cartridge is loaded onto the cocked injector. Upon actuation, the piston forces the drug formulation out of the orifice, thus creating high-velocity liquid jet (Fig. 14.2). Liquid jets are typically turbulent in nature, with Reynolds numbers in several thousands (Mitragotri 2006).
Schematic of drug delivery using liquid jet injector. (a) Formation of liquid jet, (b) initiation of hole formation due to the impact of jet on skin surface, (c) development of hole inside the skin with progress of injection, (d) deposition of drug at the end of hole in a near spherical or hemispherical pattern (spherical pattern shown) (Reprinted with permission from Arora et al. 2008)
Modern jet injectors have clear distinction between disposable and reusable components, with disposable components consisting of parts coming into contact with drug or patient. This makes the drug cartridge and part of piston assembly coming into contact with drug solution disposable. Newer designs have both these components as a part of drug cartridge itself, which minimizes the disassembling task post-injection.
2.2 Mechanism of Action
The mechanism of skin penetration and drug deposition in the skin can be divided into two phases, i.e., erosion and dispersion (Baxter and Mitragotri 2005). In the erosion phase, a high-velocity liquid jet impinges on the skin and causes skin fracture due to failure under mechanical stress. This phase is characterized by creation and progression of a hole, formed due to skin erosion along the path of jet progression (Fig. 14.2). During erosion, the jet progresses under submerged conditions and continues to increase the hole depth until it has lost the power required to deepen the hole further. This depth is characterized as the depth where maximum dispersion of drug formulation would occur (Schramm-Baxter et al. 2004). Based on jet power, the drug formulation is deposited in a spherical or part-spherical pattern, with the terminus of the hole acting as a pseudo source of liquid being dispersed. This hypothesis was supported by strong correlation between the depth of maximum dispersion, measured from skin surface, and hole depth, measured across skin samples with varying mechanical properties and jets created with various design parameters. These design parameters are discussed in the following section.
2.3 Design Parameters
The fluid delivery profile of a jet injector can be described by percent completeness of injection, penetration depth, and fluid dispersion pattern inside the skin. The design parameters that can be controlled for tailoring this fluid delivery profile of a jet injector include jet velocity, orifice size, pressure profile, and standoff distance.
The penetration of liquid jet into the skin and the percent of fluid delivered into the skin have been shown to be dependent on orifice diameter and jet velocity. Percent delivery of fluid into human skin has been shown to increase in the velocity range of 80–190 m/s for fixed orifice diameter of 152 μm (Schramm-Baxter et al. 2004; Schramm-Baxter and Mitragotri 2004). It is expected that a minimum threshold velocity would be required to rupture the skin, and no penetration would be observed below this threshold. The dependence of percent delivery on orifice diameter was not as strong as that reported for jet velocity. It has been hypothesized that the change in jet structure at higher orifice sizes may result in decrease of percent delivery. The two parameters of jet velocity and orifice diameter have been combined into a single parameter of jet power (P o ). Jet power is calculated as
where D o is the nozzle diameter, u o is the exit velocity, and ρ is the liquid density. Penetration depth increased from 0.2 mm at a power of 1 W to 2.8 mm at a power of 62.4 W (Schramm-Baxter and Mitragotri 2004). With variation in jet parameters, it is possible to span the full thickness of skin and control the depth where the bulk of drug solution is being delivered. The percent completeness of injection also increased linearly from near zero at a power of 1 W to >90 % at a power of ~30 W, beyond which the delivery remained constant at or above 90 % (Mitragotri 2006).
The standoff distance is the distance between the nozzle of injector and the skin at the time of injection. A linear decrease in hole depth was reported for increase in standoff distance for submerged and unsubmerged injections in polyacrylamide gel models (Schramm-Baxter et al. 2004). Some commercial injector manufacturers have accommodated standoff distance by including spacer rings, which can be placed on the skin at the time of injection (INJEX Pharma AG 2013).
An important design parameter is the pressure during injection. In a typical pressure profile representative of spring-powered injector, the pressure rises from the baseline level to a peak of about 3000–4000 psi in under a millisecond, which indicates the actuation (Schramm and Mitragotri 2002). This pressure level is maintained with some oscillations or exhibits a slight drop for the duration of injection. A sudden drop in pressure marks the end of injection. Modulating the pressure profile to better match the delivery of fluid with the rate of fluid absorption by the skin during injection has been an important area of research. To this effect, researchers have developed newer injector designs, which offer more sophisticated control of the jet velocity and thus pressure profiles, such as two distinct phases of higher and lower pressure to breach the skin and deliver the fluid, respectively (Stachowiak et al. 2009; Taberner et al. 2012). To achieve this control, traditional power sources such as compressed spring have been replaced with piezoelectric or Lorentz-force actuators (Stachowiak et al. 2009; Taberner et al. 2012).
In addition to the parameters described above, jet penetration also depends on the skin’s mechanical properties, with Young’s modulus of the skin being inversely correlated to both hole depth and fraction of liquid delivered. This, however, is not the focus of this section and is not discussed in detail. Readers are referred to the work published by Baxter and co-workers (2005).
2.4 Applications
MUNJIs have been used for mass immunization programs for diseases including measles, smallpox, cholera, hepatitis B, influenza, and polio (Mitragotri 2005). DCJIs have been used for delivery of several proteins. Most work on DCJIs has been done on delivery of insulin (Lindmayer et al. 1986; Weller and Linder 1966) and growth hormones (Agerso et al. 2002; Bareille et al. 1997; Dorr et al. 2003; Verhagen et al. 1995), while erythropoietin (Suzuki et al. 1995) and interferon (Brodell and Bredle 1995) have also been delivered. Insulin administration by jet injectors led to a faster delivery into systemic circulation, possibly due to better dispersion at the injection site. More recently, Zogenix received approval for SUMAVEL® DosePro®, its needle-free jet injector system for delivery of sumatriptan, indicated for migraine (Zogenix, Inc., USA 2013). To ensure commercial success, companies are now marketing their devices for both pharmaceutical and cosmetic applications. For example, Injex is currently marketing its needle-free jet injector for the delivery of insulin and local anesthetics as well as for cosmetic applications (INJEX Pharma AG, Germany, 2013). However, the acceptance of jet injectors has been low due to variable reactions at the site of administration (see section “Safety” below).
To counter the challenges faced by traditional jet injectors, a novel pulsed microjet was also developed (Arora et al. 2007). This new approach focuses on minimizing pain and bruising by minimizing injection volumes and depth of penetration. The actuation mechanism is based on a piezoelectric transducer and offers strict control over delivery volumes and injection velocity. The high velocity (>100 m/s) of microjets allowed their penetration into the skin, whereas the small jet diameters (50–100 μm) and extremely small volumes (2–15 nl) limited the penetration depth of these jets inside the skin to approximately 200 μm. The efficacy of this design was confirmed by delivering therapeutic doses of insulin in a rat model.
2.5 Safety
The acceptance of conventional jet injectors has been limited due to variable reactions at the administration site. Some reports state no difference in the level of pain compared to that experienced by hypodermic needles (Sarno et al. 2000), but others have reported higher levels of pain (Jackson et al. 2001). Variable reports in local reactions further augmented this fact, with some researchers reporting the absence of local reactions (Resman et al. 1985), while others have reported significantly more reactions including pain, bleeding, and hematomas (Houtzagers et al. 1988). It has been shown that the depth of penetration and percent delivery decrease with increasing Young’s modulus (i.e., mechanical strength) of the skin (Baxter and Mitragotri 2005). Commercial injectors come with very limited choice of settings, and owing to the person-to-person variability in skin’s mechanical properties, variability in patient response may be due to the failure of this “one-size-fits-all” approach of current commercial devices. Future devices such as pulsed microjets or Lorentz-force actuator-based injectors are being designed to address these problems by offering superior control over injection profile.
3 Powder Jet Injectors
The term powder injectors or projectile injectors describe injector devices used to deliver vaccines or drugs in dry powder or particulate form (Kendall 2006). Other terms such as biolistic injectors and gene guns have also been commonly used for these injectors, with the latter term used exclusively for deoxyribonucleic acid (DNA) delivery, typically on coated microparticles. Early work on powder injectors demonstrated the delivery of genetic material in plant cells via coated tungsten particles (Klein et al. 1987).
3.1 Injector Design and Operation
Basic design of solid jet injectors includes compressed gas as the power source, a drug compartment containing particulate drug formulation, and a nozzle to direct the flow of particles (Kendall et al. 2004a; Mulholland et al. 2004). A schematic of solid jet injector is shown in Fig. 14.3. The drug compartment is closed with diaphragms on either side, which are typically few microns thick. Upon triggering the actuation mechanism, compressed gas from a storage canister expands and pushes against the diaphragms, sequentially rupturing them. The flow of gas carries the drug particles with it. The particles then exit through a nozzle and impinge on the skin (Fig. 14.4). Upon impacting on the skin, particles puncture micron-sized holes into the stratum corneum by virtue of their momentum. Some particles are contained in the stratum corneum while a significant percent reach the viable epidermis for the desired therapeutic effect.
Schematic of drug delivery using powder injector. (a) Ejection of particles from the nozzle, (b) impact of particles on skin surface, (c) penetration of particles across the stratum corneum, (d) completion of delivery. Particles which penetrate into the skin are mostly distributed in the stratum corneum and viable epidermis (Reprinted with permission from Arora et al. 2008)
Another design used for studying powder injection mechanisms is light-gas gun, which uses an accelerating piston for imparting desired particle velocity (Crozier and Hume 1957). Upon triggering the actuation mechanism, the piston accelerates and carries the particles with it. A deceleration mechanism forces the piston to slow down and makes the particles leave the surface of piston. The particles are ejected and impact on target tissue surface.
3.2 Design Parameters
Key parameters in determining particle delivery across the stratum corneum are impact velocity, particle radius, and particle density. The particles constitute powdered preparation of drugs or vaccines and range between 10 and 20 μm. For DNA vaccination, coated metal particles between 0.5 and 3 μm have been used. A much broader range of particle sizes (0.5–52.6 μm) and densities (1.08–18.2 g/cm3) have been studied for injector development (Kendall 2002; Kendall et al. 2004a). Increase in particle size has been shown to increase delivery in an animal model using PowderJect® injector (Isis Innovation Ltd., Oxford, UK) (Sarphie et al. 1997). Diameter of the treated region is another design parameter and has been reported as up to 4 mm. When combined with the key parameters mentioned previously, this puts a limit of several milligrams on the dose delivered. It was also shown that increase in relative humidity and temperature increased the depth of penetration of gold particles from the stratum corneum to the epidermis using a biolistic injector (Kendall et al. 2004b). Hence, relative humidity and temperature may also need to be monitored or controlled for targeted delivery.
For studying correlations between particle properties and skin penetration, a combined parameter, namely, particle impact parameter, has been defined as ρvr, where ρ, v, and r are particle density, impact velocity, and radius, respectively (Rochelle and Lee 2007; Soliman and Abdallah 2011). Particle impact parameter represents momentum per unit cross-sectional area of the particles. The depth of penetration and fraction of particles penetrating the stratum corneum were found to be directly proportional to this parameter. At a fixed value of particle impact parameter, an increase in particle radius corresponds to a decrease in particle velocity at constant density and resulted in a decrease in penetration depth. For a given set of particle properties, varying gas pressure can control the velocity of particles. Typical range of pressures investigated and used is between 200 and 900 psi. Since keeping particle impact parameter uniform is necessary for targeting specific skin layers, various internal contour designs have been studied for achieving narrow velocity profiles. This has led to the optimization of internal sections of the injector, namely, driver tube and shock tube, through which the carrier gas flows before reaching the nozzle (Kendall 2002; Kendall et al. 2004c). A recent study has revealed a correlation between epidermal cell death and particles delivered per unit area of target tissue, making particle payload another important parameter (Raju et al. 2006).
3.3 Applications
Several researchers have investigated and showed the efficacy and dose-sparing effect of biolistic injectors for immunization against protein- and nucleotide-based antigens including influenza virus, papillomavirus, Yersinia pestis, and malaria, among others, in various animal models (Bennett et al. 2000; Bergmann-Leitner and Leitner 2013; Fynan et al. 1993; Han et al. 1999). Dose-dependent antibody response was reported for vaccination using DNA-coated gold microparticles against influenza in humans (Drape et al. 2006). Application of biolistic injectors has been extended beyond immunization to areas such as downregulating allergic response (Kendall et al. 2006).
Feltquate and co-workers (1997) showed that immunization using gene gun produced predominantly TH2 response, when antigens were delivered in the skin or muscle. TH2 response controls immunity to extracellular parasites and allergic inflammatory responses (Paul and Zhu 2010). DNA plasmids expressing proteins of interest were used to compare the immunization potential of a biolistic injector with intramuscular injection. It was shown that immunization with biolistic injection could potentially be used to induce humoral response irrespective of cellular localization of the protein, which was not the case with intramuscular immunization (Morel et al. 2004). Another study showed that the efficacy of gene gun-based immunization is independent of Langerhans cells (Stoecklinger et al. 2007). Biolistic injectors such as Helios® gene gun system (Bio-Rad Laboratories, Hercules, USA) which can be used with coated gold microparticles are now commercially available to researchers for further investigating the applications of biolistic injectors (BioRad Helios® Gene Gun System 2013).
3.4 Safety
Erythema has been reported in animals after application of PowderJect®, but it was deemed acceptable (Sarphie et al. 1997). Human clinical trials have reported painless delivery at the time of injection, with DNA vaccines being well tolerated (McConkey et al. 2003; Roberts et al. 2005; Rottinghaus et al. 2003; Roy et al. 2000; Tacket et al. 1999). Post-injection symptoms have been reported to develop quickly after the injection, notably at the injection site, and include mild erythema, hyperpigmentation, flaking, and discoloration at the injection site. In some cases, transient sensations of mild tingling, tightening, or burning have also been reported. Most symptoms disappeared within the first month except mild discoloration, which has been reported to persist for up to 6 months.
4 Summary
The concepts, which form the basis of liquid and solid jet injectors described here, were discovered and first described several decades ago. The literature reviewed here strongly indicates that our fundamental understanding of injector design parameters and how these parameters affect device interaction with the skin has significantly advanced over the last decade. These advances have resulted in novel device designs with increased therapeutic potential and superior control over delivery profiles, thus promising minimal patient discomfort. Ongoing challenges include increasing therapeutic potential still further and minimizing patient-to-patient variability. Overall, promising trends for the next generation of jet injectors have emerged. Current disadvantages are big size of some devices and high cost for single-use devices or difficulties in component reuse. Future challenges lie principally in device engineering for making devices more portable and affordable and ensuring reproducible results across a wide range of subjects.
References
Agerso H, Moller-Pedersen J, Cappi S, Thomann P, Jesussek B, Senderovitz T (2002) Pharmacokinetics and pharmacodynamics of a new formulation of recombinant human growth hormone administered by ZomaJet 2 Vision, a new needle-free device, compared to subcutaneous administration using a conventional syringe. J Clin Pharmacol 42:1262–1268
Arora A, Hakim I, Baxter J, Rathnasingham R, Srinivasan R, Fletcher DA et al (2007) Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc Natl Acad Sci U S A 104:4255–4260
Arora A, Prausnitz MR, Mitragotri S (2008) Micro-scale devices for transdermal drug delivery. Int J Pharm 364:227–236
Bareille P, MacSwiney M, Albanese A, Vile CD, Stanhope R (1997) Growth hormone treatment without a needle using the Preci-Jet 50 transjector. Arch Dis Child 76:65–67
Barry BW (2001) Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14:101–114
Baxter J, Mitragotri S (2005) Jet-induced skin puncture and its impact on needle-free jet injections: experimental studies and a predictive model. J Control Release 106:361–373
Bennett AM, Phillpotts RJ, Perkins SD, Jacobs SC, Williamson ED (2000) Gene gun mediated vaccination is superior to manual delivery for immunisation with DNA vaccines expressing protective antigens from Yersinia pestis or Venezuelan equine Encephalitis virus. Vaccine 18:588–596
Bergmann-Leitner ES, Leitner WW (2013) Gene gun immunization to combat malaria. Method Mol Biol 940:269–284
BioRad Helios® Gene Gun System (2013) http://www.bio-rad.com/prd/en/US/LSR/PDP/42e9d6be-369a-49f8-8fbb-281a0fea6df8/Helios-Gene-Gun-System. Accessed 10 Jan 2013
Brodell RT, Bredle DL (1995) The treatment of palmar and plantar warts using natural alpha-interferon and a needleless injector. Dermatol Surg 21:213–218
Brown MB, Martin GP, Jones SA, Akomeah FK (2006) Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–187
Canter J, Mackey K, Good LS, Roberto RR, Chin J, Bong WW et al (1990) An outbreak of hepatitis-B associated with jet injections in a weight-reduction clinic. Arch Intern Med 150:1923–1927
Crozier WD, Hume W (1957) High-velocity, light-gas gun. J Appl Phys 28:892–894
Dorr HG, Zabransky S, Keller E, Otten BJ, Partsch C-J, Nyman L et al (2003) Are needle-free injections a useful alternative for growth hormone therapy in children? Safety and pharmacokinetics of growth hormone delivered by a new needle-free injection device compared to a fine gauge needle. J Pediatr Endocrinol Metab 16:383–392
Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ (2006) Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 24:4475–4481
Feltquate DM, Heaney S, Webster RG, Robinson HL (1997) Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol 158:2278–2284
Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL (1993) DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A 90:11478–11482
Han R, Cladel NM, Reed CA, Peng X, Christensen ND (1999) Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J Virol 73:7039–7043
Houtzagers CMGJ, Visser AP, Berntzen PA, Heine RJ, van der Veen EA (1988) The Medi-Jector-II – efficacy and acceptability in insulin-dependent diabetic-patients with and without needle phobia. Diabet Med 5:135–138
INJEX Pharma AG (2013) http://www.injex.com. Accessed 10 Jan 2013
Jackson LA, Austin G, Chen RT, Stout R, DeStefano F, Gorse GJ et al (2001) Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine 19:4703–4709
Kendall MAF (2002) The delivery of particulate vaccines and drugs to human skin with a practical, hand-held shock tube-based system. Shock Waves 12:23–30
Kendall M (2006) Engineering of needle-free physical methods to target epidermal cells for DNA vaccination. Vaccine 24:4651–4656
Kendall M, Mitchell T, Wrighton-Smith P (2004a) Intradermal ballistic delivery of micro-particles into excised human skin for pharmaceutical applications. J Biomech 37:1733–1741
Kendall M, Rishworth S, Carter F, Mitchell T (2004b) Effects of relative humidity and ambient temperature on the ballistic delivery of micro-particles to excised porcine skin. J Invest Dermatol 122:739–746
Kendall MAF, Quinlan NJ, Thorpe SJ, Ainsworth RW, Bellhouse BJ (2004c) Measurements of the gas and particle flow within a converging–diverging nozzle for high speed powdered vaccine and drug delivery. Exp Fluids 37:128–136
Kendall M, Mitchell TJ, Costigan G, Armitage M, Lenzo JC, Thomas JA et al (2006) Downregulation of IgE antibody and allergic responses in the lung by epidermal biolistic microparticle delivery. J Allergy Clin Immunol 117:275–282
Klein TM, Wolf ED, Wu R, Sanford JC (1987) High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327:70–73
Lindmayer I, Menassa K, Lambert J, Moghrabi A, Legendre L, Legault C et al (1986) Development of new jet injector for insulin therapy. Diabetes Care 9:294–297
McConkey SJ, Reece WHH, Moorthy VS, Webster D, Dunachie S, Butcher G et al (2003) Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med 9(6):729–735
Mitragotri S (2005) Immunization without needles. Nat Rev Immunol 5:905–916
Mitragotri S (2006) Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov 5:543–548
Morel PA, Falkner D, Plowey J, Larregina AT, Falo LD (2004) DNA immunisation: altering the cellular localization of expressed protein and the immunisation route allows manipulation of the immune response. Vaccine 22:447–456
Mulholland WJ, Kendall MAF, White N, Bellhouse BJ (2004) Characterization of powdered epidermal vaccine delivery with multiphoton microscopy. Phys Med Biol 49:5043
Needle-free jet injection bibliography, device & manufacturer roster and patent list (2013) http://hcvets.com/data/occupational/munji/Jetinject-Bib.pdf. Accessed 12 May 2013
Paul WE, Zhu J (2010) How are TH2-type immune responses initiated and amplified? Nat Rev Immunol 10:225–235
Prausnitz MR, Mitragotri S, Langer R (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3:115–124
Raju PA, McSloy N, Truong NK, Kendall MA (2006) Assessment of epidermal cell viability by near infrared multi-photon microscopy following ballistic delivery of gold micro-particles. Vaccine 24:4644–4647
Resman Z, Metelko Z, Skrabalo Z (1985) The application of insulin using the jet injector Dg-77. Acta Diabetol Lat 22:119–125
Roberts LK, Barr LJ, Fuller DH, McMahon CW, Leese PT, Jones S (2005) Clinical safety and efficacy of a powdered Hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine 23:4867–4878
Rochelle C, Lee G (2007) Dextran or hydroxyethyl starch in spray-freeze-dried trehalose/mannitol microparticles intended as ballistic particulate carriers for proteins. J Pharm Sci 96:2296–2309
Rottinghaus ST, Poland GA, Jacobson RM, Barr LJ, Roy MJ (2003) Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine 21:4604–4608
Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S et al (2000) Induction of antigen-specific CD8 + T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764–778
Sarno MJ, Blasé E, Galindo N, Ramirez R, Schirmer CL, Trujillo-Juarez D (2000) Clinical immunogenicity of measles, mumps and rubella vaccine delivered by the Injex jet injector: comparison with standard syringe injection. Pediatr Infect Dis J 19:839–842
Sarphie DF, Johnson B, Cormier M, Burkoth TL, Bellhouse BJ (1997) Bioavailability following transdermal powdered delivery (TPD) of radiolabeled inulin to hairless guinea pigs. J Control Release 47:61–69
Schramm J, Mitragotri S (2002) Transdermal drug delivery by jet injectors: energetics of jet formation and penetration. Pharm Res 19:1673–1679
Schramm-Baxter J, Mitragotri S (2004) Needle-free jet injections: dependence of jet penetration and dispersion in the skin on jet power. J Control Release 97:527–535
Schramm-Baxter J, Katrencik J, Mitragotri S (2004) Jet injection into polyacrylamide gels: investigation of jet injection mechanics. J Biomech 37:1181–1188
Schuetz YB, Naik A, Guy RH, Kalia YN (2005) Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv 2:533–548
Soliman SM, Abdallah S (2011) CFD investigation of powdered vaccine and gas dynamics in biolistic gun. Powder Technol 214:135–142
Stachowiak JC, Li TH, Arora A, Mitragotri S, Fletcher DA (2009) Dynamic control of needle-free jet injection. J Control Release 135:104–112
Stoecklinger A, Grieshuber I, Scheiblhofer S, Weiss R, Ritter U, Kissenpfennig A et al (2007) Epidermal Langerhans cells are dispensable for humoral and cell-mediated immunity elicited by gene gun immunization. J Immunol 179:886–893
Sutermesiter A (1954) High-pressure injection device. 2680439 (Patent)
Suzuki T, Takahashi I, Takada G (1995) Daily subcutaneous erythropoietin by jet injection in pediatric dialysis patients. Nephron 69:347
Taberner A, Hogan NC, Hunter IW (2012) Needle-free jet injection using real-time controlled linear Lorentz-force actuators. Med Eng Phys 34:1228–1235
Tacket CO, Roy MJ, Widera G, Swain WF, Broome S, Edelman R (1999) Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine 17:2826–2829
Verhagen A, Ebels JT, Jonkman JHG, Dogterom AA (1995) Pharmacokinetics and pharmacodynamics of a single-dose of recombinant human growth hormone after subcutaneous administration by jet-injection – comparison with conventional needle-injection. Eur J Clin Pharmacol 49:69–72
Weller C, Linder M (1966) Jet injection of insulin vs syringe-and-needle method. J Am Med Assoc 195:844–847
Zogenix, Inc (2013) http://www.zogenix.com. Accessed 10 Jan 2013
Acknowledgments
The author acknowledges J. Schramm-Baxter, R. Rathnasingham, J. Stachowiak, T. Li, D. Fletcher, and S. Mitragotri for their scholastic contributions via original research and scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Arora, A. (2017). Liquid and Powder Jet Injectors in Drug Delivery: Mechanisms, Designs, and Applications. In: Dragicevic, N., I. Maibach, H. (eds) Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53273-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-662-53273-7_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53271-3
Online ISBN: 978-3-662-53273-7
eBook Packages: MedicineMedicine (R0)