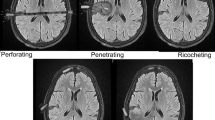
Abstract
Traumatic brain injury (TBI) remains a major cause of death and disability worldwide, and missile-induced TBI remains the most deadly of all traumas since first reported and has always been associated with high mortality and morbidity. The prevalence of TBI secondary to gunshots is strikingly variable and reflects the global scenery of violence. Injuries from gunshot wounds (GSW) to the head place an extreme economic burden on the public while disabling the victims in the zenith of their life and imposing enormous medical, legal, and emotional costs. Since every gun/projectile combination is associated with a typical pattern of injury, war injuries differ significantly from others. We will focus here on predominantly penetrating civilian gunshot wounds with low muzzle velocity (<1,000 f/s) as they occur in the setting of homicide and suicide attempts or during accidents. Many surgeons did take patients to the OR over the last 30 years, and they have achieved a remarkable reduction in morbidity from well above 50 % to less than 25 % in patients admitted with severe brain injury. However, in the setting of increasingly limited resources, recent research focus has shifted toward more precise prediction of survival as well as on better functional outcome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic Brain Injury
- Mean Arterial Pressure
- Cerebral Perfusion Pressure
- Severe Traumatic Brain Injury
- Traumatic Brain Injury Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Traumatic brain injury (TBI) remains a major cause of death and disability worldwide, and missile-induced TBI remains the most deadly of all traumas since first reported and has always been associated with high mortality and morbidity. The prevalence of TBI secondary to gunshots is strikingly variable and reflects the global scenery of violence. Injuries from gunshot wounds (GSW) to the head place an extreme economic burden on the public while disabling the victims in the zenith of their life and imposing enormous medical, legal, and emotional costs. Since every gun/projectile combination is associated with a typical pattern of injury, war injuries differ significantly from others. We will focus here on predominantly penetrating civilian gunshot wounds with low muzzle velocity (<1,000 f/s) as they occur in the setting of homicide and suicide attempts or during accidents. Many surgeons did take patients to the OR over the last 30 years, and they have achieved a remarkable reduction in morbidity from well above 50 % to less than 25 % in patients admitted with severe brain injury. However, in the setting of increasingly limited resources, recent research focus has shifted toward more precise prediction of survival as well as on better functional outcome.
Among firearm injuries, gunshot wounds to the head and brain are nightmares for all involved. Prehospital mortality remains >50 % and the in-hospital mortality for civilians with penetrating neurocranial injury is around 50–95 % depending on the study and the proportion of suicide victims in the series. Of note is the observation that female victims seem to have worse outcome based on a different causative injury pattern. It is clear from all studies that “time is brain,” – so we must act swiftly on all patients brought to a trauma center and ensure protocol-guided resuscitation. You must obtain an accurate qualified exam upon arrival, since this is the most relevant determinant and reports of any GSC from the scene are often established pre-resuscitation and hence grossly inaccurate (e.g., secondary to intoxication, hypotension, or hypoxia/hypothermia). “Time is brain,” so initiate ATLS-guided treatment (vasopressors, mannitol, hyperventilation) even prior to completing the imaging. To prevent secondary damage perioperatively, always ensure sufficient cerebral perfusion (goal >70 mmHg) by keeping intracranial pressure below 25 mmHg and arterial blood pressure above 90 mmHg and use ICP monitoring, broad-spectrum antibiotics, and anticonvulsants.
Most studies support intervention for patients with a post-resuscitation GCS of greater than 5, but there are exceptions to the rule, and despite a first impression of devastation, some will have good outcome “against all odds.” So our credo is to treat any not clearly hopeless case as fast and aggressively as possible.
In managing gunshot-injured brain-patients, you should be well aware that only a part of the neurological harm arises at the moment of impact. The prognostic relevant damage most frequently evolves in the time span after the incident, and any achieved outcome correlates to the time between injury and the time of intervention and postoperative management. By managing and preventing secondary problems aggressively, you justify swift surgical treatment and improve your outcome.
If the patient is comatose with a GCS of 3–5, we do initiate intracranial pressure (ICP) treatment already in the trauma bay even prior to the acquisition of imaging. However, as soon as the patient is systemically stabilized, it is mandatory to immediately obtain a standardized CT scan (5-mm cuts parallel to the skull base in brain/home/bone windows with reformats in coronal and sagittal planes) in ALL patients with GSW to the head to make a decision on operative intervention; CT scanning does not differ from other trauma patient workups, but we scan the patient head first during the trauma protocol workup to get a better idea about the prognosis, the urgency of the situation, and the chance to plan a more precise setup for the OR. Workup and handling of patients with stab injuries to the brain does also not differ significantly from those with gunshot wounds, but injury might be more localized since the impact transforms less energy than that of a projectile.
The acquisition of CT scans must NOT be postponed ever because of a good presenting GCS, since approximately 10 % of patients with non-penetrating injury (without breach of the neurocranium) may still suffer a significant intracranial injury and will require lifesaving neurosurgical intervention; see also patient illustrated in Fig. 27.1. The reverse is also true: even in the setting of a GSC as low as 3–5, a young patient deserves surgical intervention if a defined space-occupying lesion (e.g., hematoma) is identified on admission CT.
Initial patient management should be according to ATLS protocols or equivalent algorithms. Isotonic volume resuscitation, normotonia, normorhythmia, and normothermia should be ensured. Traumatic GSW brain injury is classified as critical in any patients presenting with a GCS score below eight and an abnormal CT scan, e.g., showing a skull fracture or deformation, hematoma, contusion, swelling, or other signs of local or global mass effect possibly causing incipient herniation. Remember that patients with GCS scores >8 and/or a supratentorial single-lobe lesion have the best chance to show good outcome after aggressive surgical treatment.
Recent literature offers also some outcome prediction models for head-injured patients using a number of parameters including age, GCS score, pupil reactivity, and the presence of extracranial injuries. Further adjustments are made by including findings on CT scanning. As expected, outcome is also defined by the locally available treatment resources and hence reflects the socioeconomic status of the country. Based on our experience in an urban trauma level 1 center, we strongly suggest aggressive surgical treatment in all not clearly hopeless cases.
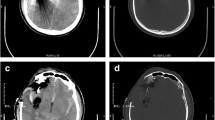
Patient No. 1: a 22-year-old female crime victim, who sustained multiple GSW with a single non-penetrating GSW to the head. Entry wound at R cheek, exit wound on R supraorbital forefront. Plates 1 and 2: scout images A/P and lateral without evidence of bullet. Plates 3 and 4: preoperative images (bone windows) demonstrating R frontal skull fracture with pneumocephalus and orbital roof fracture. Plates 5 and 6: axial and coronal views of large R epidural hematoma and infraorbital hematoma. Plates 7 and 8: axial views of postoperative results status post evacuation of hematoma and autologous cranioplasty. Plates 9 and 10: reconstructed orbital roof status post transfrontal evacuation of retro-orbital hematoma
1 Some Rules for Workup Leading to Operative Management
The most important rule to memorize at the very beginning is Time is brain.
As profane as it may sound: Clear thinking and a high speed of coordinated action is crucial and it requires a well pre-instructed and well-drilled team.
What we really mean here is: Swiftly coordinated actions are vital in the true sense of the word and will define the outcome for such challenging endeavors and are an absolute requirement if you want to succeed. This applies to all parts of the care-provider chain: from Advanced Life Support-trained EMTs, who pick up and transport the patient, their management en route, to the presentation upon arrival in the ER. Admission exam and timely workup is critical and potentially lifesaving (with its bedside decisions) until a possible intervention in the OR can be performed. The latter can only be successful with a premeditated and very well-carried-out surgical plan.
Here are my (EMK) rules:
-
No. 1: DO NOT PANIC! In many ways, it is a case like many others; therefore RUN YOUR ROUTINE. Do all the workup and related decision like a tree and according to a protocol.
-
No. 2: DO NOT WASTE TIME – and save it where you can do it safely. This means: When the hospital is notified about the arrival of such a GSW patient, GET READY BEFORE THEY ARRIVE. Call the OR upfront to get a room setup. Announce the most likely scenario (e.g., 20-year-old male; RT craniotomy/supine or suboccipital craniotomy/prone, etc.). Ask to assemble a team for the OR that you already know/can work with, not newcomers (Fig. 27.2).
Fig. 27.2 Patient No. 2: a 23-year-old female, who sustained a solitary GSW to the head from close range. Entry wound on the R cheek, exit wound R parietal. Arrows in plates 1 and 2 of this figure illustrate the blow out fracture of the right sided calvarium as seen on CT scout images; This creates a hemispheric decompression. Arrows in plate 3 and 4 indicate the corresponding bony defect with multiple fragments as seen in bone windows of the axial CT scan. Arrows in plates 5 and 6 indicate the hemorrhagic contusion and SAH. Arrows in plates 7 and 8 indicate the hemicraniectomy site (7) and evacuated subdural hematoma site (8). Arrow in 9 points at the inserted hemicranioplasty allograft.Arrow in 10 points at the cystic portion of the encephalomalacia from the bullet tract
-
No. 3: GO TO THE ER AND WAIT IN THE TRAUMA BAY FOR THE PATIENT TO ARRIVE. If you are out of the hospital, start driving in NOW. Meanwhile organize things by phone on your way. These are most valuable minutes that you can save for later (Fig. 27.3).
Fig. 27.3 Patient No. 3: a 30-year-old female, who sustained a solitary GSW to the back of her head from close range. Entry wound on the occiput and no exit wound. Arrow in plates 1 and 2 points at the retained bullet case. Arrow in plate 3 shows the bullet case as seen in the corresponding head CT on axial CT bone window. Arrows in plate 4 point at a dilated right lateral ventricle and at left perimesencephalic hemorrhage. Arrows in plate 5 show the enlarging hematoma in the CP angle and a hematoma in the left sided posterior fossa. Arrow in plate 6 shows the bilateralsuboccipital craniectomy site that was created to gain access for evacuation. Arrow in plate 7 points at the evatuation side of the previously seen hematoma in 5. Arrow in plate 8 points at the R frontal EVD catheter inserted to treat the occlusive hydrocephalus
-
No. 4: Touch base with the ER attending. In an experienced setting, the ER will get prepared early and have identification labels/numbers and a trauma team assigned prior to arrival (Fig. 27.4).
Fig. 27.4 Patient No. 4: a 26-year-old male, who sustained a solitary GSW to the head from distant range. Entry wound at the R ear canal, no exit wound. Plates 1 and 2: scout images A/P and lateral with evidence of multiple bullet fragments on the R extracranially and bilaterally intracranially. Note the large R-sided skull fracture. Arrows in panel 3 an 4 point at the bullet tract as seen on head CT with axial bone windows (3) and in matching coronal reconstructions (4). There are interspersed metal bullet fragments visible with their streak artifact. Arrow in plate 5 points at the temporal lobe hematoma. Arrow in plates 6 and 7 points at the hemicraniectomy site. Arrow in plate 8 points at the R MCA bifurcation with multpiple retained bullet fragments and a surgical clip for the traumatic avulsion site. Arrow in plate 9 indicates the allograft cranioplasty. And plate 10 shows a small left frontal inraparenchymal hematoma
-
No. 5: Make sure they notify the blood bank for possible need of products with “emergent release.” Make sure they have vasopressors and mannitol/Lasix IV ready and a respiratory therapist to initiate hyperventilation. Remember the rule of 30s: height of bed 30o and hyperventilation with f = 30 for a goal pCO2 < 30.
-
No. 6: Get the trauma team ready in the bay and assign tasks by talking to the senior/attending running the case. THIS IS NOT THE PATIENT TO PRACTICE ON. Newcomers can stand by and watch, but should stay at a distance and out of the way! Try to pass all preliminary information around as it can be gathered from the EMT-call-in from the scene or en route (ask about patient age, single wound or systemic injury, patient awake or with loss of consciousness (LOC)/comatose; patient intubated, patient stable; blood loss at the scene; other issues).
-
No. 7: Call the CT scanner upfront that you will bring a critically ill patient ASAP so they keep the scanner FREE for your case!
-
No. 8: Listen well to what the transport team has to say upon presenting the case; they sometimes know important details (downtime, seizures at the scene, difficulties with the airway, etc.).
-
No. 9: WATCH if there is any sign of life upon arrival. Get a good glimpse at the patient (I recommend you stand behind the chief running the case at the head end of the patient) and once the primary survey is done.
-
No. 10: You should get a 10–30-s neuroexamination yourself.
NOW MAKE THE RUN AGAINST THE CLOCK!
2 Preoperative and Intraoperative Management
For most TBI strategies, you will find very little class 1 or 2 recommendations. To get a good grip on how to run these scenarios and treat your patient well, watch as many cases as you can during training. Take home the pivotal steps of decision making from seasoned staff. You can often not randomize patients in critically ill settings since it often poses an ethical dilemma; therefore, remember the following points to increase the possibility of a satisfying discharge status of a patient injured by gunshot or a stab wound:
In all trauma patients, let the trauma team perform systematic reviews in the bay and stanch blood ASAP; get the patient lined up (two peripheral 16 G IVs) and treat abnormal vital signs (e.g., hypotension, hypoxia/hypothermia!) before you move to the radiological examinations, and consider any surgical intervention. The minutes spent here are WELL SPENT and make your part SAVE. No one wants to rush the GSW patient to the scanner and see them crashing there. And remember: NO GSW TO THE HEAD goes to the OR without films EVER!
Have one team member assigned as liaison to the relatives if you do not have the time to communicate during the need of swift action. They will be extremely grateful and less anxious. Once you have obtained your scans, make a swift decision: Patients with a GCS of 3–5 AND a devastating scan (bilateral global injury with transventricular bullet trajectory, massive blood or swelling with near-complete herniation, tram track signs) may not be salvageable and warrant conservative treatment alone with ICP-bolt placement and medical management only. Other patients with either improved post-resuscitation GCS > 5 and limited supratentorial injury and a vector that does not show involvement of the fatal zone should be considered for surgery.
NOW TO THE POINTS THAT MATTER:
3 Operative Management
Always ask yourself:
-
“How can I do the best intervention the fastest possible way?”
-
Here are the 15 most important points on the road to success:
-
-
1.
Transport the patient yourself from the CT scanner straight to the OR.
-
2.
Position the patient by transferring him from the stretcher onto the OR bed (which has been preplaced correctly in the room since you called from the CT scanner).
-
3.
Apply only the utmost necessary padding to save time (this is not the time to search for pneumoboots or gel rolls).
-
4.
Pin the patient in a Mayfield headrest at straight angles! (either supine or fully lateral). This helps to keep your orientation once you are deep inside.
-
5.
Shave the entire hemiconvexity (be generous!).
-
6.
Scratch the skin to keep your landmarks and pay attention to especially the midline!
-
7.
Use a quick prep solution: e.g., soaking beta-iodine sponges followed by Prevail®; this is not the time to go through six sponges of your three soap elective crani routine.
-
8.
Do not waste the time to wait for using local anesthesia/epinephrine for better hemostasis.
-
9.
Incise with the goal of creating a generous flap to allow for post-OP swelling.
-
10.
Perform a really good sized hemicraniectomy for optimal decompression and do not forget to prepare in all p-fossa lesions a Frazier burr hole so you can place an external ventricular drain (EVD) any time.
-
11.
Save the bone flap on the back table to be used in a freezer-storage protocol, and do not waste time on a second (abdominal) incision! You want to get out of the OR ASAP.
-
12.
Always irrigate copiously with antibiotic solution: e.g., bacitracin®.
-
13.
Perform your wide durotomy BEFORE you place any dural tenting stitches since this decompresses the brain earlier and you save the brain some more vital minutes.
-
14.
Close the dura provisionally, e.g., with an onlay dural allograft (e.g., DuraGen®) to prevent adhesion scarring from the brain surface to the undersurface of the muscle flap. A subgaleal CSFoma is of no concern here, since you will be back for a regular cranioplasty.
-
15.
Close the muscle flap in three layers only to save some time: (1) muscle + fascia, (2) galea, and (3) skin.
Do not forget to talk to “your team” at all times during the case and announce your next moves clearly and loud. These are fast and stressful cases and performed not for pretty but effective surgery; describe technical details that make a difference (anesthesia needs to know when you open the dura to anticipate a change in ICP and SBP response).
4 Perioperative Management
4.1 Cerebral Perfusion Threshold
An adequate cerebral perfusion pressure (CPP) is instrumental to keep brain tissue alive. More is better here. The goal value is the result of subtracting the ICP from the mean arterial pressure (MAP). You may guess via the SBP if your monitor does not calculate and display MAPs.
The critical cerebral perfusion pressure (CPP) threshold for ischemia lies around 50_60 mmHg; do not over resuscitate with IV fluids, and DO NOT USE fluids with concentrations of half normal saline (0.45 %), which act as hypo-osmolar volume expanders and may create significant brain edema. Remember: There is poor outcome in patients with systemic hypotension, but there is risk of adult respiratory distress syndrome with too ambitious use of fluids too. So keep ICP low and MAP high enough with mannitol (e.g., 1–1.5 g/kg body weight is about 100 g I/V for an average-sized person of 70 kg), and do not hesitate to use vasopressors early (e.g., Neo-Synephrine) and do NOT bring down systolic blood pressure (SBP) if a patient comes in at 165 before you have a CT scan; he may need that pressure for good perfusion!.
Always monitor blood pressure (BP) frequently (q2–5 min) and avoid systolic drops of BP < 90 mmHg. Now a personal hint: Do NOT waste time placing an A-line before CT scanning. In a hemodynamically stable patient, you are better off seeing the intracranial damage early and go to the OR quicker rather than waiting 5 min for line placement before you can make an informed decision. The OR can work more efficiently with teams acting in parallel which saves you vital minutes (needless to say: In the unstable patient, this does not hold true).
5 Intracranial Pressure Monitoring
Aim to always maintain adequate cerebral perfusion to prevent secondary damage! So monitor ICP in all necessary settings of severe traumatic brain injury with GCS < 8 to define the need of intervention (level II evidence). But what does that mean here? You are not able to manage CPP correctly without measuring ICP and MAP! However, if the patient goes to the OR anyway, do not waste time placing an ICP-bolt monitor or external ventricular drain upfront. It is more suitable for the post-op setting.
Especially in smaller institutions, the threshold for invasive monitoring remains too high. Here we advocate a low threshold for transferring the patient to an experienced center and correct placement in an ICU setting. CT scans are not appropriate for “guessing” ICP, but good enough preop to make a decision. If the patients scan supports nonoperative management, the patient needs an ICP bolt placed ASAP.
By the way, if a CT does not present any abnormalities to explain a low admission GCS, then measure ICP when two or more of the following features are noted: negative tox screen, patients above 40 years of age, systolic BP < 90 mmHg, or the patient showing sign of posturing (uni- or bilateral).
Do not treat potential high ICP for any prolonged period of time prophylactically without correct monitoring in the ICU setting. (This is NOT true for a sudden change in mental status in a critically ill TBI patient; if you notice a rapid decline in neuroexam, you SHOULD initiate therapy immediately with hyperventilation/HOB30/mannitol and then go to the scanner ASAP to explore the intracranial situation!) Once again, time is brain and less ICP for several minutes can save a lot of tissue if used in the correct setting. Whether you use a parenchymal or ventricular ICP-bolt device is more a question of preference than of evidence. However, the latter is known for lower costs and offers the chance to also treat by draining off excess cerebrospinal fluid (CSF). Start treatment if ICP is sustained >20 mmHg (level II) and follow respective clinical and radiological findings.
6 Hyperosmolar Therapy and Barbiturates
Mannitol or hypertonic saline lowers ICP and may thereby increase CPP, thus improving neurological outcome. As a rule of thumb, use mannitol at 1 g/kg body weight as a loading dose (level II). Equimolar doses of NaCl may be given according to institutional protocols. Then maintain the dosing but divide it into equal fractions (e.g., 25 g mannitol q6 h). Do NOT forget to also order holding parameters (e.g., hold next dose for osmolarity > 320 or Na > 150) to prevent drying the patient out. Also be aware that you might cause transient arterial hypotension! Mannitol outweighs barbiturates in improving ICP, but bears a higher risk of hypotension. While mannitol may have a detrimental effect on mortality when compared to hypertonic saline, recent comprehensive literature review found conflicting evidence. Prophylactic administration without evidence of increased ICP is not recommended. Only use barbiturates if ICP cannot be decreased by any other measure to prophylactically slow metabolism. (It also makes brain death determination really difficult.)
7 Hyperventilation and Steroids
Hyperventilation can reduce ICP. The mechanism most likely comes from intravasal volume reduction secondary to vasoconstriction. The method works well for 6 h (giving you a good time window to initiate further treatments) but can turn detrimental thereafter. So do not use it without careful consideration and limits! Avoid too excessive a protocol, and do not hyperventilate to a PaCO2 < 25 mmHg during the first 24 h after TBI when cerebral blood flow (CBF) is often critically reduced (level II). Mind you that the day after a significant injury, CBF is reduced to less 50 % of normal individuals; this means that you risk decreasing CBF even further with aggressive hyperventilation and a subsequent reduction in CBF and you actually worsen the situation to the point that the patient may become ischemic or stroke.
Do NOT apply steroids. Currently, there is no proven benefit for the use of steroids in traumatic closed-head/brain injury. Earlier data had reported some benefit but at an increased risk for overall morbidity especially in the population of elderly.
8 Infection Prophylaxis
Most general guidelines (level II) suggest periprocedural administration of antibiotics to reduce the incidence of pneumonia after intubation in the patients with significantly decreased mental status. Although gunshots are often considered to be sterile in themselves, we support the notion of a 48–72-h period of broad-spectrum antibiotic prophylaxis for prevention of meningitis secondary to a CSF leak. Vancomycin 1 g Q12 h, gentamicin 80 mg Q 8 h, and Flagyl 500 mg Q6 will suffice. Since most CSF leaks close spontaneously within 48 h, or will be taken care of during surgery, we do not maintain this regimen beyond day 3, unless there is a significant amount of bony debris translocated into the parenchyma. If that is the case, 7–10 days of antibiotic coverage seems reasonable.
9 Prophylactic Hypothermia
Even though preliminary data had shown a possible increase of survival when induced hypothermia is maintained for more than 48 h in TBI patients, we currently do not use prolonged hypothermia on patients with GSW. Pooled data (level III) indicated no improvement in overall mortality and are hinting at increased coagulopathies. Furthermore, RCTs on hypothermia for severe traumatic brain injury in pediatrics found neither improvement in global functional outcome nor reduced morality rates. In fact, mortality rates may increase in hypothermia-treated patients.
10 Antiseizure Prophylaxis
We strongly recommend the use of antiepileptic medications for a minimum of the first 7 days of injury for subarachnoidal hemorrhage (SAH). If significant parenchymal damage incurred, we keep it on until the first follow-up appointment.
Phenytoin is the drug of choice and clearly decreases the incidence of early posttraumatic seizures (PTS) and associated morbidity. Penetrating trauma to the head is an established risk factor for the development of PTS, but you can relax: These early PTS are not worsening long-term outcome. Since precautionary administration of valproate or phenytoin has not shown to prevent (level II) late posttraumatic seizures, most centers do not use them for anything else but perioperative.
11 Postoperative Consideration
Excessive postoperative strategies are not topic of this book. All basic postoperative prophylactic strategies apply for GSW trauma victims too. A brief reminder follows and your care protocols should include the following:
-
Most patients have a rough course during the first 3–7 days, since swelling seems to peak around POD 3–4, and you have to watch out for it and treat any trends of increase in ICP early and aggressively.
-
Wean all patients from the ventilator ASAP; an extubated patient gives you the best scenario for a proper assessment and neurological examination which can be followed once the GCS > 8. If the patient does not regain consciousness soon, opt for an early tracheostomy and PEG in anticipation of a long postoperative course.
-
Ensure full caloric intake by day 7 post-injury to support wound healing. To achieve best results, begin feeding not later than72 h after injury.
-
Combine mechanical DVT prophylaxis via compression stockings or intermittent pneumatic compression stockings with low molecular weight heparin or low-dose unfractionated heparin as early as POD 2.
-
Provide a decent bowel regimen (including acid blocker and a stool softener to help the slowed guts) and for prevention of stress-induced ICU gastritis.
-
Supply adequate pain medications as these patients will not ask for any.
-
Support the patient with anxiolytics and sedation in the setting of ICU care.
-
Meticulous decubitus prophylaxis must be applied.
-
Mobilize the patient early (PT/OT/out of bed to chair).
12 Special Circumstances
If you ever face a situation in which you have multiple GSW victims (such as a terror attack or a mass casualty), you have to make a stern decision: Who is going to be treated first, or who is not going to be treated at all. It seems to be acceptable to make that decision based on your available resources and based on the available data reflecting the different prognoses for patients; I recommend to perform the workup in each patient just as outlined above. Based on the clinical information (presenting GCS score) and the CT scan, I feel strongly that a patient with a higher GCS and limited damage on scan (e.g., unilobar right-sided injury) has the best chances for good functional outcome and hence should go to surgery first. However, we acknowledge the ethical dilemma in this scenario and accept differing decisions based on momentary rationale or the experience of the treating team.
Important Points
-
DO NOT PANIC! In many ways, it is a case like many others; therefore RUN YOUR ROUTINE. Do all the workup and related decision like a tree and according to a protocol.
-
DO NOT WASTE TIME – and save it where you can do it safely. This means: When the hospital is notified about the arrival of such a GSW patient, GET READY BEFORE THEY ARRIVE. Call the OR upfront to get a room setup. Announce the most likely scenario (e.g., 20-year-old male; R crani/supine or suboccipital crani/prone, etc.). Ask to assemble a team for the OR that you already know/can work with, not newcomers.
-
GO TO THE ER AND WAIT IN THE TRAUMA BAY FOR THE PATIENT TO ARRIVE. If you are out of the hospital, start driving in NOW. Meanwhile organize things by phone on your way. These are most valuable minutes that you can save for later.
-
Touch base with the ER attending. In an experienced setting, the ER will get prepared early and have identification labels/numbers and a trauma team assigned prior to arrival.
-
Make sure they notify the blood bank for possible need of products with “emergent release.” Make sure they have pressors and mannitol/Lasix IV ready and a respiratory therapist to initiate hyperventilation. Remember the rule of 30s: height of bed 30o and hyperventilation with f = 30 for a goal pCO2 < 30.
-
Get the trauma team ready in the bay and assign tasks by talking to the senior/attending running the case. THIS IS NOT THE PATIENT TO PRACTICE ON. Newcomers can stand by and watch, but should stay at a distance and out of the way! Try to pass all preliminary information around as it can be gathered from the EMT-call-in from the scene or en route (ask about patient age, single wound or systemic injury, patient awake or with loss of consciousness (LOC)/comatose; patient intubated, patient stable; blood loss at the scene; other issues).
-
Call the CT scanner upfront that you will bring a critically ill patient ASAP so they keep the scanner FREE for your case!
-
Listen well to what the transport team has to say upon presenting the case; they sometimes know important details (downtime, seizures at the scene, difficulties with the airway, etc.).
-
WATCH if there is any sign of life upon arrival. Get a good glimpse at the patient (I recommend you stand behind the chief running the case at the head end of the patient) and once the primary survey is done.
-
You should get a 10–30-s neuroexam yourself.
NOW MAKE THE RUN AGAINST THE CLOCK!
13 Clinical Vignette
A 33-year-old male was shot in his car which then led to a car crash. He was transferred to our trauma center. On arrival, he was moving all his extremities but needed to be intubated for airway protection. Clinical examination revealed a left bullet entrance in the occipital region. After the patient was stabilized, a head CT was obtained. See Figs. 27.5 and 27.6. The patient was flexor posturing in his upper extremities and extending his bilateral lower extremities. Pupils were 2 mm and nonreactive. His brain stem reflexes were intact.
Axial slices form the admission head CT. Panel 1 brain window and Panel 2 bone window show the bihemispheric injury and bullet trajectory, but it is high enough for not to injury deep brain structures. Panels 3 and 4 again show a left posterior occipital parietal gunshot wound with multiple metallic and osseous fragment and associated hemorrhage within the right and left occipital lobes
While surgical management in most cases is limited to local wound care, debridement of wound and scalp closure, this decision needs to be based on the neurological exam and the extent of intracranial injury. If the trajectory of the bullet transects both ventricles, it is thought to be futile. Although the trajectory in this patient involved both hemispheres, it was not to be deemed futile since there was no injury to deep brain structures such as the thalamus and basal ganglia.
If the patient has a survivable injury, safe accessible bone fragments should be removed and subdural, and epidural hematomas with mass effect should be evacuated. Routine surgical removal of bone or missile fragments lodged distant from the entry site or in the eloquent areas of the brain is not recommended. Chasing these fragment leads to worse outcome and higher morbidity. For this reason, we decided in this case to perform an emergent right-sided hemicraniectomy for ICP control. Although the entry wound was on the left side, the main bullet tract-associated hemorrhage was mostly on the right. See Fig. 27.7, Panels 3 and 4.
At 6-month follow-up, the patient is imaged again, now 1 month after his cranioplasty. A CT scan of the head (Fig. 27.7) shows encephalomalacia along the bullet tack and reabsorption of the associated hemorrhage. He is alert and oriented to place, time, and person. His executive functions were good enough to allow him to consent for his own cranioplasty 1 month prior. Neurologically, he is left with increased tone and 2/5 strength on the left side and 4/5 strength in his right upper and lower extremity.
This case demonstrates very well that – although most GSWs have a high mortality – the need for surgical intervention needs to be evaluated on a case-by-case basis. Although pathophysiology of penetrating wounds is very different from other closed-head injuries, predictors for poor prognosis are the same: pupil size, GCS, and age. GCS score of 3–5, bihemispheric lesions, multilobar injuries, intraventricular hemorrhage, and uncal herniation (CT scan findings) are indicators for poor outcome. All these aspects should be considered before offering surgery for penetrating TBI from a GSW.
Recommended Reading
Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, Okada P, Beers SR, Balasubramani GK, Hirtz D (2013) Paediatric traumatic brain injury consortium. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol 12(6):546–553
Bayston R, de Louvois J, Brown EM et al (2000) Use of antibiotics in penetrating craniocerebral injuries. Lancet 355:1813–1817
Boone MD, Oren-Grinberg A, Robinson TM, Chen CC, Kasper EM (2015) Mannitol or hypertonic saline in the setting of traumatic brain injury: what have we learned? Surg Neurol Int 6:177
Bratton SL, Chestnut RM, Ghajar J et al (2007) Guidelines for the management of severe traumatic brain injury. J Neurotrauma 24(Suppl 1):1–106
Bullock R, Chestnut RM, Clifton G et al (2000) Guidelines for the management of severe head injury. J Neurotrauma 17:451–553
Cliffton GL, Miller ER, Sc C et al (2002) Fluid thresholds and outcome from severe brain injury. Crit Care Med 30:739–745
Cook PJ, Lawrence BA, Ludwig J, Miller TR (1999) The medical cost of gunshot injuries in the United States. JAMA 282:447–454
Denton JS, Segovia A, Filkins JA (2006) Practical pathology of gunshot wounds. Arch Pathol Lab Med 130:1283–1289
Finlay-Morreale HE, Tsuei BJ, Fisher BS, Davis K, Johannigman JA (2009) Close is dead: determinants of firearm injury lethality in women. J Trauma 66(4):1207–1211
Glapa M, Zorio M, Snycker FD, Bowley DM, Yilmaz TH, Doll D, Degiannis E (2009) Gunshot wounds to the head in a civilian practice. Am Surg 75(3):223–226
Hutchison JS, Ward RE, Lacroix J, Hébert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, Joffe AR, Kirpalani HM, Meyer PG, Morris KP, Moher D, Singh RN, Skippen PW, Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group (2008) Hypothermia therapy after traumatic brain injury in children. N Engl J Med 358(23):2447–2456
Jandial R, Reichwage B, Levy M, Duenas C, Sturdivan L (2008) Ballistics for the neurosurgeon. Neurosurgery 62(2):472–480
Kim TW, Lee JK, Moon KS, Kwak HJ, Jpp SP, Kim JH, Kim SH (2007) Penetrating gunshot injuries to the brain. J Trauma 62(6):1446–1451
Kim KA, Wang MY, McNatt SA, Pinsky G, Liu CY, Gianotta SL, Appuzzo MLJ (2005) Vector analysis correlating bullet trajectory to outcome after civilian through and through gunshot wounds to the head: using imaging cues to predict fatal outcome. Neurosurgery 57(4):737–747
Levy ML (2000) Outcome prediction following penetrating craniocerebral injury in a civilian population: aggressive surgical management in patients with admission GCS scores of 6–15. Neurosurg Focus 8(1):e2
Lozier AP, Sciacca RR, Romagnoli MF et al (2008) Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 62(Suppl 2):688–700
Lu J, Marmarou A, Choi S et al (2005) Mortality from traumatic brain injury. Acta Neurochir Suppl 95:281–285
Martins RS, Siqueira MG, Santos MT, Zanon-Collange MOJ (2003) Prognostic factors and treatment of penetrating gunshot wounds to the head. Surg Neurol 60(2):98–104
MRC CRASH Trial Collaborators, Perel P, Arango M, Clayton T et al (2008) Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 336:425–429
Pabuscu Y, Bulakbasi N, Kocaoglu M, Ustunsoz B, Tayfun C (2003) A different approach to missile induced head injuries. Comput Med Imaging Graph 27:397–409
Patel HC, Menon DK, Tebbs S, Hawker R, Hutchinson PJ, Kirkpatrick PJ (2002) Specialist neurocritical care and outcome from head injury. Intensive Care Med 28(5):547–553
Roberts I (2000) Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev (2):CD000033
Rosenfeld JV (2002) Gunshot injury to the head and spine. J Clin Neurosci 9(1):9–16
Semple PL, Domingo Z (2001) Craniocerebral gunshot injuries in South Africa – a suggested management strategy. S Afr Med J 91(2):141–145
Stuehmer C, Blum KS, Kokemueller H, Tavassol F, Bormann KH, Gellrich NC, Rücker M (2009) Influence of different types of guns, projectiles, and propellants on patterns of injury to the viscerocranium. J Oral Maxillofac Surg 67:775–781
Tsuei YS, Sun MH, Lee HD, Chiang MZ, Leu CH, Cheng WY, Shen CC (2005) Civilian gunshot wounds to the brain. J Chin Med Assoc 68(3):126–132
Wakai A, McCabe A, Roberts Schierhout G (2013) Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev 8:CD001049
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kasper, E.M., Laviv, Y., Stippler, M., Kasper, B.S. (2017). Gunshot Injuries to the Head. In: Velmahos, G., Degiannis, E., Doll, D. (eds) Penetrating Trauma. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-49859-0_27
Download citation
DOI: https://doi.org/10.1007/978-3-662-49859-0_27
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49857-6
Online ISBN: 978-3-662-49859-0
eBook Packages: MedicineMedicine (R0)