Abstract
The percutaneous route has been recognized as one of the highly potential routes not only for topical drug delivery but also for systemic drug delivery as it provides the advantage of avoidance of the first-pass effect, ease of use and removal (in case of side effects), and better patient compliance. However, the major limitation of this route is the difficult permeation of the drug through the skin. The skin has become an impressive and idealistic platform for the delivery of drugs compared to other routes. However, the stratum corneum or the horny layer, an impermeable barrier of the skin devoid of biological activity, had challenged the development of transdermal products, which deliver drugs directly to the systemic circulation at a controlled rate. Studies have been carried out to find safe and suitable permeation enhancers to promote the percutaneous absorption of a number of drugs. Some of them are niosomes and proniosomes, which ideally possess the property of reversibly reducing the barrier resistance of the horny layer, allowing the drug to reach the living tissues at a greater rate. Niosomes and proniosomes, being colloidal carriers, are still in their infancy and need to be exploited more in the field of dermal and transdermal drug delivery. This chapter explains the state of the art of drug transport through the skin by means of vesicular classic systems: niosomes and proniosomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Skin and Penetration Enhancers
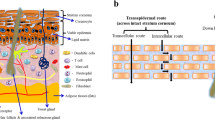
The skin is very effective as a selective penetration barrier. Percutaneous absorption involves the passage of the drug molecule from the skin surface into the stratum corneum under the influence of a concentration gradient and its subsequent diffusion through the stratum corneum and underlying epidermis, through the dermis, and into the blood circulation. The skin behaves as a passive barrier to the penetrant molecule. The stratum corneum provides the greatest resistance to penetration, and it is the rate-limiting step in percutaneous absorption (Barry 2001; Inayat and Mallikarjuna 2009).
An approach commonly researched, for promoting permeation through the skin of poorly penetrating drug molecules, is the formulation of a suitable delivery vehicle or the incorporation of a chemical enhancer into dermal and transdermal delivery systems. Penetration enhancers are substances that facilitate the absorption of penetrants through the skin by temporary diminishing the impermeability of the skin. Ideally, these materials should be pharmacologically inert, nontoxic, nonirritating, nonallergenic, compatible with the drug and excipients, odorless, tasteless, colorless, inexpensive, and have good solvent properties (Williams and Barry 2004). The enhancer should not lead to the loss of body fluids, electrolytes, and other endogenous materials, and the skin should immediately regain its barrier properties on its removal (Davis and Hadgraft 1991; Pfister et al. 1990). Alternatively, physical mechanisms such as iontophoresis and phonophoresis can be used to promote the diffusion of certain classes of drugs (Venuganti and Perumal 2009).
The primary role of the stratum corneum is to provide a substantial diffusional barrier and thereby protect the body from the ingress of xenobiotics. The dermal barrier of the body is now known to be a complex, dynamic, biochemical environment that responds to ambient conditions to maximize the protective barrier. Diffusional resistance is known to reside in the stratum corneum, and it is constituted by a complex interaction, principally of lipid and protein components, which create fairly distinct hydrophilic and lipophilic penetration pathways. The increase in the understanding of the function of the stratum corneum in recent years has resulted in a diverse range of compounds being tested for their ability to facilitate improved permeation of coadministered drugs through the skin (Vyas and Khar 2002). Penetration-enhancing effects, resulting from structural alterations of the stratum corneum barrier, manifest themselves in an increase of the drug diffusion coefficient and/or of the drug solubility in the barrier. The quantification of enhancing effects on drug penetration is possible either by the direct determination of the drug fluxes or by an indirect determination through the measurement of the pharmacodynamic response.
Percutaneous applied drug preparations for local or systemic therapy still have limited efficacy.
The transdermal delivery of systemically acting drugs may cause problems. The number of drugs that lead to plasma levels in the therapeutic range after transdermal application is small. This is limited due to the significant barrier to penetration across the skin, consequently even for readily penetrating drugs, such as nitroglycerin, the daily dose of drug that can be delivered from a transdermal patch is not more than a few milligrams (Guy and Hadgraft 1985). The penetration rate of drugs from transdermal systems does not really seem to be regulated by the drug release rate from the transdermal system (Chien et al. 1983; Tojo et al. 1986): the drug penetration across the stratum corneum is the penetration rate-limiting step. The reason for these problems is the distinct barrier function of the stratum corneum. The drug penetration rate and the permeability of the barrier for the drug increase primarily with increasing drug’s partition coefficients between stratum corneum and vehicle or between octanol and water and with decreasing relative molecular weight (Flynn 1990; Hagedorn-Leweke and Lippold 1995; Le and Lippold 1995). High skin permeability can be expected, in particular, for small molecules with sufficient affinity to the stratum corneum.
The final aim of transdermal drug delivery is to ensure that compounds are delivered, preferably at a specific rate, to the systemic circulation. Penetration of the drug to the dermal vasculature follows exposure of the skin to a dosage form from which the active must partition, followed by diffusion of the compound through the external strata to the dermis. Partitioning of the drug from the dosage form to the skin is highly dependent on the relative solubility of the drug in the components of the delivery system and in the skin. Thus, the composition of the vehicle may markedly influence the degree of penetration of the drug (Guy and Hadgraft 1989). Partitioning is governed to a large extent by the thermodynamic activity of the drug in the vehicle, and this is, therefore, of major importance in controlling the degree of penetration of any compound (Chien 1991).
Since diffusion of drugs across the skin is a passive process, compounds with low solubility and low affinity for the hydrophilic and lipophilic components of the SC would, theoretically, partition at a slow rate. These difficulties may be overcome by addition of a chemical adjunct to the delivery system that would promote drug partitioning into the SC. Furthermore, penetration enhancer chemicals added to topical vehicles usually also partition into the SC and affect the intrinsic diffusional barrier properties of this structure. Other factors that require consideration, when penetration enhancers are included into formulations, are the effects that these chemicals may have on the solubility of the drug in the delivery vehicle, influencing the diffusional gradient, or the possible effects that the chemical may have on the state of hydration of the diffusional barrier (Vyas and Khar 2011).
Different classes of compounds have been tested for their enhancer action (Sinha and Kaur 2000), and different approaches to enhance drug penetration which include the use of enzymes, natural oils, phospholipid micelles, liposomes (Sinico et al. 2005), niosomes (Tavano et al. 2011), polymers, lyotropic liquid crystals (Muzzalupo et al. 2010), and surfactants (Bettley 1965).
Carrier-based percutaneous drug delivery systems are represented in Fig. 10.1.
Carriers for percutaneous drug delivery (Adapted from Venuganti and Perumal 2009)
Surfactants contribute to the overall penetration enhancement of compounds primarily by adsorption at interfaces, by interacting with biological membranes, and by alteration of the barrier function of the SC, as result of reversible lipid modification (Kushla et al. 1993). Nonionic surfactants are used widely in pharmaceuticals to increase stability, solubility, and permeation of drugs.
Among these strategies, special formulation approaches based mainly on the use of surfactant solutions or vesicles (niosomes and proniosomes) are the most promising (Choi and Maibach 2005).
There is a direct contact of niosome/proniosome formulations with the skin after their application, so it is better to discuss the potential interactions between the skin and vesicles formed in niosome/proniosome formulations. As known, niosomes and proniosomes are vesicles composed of nonionic surfactants. So it is advisable to study the interactions between nonionic surfactants and the skin. Nonionic surfactants are used widely in pharmaceuticals to increase stability, solubility, and permeation of drugs. There is a strong indication that the degree of interaction between vesicles and skin mainly depends on physicochemical properties of the surfactant molecules which the niosomes or proniosomes are composed of.
10.2 Niosomes as Carriers in Percutaneous Drug Delivery
Colloidal vesicular carriers such as niosomes have been extensively applied in drug delivery systems due to their unique advantages. The formation of vesicular systems based on hydration of a mixture of a single-alkyl chain nonionic surfactant and cholesterol was firstly reported in 1979 (Handjani-Vila et al. 1979). Niosomes are self-assembled, submicron vesicles composed of nonionic surfactants with closed bilayer structures similar to liposomes. However, they are much more stable and less expensive than liposomes. Niosomes are synthetic microscopic vesicles consisting of an aqueous core enclosed in a bilayer consisting of one or more nonionic surfactants and cholesterol. They are made of biocompatible, biodegradable, nontoxic, nonimmunogenic, and noncarcinogenic agents which form closed spherical structures (self-assembly vesicles) upon hydration. With high resistance to hydrolytic degradation, niosomes are capable of entrapping many kinds of drugs and exhibit greater stability and longer shelf life than liposomes. These vesicles can act as drug reservoirs, and the rate of drug release can be modified by changing their composition (Handjani-Vila et al. 1979; Sankhyan and Pawar 2012; Tojo et al. 1986; Udupa 2004).
These vesicular carriers can encapsulate both hydrophilic drugs (in the aqueous core) and hydrophobic drugs (in the bilayer). Because of their potential to carry a variety of drugs, these vesicles have been widely used in various drug delivery systems, for drug targeting, controlled drug release, and permeation enhancement of drugs (Akhilesh 2011). The hydrophobic part of the surfactant faces toward the core, whereas the hydrophilic groups interface with the surrounding aqueous medium. Niosomes can be constructed by using a variety of surfactants, which possess a hydrophilic head group and a hydrophobic tail. The hydrophobic tail may consist of one or two alkyl or perfluoroalkyl groups, or in some cases, it consists of a single steroidal group. The surfactants with an alkyl chain length from C12–C18 are suitable for the preparation of niosomes. Hydrophilic head groups include glycerol, ethylene oxide, polyhydroxy groups, crown ethers, sugars, and amino acids (Lohumi et al. 2012; Sahin 2007). The most frequently used nonionic surfactants include ester of sorbitan (sorbitans are also known as “Spans”) and polyoxyethylene alkyl ether (C n EO m , Brij®) surfactants. Cholesterol and their derivatives are included in niosomes besides surfactants, usually in a 1:1 molar ratio, as steric stabilizers to prevent aggregation (Florence 1993; Vyas and Khar 2011). Cholesterol also prevents the phase transition of niosomes from the gel to the liquid state and thereby reduces drug leakage from niosomes. The stability of niosomes can be further improved by the addition of charged molecules such as dicetyl phosphate, which prevents aggregation by charge repulsion. Generally, an increase in surfactant/lipid level increases the drug encapsulation efficiency in niosomes (Uchegbu and Vyas 1998). Preparation of niosomes requires some energy in the form of elevated temperature and/or shear. The majority of the methods involve hydration of a mixture of surfactant/lipid at elevated temperature, followed by size reduction using sonication, extrusion, or better high-pressure homogenization. Finally, the removal of unentrapped drug from the vesicles can be accomplished by dialysis, gel filtration, or centrifugation. Niosomes prepared by hydration methods usually are in micron size range. Size reduction by sonication and/or extrusion results in niosomes of 100–200 nm, whereas microfluidizer or high-pressure homogenizer can achieve niosomes of 50–100 nm. The smaller size of niosomes is achieved at the cost of reduced drug loading. Furthermore, the smaller niosomes are relatively more unstable than larger ones and, therefore, require stabilizers, such as cholesterol or fatty alcohols or diacetyl phosphate to prevent aggregation. Both hydrophilic and hydrophobic drug molecules have been encapsulated in niosomes by using either dehydration–rehydration technique or the pH gradient within and outside the niosomes (Uchegbu and Vyas 1998; Vyas and Khar 2011).
Percutaneous delivery defines targeting to the pathological sites within the skin with the least systemic absorption. Drug localization in the skin is of crucial importance in the treatment of dermatological diseases such as skin cancer, psoriasis, alopecia, and acne, where the origin of disease is located in the skin (Brown et al. 2006). Drug administration onto the skin has been used since a long time to deliver drugs to different skin layers as well as to deep regions somewhat remote from the application site. However, several limitations have been associated with conventional topical preparations, e.g., low percutaneous drug penetration because of the barrier function of the stratum corneum, the outermost layer of the skin (Rubio et al. 2011), or unwanted absorption to the systemic circulation (Dubey et al. 2012). Research studies report nowadays about delivery systems that are able to deliver drugs through the skin (Higaki et al. 2005).
Niosomes have been reported to enhance the residence time of drugs in the stratum corneum and epidermis, while reducing the systemic absorption of the drug and improving penetration of the trapped substances through the skin. In addition, these systems have been reported to decrease side effects of drugs. (Schreier and Bouwstra 1994). They are thought to improve the horny layer properties both by reducing transepidermal water loss and by increasing smoothness via replenishing lost skin lipids (Hofland et al. 1991). Moreover, it has been reported in several studies that compared to conventional dosage forms, vesicular formulations exhibited an enhanced cutaneous drug bioavailability (Manconi et al. 2006; Mura et al. 2007; Srikanth et al. 2010).
Therefore, the main advantages of niosomal systems are as follows (Biju et al. 2006; Indhu et al. 2004; Vyas and Khar 2011):
-
They consist of hydrophilic, amphiphilic, and lipophilic moieties together and as a result they can accommodate drug molecules with a wide range of solubilities.
-
The vesicles have flexible properties that can be varied by changing, the composition of vesicles, which affects the size, lamellarity, tapped volume, and surface charge.
-
As the vesicle dispersion is a water-based vehicle, it provides better patient compliance than oil-based dosage forms.
-
By improving oral bioavailability of poorly absorbed drugs, by delaying clearance from the circulation, and by protecting the drug from biological environment, they improve the therapeutic performance of the drug molecules.
-
The vesicles may act as a depot, releasing the drug in a controlled manner.
-
They are osmotically active, chemically stable, and also increase the stability of the drug entrapped. Oral, parenteral, and topical routes can be used for their administration.
-
Biodegradable, biocompatible, and nonimmunogenic surfactants are used in the preparation of niosomes.
-
Handling and storage of surfactants require no special conditions.
The drawbacks of niosomes are principally linked to their preparation (Verma et al. 2010):
-
The aqueous suspensions of niosomes may have limited shelf life due to fusion and aggregation of vesicles and leaking of entrapped drugs from vesicles.
-
The methods of vesicle preparation foreseeing the steps, such as extrusion and sonication, are time consuming and may require specialized equipment for processing.
The entrapment efficiency of the drug, the rate of drug release, and the size of vesicles are dependent on the hydrophilic-lipophilic balance (HLB) value of the surfactant. The entrapment efficiency increases with the increase in the concentration and lipophilicity of the surfactant, while if the HLB value of the surfactant decreases, the mean size is reduced (Biswal et al. 2008; Lawrence et al. 1996; Shahiwala and Misra 2002).
The rate of drug release from niosomes is dependent on the surfactant type and also on the phase-transition temperature of surfactant. For example, the release of carboxyfluorescein from sorbitan monoester (sorbitan molaurate, Span® 20; sorbitan monopalmitate Span® 40; sorbitan monostearate, Span® 60;) niosomes was in the following decreasing order: Span® 20 > Span® 40 > Span® 60, i.e., the release decreased with an increase in alkyl chain length of the surfactant [82]. Their phase transition temperature (from gel to liquid thermodynamic state) increased as the length of the acyl chain increased. Thus, sorbitan monolaurate (Span® 20) with a C9 chain is in liquid thermodynamic state at room temperature; sorbitan monopalmitate (Span® 40) with a C13 chain has a phase transition temperature of 46–47 °C; sorbitan monostearate (Span® 60) with a C15 chain has a gel to liquid phase transition temperature of 56–58 °C. Vesicles made with these higher molecular weight Span® surfactants are less leaky and more stable to osmotic gradients. Niosomes have been shown to penetrate into the skin and enhance the permeation of drugs. Span® niosomes provided significantly higher skin permeation and partitioning of enoxacin than liposomes and the free drug. The action of niosomes as permeation enhancers might predominantly be on the intercellular lipids of SC, raising the fluidity and weakness of the SC. The direct permeation of the vesicles into the viable epidermis and dermis was largely restricted. Niosomes were mainly localized in the SC, but niosomes largely contribute to the rapid permeation of enoxacin across the SC which may be due to the higher diffusion of vesicles with the drug into the SC. It is proposed that niosomes disrupt the membrane properties of the SC as well as that they directly fuse into the upper layer of the skin, thereby enhancing the skin permeation of enoxacin (Fang et al. 2001a). Niosomes dissociate and form loosely bound aggregates, which then penetrate into the deeper skin strata. Furthermore, the high skin penetration of drugs from niosomes has been attributed to the flexibility of niosomes, and this is supported by the fact that a decrease in cholesterol content which leads to higher vesicle flexibility increases the drug penetration through the skin (Vanhal et al. 1996). Moreover, the nonionic surfactant can also modify the intercellular lipid structure in the SC to enhance skin permeability. In addition, adsorption and fusion of niosomes with the skin surface increase the thermodynamic activity of the drug, leading to enhanced drug penetration into the skin (Schreier and Bouwstra 1994). In vitro studies have found that the chain length of alkyl polyoxyethylene in niosomes did not affect the cell proliferation of human keratinocytes, but the ester bond was found to be more toxic than the ether bond in the surfactants (Hofland et al. 1991).
10.3 Percutaneous Applications of Niosomes
Niosomes and niosomal gels are considered to be useful controlled drug delivery systems for the percutaneous route, and many researchers studied their potential as innovative drug delivery systems for this route (Hamishehkar et al. 2013).
5-Fluorouracil (5-FU) is an anticancer drug that showed appropriate antitumoral effect in the topical treatment of lesions associated to squamous cell carcinoma (Gross et al. 2007). Unfortunately, 5-FU shows a low percutaneous permeation, thus reducing its anticancer effectiveness. Therefore, improved percutaneous permeation of 5-FU is an important prerequisite to attain an effective topical therapeutic approach (Gupta et al. 2005; Singh et al. 2005). Paolino et al. designed an innovative niosomal system composed of α,ω-hexadecyl-bis-(1-aza-18-crown-6) (Bola), Span® 80, and cholesterol as a topical carrier system for 5-FU. In this study, the percutaneous permeation of 5-FU through human stratum corneum and epidermal layers demonstrated that bola-niosomes provided an eightfold and fourfold increase of the drug penetration compared to an aqueous drug solution and to a mixture of empty bola-niosomes with an aqueous drug solution, respectively (Paolino et al. 2008). Niosomes loaded with capsaicin were prepared using a particular ratio between surfactants, to obtain systems with a specific HLB value (10, 12, 14) and characterized in terms of particle size, morphology, and their capsaicin entrapment efficiency. They were evaluated in vitro for their percutaneous permeation-enhancing effect. The prepared formulations were compared to microemulsions prepared from the same surfactants in the same ratio, and a higher transdermal delivery of capsaicin was achieved with niosomes (Tavano et al. 2011). Carafa et al. (2002) prepared lidocaine and lidocaine hydrochloride-loaded nonionic surfactant vesicles using Tween® 20 and cholesterol. The ability of the drug to diffuse through a model lipophilic membrane and through mouse skin was studied and compared with classical liposomes and Tween® 20 micelles. Also, dicetylphosphate and N-cetylpyridinium chloride were used to prepare negatively and positively charged vesicles, respectively, in order to study the effect of vesicle charge on drug encapsulation efficiency. The obtained data provided direct evidence that neutral vesicles, prepared with Tween® 20 and cholesterol, entrapped a higher lidocaine amount, at pH 5.5, than positively and negatively charged vesicles. Ellagic acid may selectively inhibit melanin synthesis only in UV-activated melanocytes. It is a phytochemical substance with potent antioxidative properties which has limited use due to its poor biopharmaceutical properties such as low solubility and low skin permeation ability. Junyaprasert et al. (2012) prepared a niosomal formulation of ellagic acid from the mixture of Span® 60 and Tween® 60 for dermal delivery. Due to the low solubility of ellagic acid, methanol (MeOH), propylene glycol (PG), and polyethylene glycol 400 (PEG 400) were used as solubilizers. Skin permeation and distribution studies revealed that ellagic acid-loaded niosomes showed a more efficient delivery of ellagic acid through human epidermis and dermis than the ellagic acid solution, indicating that these niosomes may be a potential carrier for the dermal delivery of ellagic acid. Tretinoin cutaneous delivery is strongly affected by vesicle composition and thermodynamic activity of the drug. In particular, small, negatively charged niosomal formulations, which are saturated with tretinoin, have shown to give higher cutaneous drug retention (Manconi et al. 2006). Nasr et al. (2008) studied multilamellar liposomes and niosomes of aceclofenac, a potent analgesic, antipyretic, and anti-inflammatory agent. A comparative study was performed through evaluation of entrapment efficiency, particle size, shape, differential scanning calorimetry, in vitro drug release, and 3 months stability. Results proved that niosomes possess better stability than liposomes. Both vesicular systems showed considerable sustained anti-inflammatory activity compared to the commercial product. However, niosomes were superior to liposomes as clearly shown with both edema and inhibition rates assessed by the rat paw edema technique. Ketoprofen was encapsulated in niosomes of Span® 60 for topical application which released the drug in a slow and sustained manner (Arora and Sharma 2010). Elastic and nonelastic niosomes of gallic acid were prepared, and nonelastic niosomes showed a slight increase in the entrapment efficiency. Elastic niosomes showed, however, an increased permeation through the skin which will be beneficial for topical antiaging treatment (Manosroi et al. 2011). Manosroi et al. (2012) prepared cationic niosomes encapsulating an extract of a semipurified fraction of Oryza sativa. They investigated physicochemical characteristics and transfollicular penetration of niosomes through porcine skin using the follicular closing technique by Franz diffusion cells. The results of this study confirmed efficient transfollicular delivery of unsaturated fatty acids using cationic niosomes as well as the advantage of low systemic effect of a semipurified fraction of Oryza sativa compared to the neutral niosomes. Fluconazole-loaded niosomes of Span® 40, Span® 60, and polyoxyethylene (2) stearyl ether (known as Brij® 72) surfactants were prepared and evaluated. The prepared formulation accumulated the drug in the skin forming localized drug depots, thereby releasing the content in a sustained manner (Gupta et al. 2011). Jayraman et al. (1996) studied the topical delivery of erythromycin from various formulations including niosomes in hairless mouse in vivo. The penetration study, and confocal microscopy, revealed that nonionic vesicles could target pilosebaceous glands. A natural compound with an efficacious anti-inflammatory activity, ammonium glycyrrhizinate was loaded into bola-niosomes. These drug-loaded niosomes provided a noticeable improvement of the in vivo anti-inflammatory activity of the drug (Paolino et al. 2007).
Presence of 50 % alcohol in a marketed gel of naftifine hydrochloride, an antifungal highly lipophilic drug, has been detrimental to the skin after repeated exposure. An alcohol-free niosomal gel containing naftifine hydrochloride has been developed, and optimized to achieve maximum entrapment efficiency coupled with stability. Negatively charged niosomes have also been incorporated into a hydroxyethylcellulose gel (Barakat et al. 2009). Topical immunization with cholera toxin B is a potential adjuvant for cutaneous immune responses when coadministered with the hepatitis B surface antigen (HBsAg)-encapsulated niosomes. Niosomes for topical delivery of vaccines using HBsAg as an antigen and cholera toxin B as an adjuvant can be effective as topical delivery of vaccines (Maheshwari et al. 2011). Mannosylated niosomes were formulated as a topical vaccine carrier system and adjuvant for the induction of both humoral and cellular immunities (Jain et al. 2005).
Aceclofenac niosomes have also been prepared for topical use after their incorporation into a carbomer gel. The niosomal gel showed improved drug penetration and therapeutic efficacy of the drug (Solankia et al. 2010). A niosomal gel containing nimesulide was compared to a plain nimesulide gel in terms of drug delivery. It was concluded that the niosomal gel showed a prolonged drug release of nimesulide, thereby enhancing the anti-inflammatory activity (Shahiwala and Misra 2002).
Estradiol-loaded niosomes obtained from nonionic n-alkyl polyoxyethylene ether surfactants (CnEOm) and cholesterol facilitated estradiol transdermal permeation (Hofland et al. 1994). Similarly, a meloxicam niosomal gel produced a greater reduction in edema in albino rats when compared to the conventional meloxicam gel due to the penetration of niosomes into the deeper layers of the skin. Meloxicam was entrapped in the niosomal gel which showed decreased side effects and increased pharmacological activity, thus proving to be a promising vehicle for transdermal delivery and an alternative to the conventional dosage form (El-Menshawe and Hussein 2013).
10.4 Proniosomes as Carriers in Percutaneous Drug Delivery
Proniosomes represent a significantly improved vesicular delivery system as physical stability problems, such as aggregation or fusion of vesicles and leaking of entrapped drugs during long-term storage are eliminated. Proniosomes are dry formulations of water-soluble carriers (vesicles) that are coated with surfactants and rehydrated to form niosomal dispersions immediately before use on brief agitation in hot aqueous media within minutes. Proniosomes are convenient to store, to transport, and to dose (single dose). Since they have similar release characteristics as conventional niosomes, they may offer improved bioavailability of some drugs with poor solubility (controlled release formulations) or reduced adverse effects of some drugs. Since proniosomes are a dry powder, further processing is possible. To provide convenient unit dosing, the proniosome powder may be processed to make beads, tablets, or capsules. The hydration of the proniosome powder is much easier than the long shaking process required to hydrate surfactant in the conventional dry film method of niosome preparation. The resulting niosomes are very similar to conventional niosomes and more uniform in size (Akhilesh et al. 2011; Hu and Rhodes 1999). They offer a versatile vesicle delivery concept with the potential for transdermal drug delivery. They form niosomes following topical application under occlusive conditions, due to hydration by water from the skin itself. There are some factors such as hydration temperature, choice of surfactant, nature of membrane, nature of drug, etc. that can affect significantly the physical properties of proniosomes.
Constituents which are selected for the preparation of proniosomes should have following characteristics: free-flow ability, nontoxicity, poor solubility in the loaded mixture solution, and good water solubility for ease of hydration. Different components, such as nonionic surfactants and membrane stabilizers, are used for the proniosome preparation, and they are shown in Fig. 10.2 (Pandey 2011).
Typical components used for preparation of proniosomes (Adapted from Pandey 2011)
The proniosomes are prepared by different methods:
-
(a)
Slurry method: Coating material, principally maltodextrin powder, is added to the surfactant solution directly to form slurry. The solvent is dried until the powder appears to be dry and free flowing. Proniosome powder is stored in sealed containers at 4 °C. The time required to produce proniosomes is independent on the ratio of surfactant solution to coating material and appears to be scalable (Almira et al. 2001). A proniosome formulation based on maltodextrin was recently developed and has a potential application for delivering hydrophobic or amphiphilic drugs. The better of these formulations used a hollow particle with exceptionally high surface area. The principal advantage with this formulation was that the amount of carrier required to support the surfactant could be easily adjusted, and proniosomes with very high mass ratios of surfactant to carrier could be prepared (Almira et al. 2001).
-
(b)
Coacervation-phase separation method: Weighed amounts of surfactant, lipid, and drug are dissolved in hot (60–70 °C) alcohol. Then the aqueous phase (0.1 % glycerol solution) is added and warmed on a water bath till a clear solution is formed which is then converted into a proniosomal gel upon cooling (Benson 2006; Vora et al. 1998).
-
(c)
Slow spray-coating method: This method involves preparation of proniosomes by spraying the surfactant in organic solvent onto coating materials, such as powder of sorbitol, and then evaporating the solvent. The surfactant coating on the carrier is very thin, and hydration of this coating allows multilamellar vesicles to form as the coating material dissolves. The resulting niosomes are very similar to those produced by conventional methods, and the size distribution is more uniform (Abd-Elbary et al. 2008; Biju et al. 2006).
In addition to dry granular type of proniosomes, it is possible to obtain the liquid crystalline proniosomes. When the surfactant molecules are kept in contact with water, there are three ways through which lipophilic chains of surfactants can be transformed into a disordered liquid state called lyotropic liquid crystalline state (neat phase). These three ways are increasing temperature at kraft point (Tc); addition of solvent, which dissolves lipids; and use of both temperature and solvent. Neat phase or lamellar phase contains bilayers arranged in overlapping sheets within intervening aqueous layers. These types of structures give typical X-ray diffraction and threadlike birefringent structures under polarized microscope. The liquid crystalline proniosomes or the proniosomal gel acts as a reservoir for dermal/transdermal delivery of drugs (Bharti et al. 2012).
Advantages of proniosomes over niosomes include (Pandey 1996; Sudhamani et al. 2010):
-
1.
Avoiding problems of physical stability, like aggregation, fusion, leaking.
-
2.
Avoiding hydration of encapsulated drugs which limits the shelf life of the dispersion.
-
3.
Proniosomes are water-soluble carrier particles that are coated with surfactant and can be hydrated to form niosomal dispersions immediately before use on brief agitation with hot aqueous medium. Proniosomes (dry niosomes) can be a promising industrial product because of the facility of transportation, distribution, and storage.
-
4.
Unacceptable solvents are avoided in proniosomal formulations. The systems may be directly formulated into transdermal patches and do not require the incorporation of vesicles into a polymeric matrix.
-
5.
Ease of storage makes proniosomes a versatile delivery system for a wide range of active compounds.
10.5 Percutaneous Applications of Proniosomes
The importance of proniosomes can be described on the basis of different studies related to specific applications of proniosomes as a carrier system in transdermal delivery of different drugs.
Ketorolac, a potent nonsteroidal anti-inflammatory drug, is formulated as a proniosome gel using Span®, Tween®, lecithin, and cholesterol with ethanol as a solvent. Each of prepared proniosome formulations showed significantly improved drug permeation. Entrapment efficiency of drugs in prepared niosome formulations was about 99 %, and it was concluded by Alsarra et al. that proniosomes may be a promising carrier for ketorolac (Alsarra et al. 2005). Mokhtar et al. (2008) formulated a proniosomal gel of flurbiprofen using different Span® surfactants without and with cholesterol. The influence of different processing and formulation variables on flurbiprofen entrapment efficiency, such as cholesterol content, structure of nonionic surfactants, drug concentration, total lipid concentration, and the pH of the hydration medium, were studied. It was concluded that niosome formulations containing 10 % cholesterol were most stable among all the prepared formulations. Chandra and Sharma (2008) formulated piroxicam proniosomes using Span® surfactants, cholesterol, lecithin, and isopropyl alcohol. It was suggested that proniosome vesicles transfer drug from vesicles to the skin and that the penetration enhancement may be due to the effect of surfactants. Thakur et al. (2009) prepared proniosomes using different esters of sorbitan and polysorbates (Tween are registered trademarks of ICI Americas), such as Span® 20, Span® 40, Span® 60, Span® 80, Tween® 20, Tween® 40, and Tween® 80, for transdermal delivery of losartan potassium. The best in vitro skin permeation profile was obtained with proniosomes prepared using Span® 40. Proniosomes were used as carriers for transdermal delivery of lisinopril dihydrate by Shamsheer et al. (2011). The results of the this study indicated that the lisinopril dihydrate proniosomal gel containing lecithin, cholesterol, and a combination of two different Span® surfactants like Span® 20 and Span® 40 or Span® 60 and Span® 80 provided a sustained drug release over a period of 24 h for the control of hypertension. The proniosomal gel could be an effective alternative vehicle for delivering the drug by the transdermal route to avoid side effects associated with oral route. Gupta et al. (2007) investigated the potential of proniosomes as a transdermal drug delivery system for captopril which is used for the treatment of hypertension. The drug was encapsulated in various proniosomal gels composed of different ratios of sorbitan fatty acid esters, cholesterol, and lecithin. The authors concluded that these proniosomes are promising for prolonged delivery of captopril, have reasonably good stability characteristics, and can reduce the side effects associated with captopril. Fang et al. (2001b) reported about the skin permeation of estradiol from various proniosomal gel formulations across excised rat skin. Presence or absence of cholesterol in the lipid bilayers of vesicles did not reveal difference in encapsulation efficiency and permeation of the associated estradiol. The type and content of nonionic surfactants in proniosomes are important factors affecting the efficiency of transdermal delivery of estradiol. The study suggests that inclusion of surfactants and lecithin in vesicles may play a more important role than inclusion of cholesterol on estradiol permeation. El-Laithy et al. (2011) studied a novel sustained release proniosomal system using sugar esters as nonionic surfactants. Proniosomes were converted into niosomes loaded with vinpocetine upon skin water hydration following topical application under occlusive conditions. The researchers reported that proniosomes composed of sugar esters could be considered as very promising candidates for improving the transdermal delivery of vinpocetine.
Proniosomal gel of valsartan, an ACE inhibitor, was prepared by Kakkar et al. (2011). They evaluated the vesicle size and entrapment efficiency of the drug, and performed diffusion and stability studies of the gel. Results have shown that the surfactant type and the content of cholesterol and lecithin affect the encapsulation efficiency and the drug release rate from proniosomes. In particular, the encapsulation efficiency of valsartan of proniosomes formed by Span® 60 was observed to be higher compared to that obtained with Span® 40.
Azeem et al. (2008) studied the permeation-enhancing mechanism of the proniosomal gel of frusemide (or furosemide), in which Span®, lecithin, diacetyl phosphate, and cholesterol were used as constituents. The authors studied the effect of various formulation variables on the transdermal drug flux, amount of drug deposited in the skin, and plasma level of drug. The skin permeation studies were conducted on rat skin and human skin for quantification of permeation parameters. Overall findings suggested that the proniosomal gel was able to sustain the drug level in the blood and offer a promising means for noninvasive delivery of frusemide. Varshosaz et al. (2005) developed a proniosomal gel for transdermal delivery of chlorpheniramine maleate. The system was formulated with Span® 40 and evaluated for the effect of the composition, type of surfactants used, and alcohols on the drug loading, rate of hydration, vesicle size, polydispersity index, entrapment efficiency, and drug release across cellulose nitrate dialysis membrane. It was concluded that lecithin produced more stable and larger vesicles with higher loading efficiency of the drug. The proniosomes that contained Span 40/lecithin/cholesterol prepared by ethanol showed optimum stability, loading efficiency, particle size and release kinetic suitable for transdermal delivery of chlorpheniramine maleate. Proniosomes were used as carriers for the delivery of poorly water-soluble drugs, like celecoxib. Alam et al. (2010) prepared a proniosomal gel containing celecoxib using Span® 40 and Span® 60, cholesterol, and lecithin. The hydroxypropyl methyl cellulose (HPMC) gel (4 % w/v in ethanol) was selected as a suitable base to incorporate proniosomes into a formulation. Their results indicated that proniosomes are a promising carrier for celecoxib used at low dose for transdermal delivery that can save the recipient from the harm of large doses with improved bioavailability by bypassing the hepatic first metabolism.
10.6 Conclusion
Delivery of unstable and potent active ingredients is always a major challenge for scientific researches. In this case, selection of a carrier and a suitable route of administration for better performance are crucial aspects for the delivery of drugs. For these purposes, vesicular drug delivery systems including niosomes and proniosomes have been developed, and these drug delivery systems have been demonstrated to be promising controlled drug delivery systems for percutaneous administration of drugs. Niosomes are nontoxic and nonimmunogenic drug carriers which have been widely studied for percutaneous drug delivery. Niosomes appeared superior systems over other carriers, due to their cost-effectiveness, abilities to enhance the penetration of drugs, provide a sustained pattern of drug release and nontoxic profile, and localize drug in the skin. A proniosomal formulation which is converted into niosomes represents an innovative drug delivery system. Proniosomes were also found free from aggregation, fusion, leaking, and sedimentation of vesicles, indicating a good stability profile. These are actually a nonaqueous form of niosomes which is converted into niosomes after hydration. Proniosomes can be prepared easily by various techniques; they show enhanced stability of the formulation and simplicity in handling, they enhance drug bioavailability, and they enable controlled and prolonged drug delivery. Some studies have also shown that, for particular drugs, proniosomes show higher permeation-enhancing ability than niosomes. As niosomes were firstly exploited as a cosmetic delivery system, they still have an extensive use in that field that needs to be further explored. The topical administration of niosomes can support a large variety of relevant developments and medical applications, because their delivery-enhancing characteristics can be easily modulated by changing their composition and structure. Moreover, vesicles as dermal drug delivery systems offer many opportunities for innovative research, aimed at both increasing efficiency and reducing toxicity of drugs through simple topical application.
References
Abd-Elbary A, El-laithy HM, Tadros MI (2008) Sucrose stearate- based pro-niosome-derived niosomes for the nebulisable delivery of cromolyn sodium. Int J Pharm 357:189–198
Akhilesh D, Hazel G, Kamath JV (2011) Pro-niosomes – a propitious provesicular drug carrier. Int J Pharm Pharmacol Sci Res 1:98–103
Alam MI, Baboota S, Koili K, Ali J, Ahuja A (2010) Pharmacodynamic evaluation of proniosomal trandermal therapeutic gel containing celecoxib. Sci Asia 36:305–311
Almira I, Welesh AB, Rhodes DG (2001) Maltodextrin based pro-niosomes. AAPS Pharm Sci Tech 3(1):1–8
Alsarra IA, Bosela AA, Ahmed SM, Mahrous GM (2005) Pro-niosomes as a drug carrier for transdermal delivery of ketorolac. Eur J Pharm Biopharm 59:485–490
Arora R, Sharma A (2010) Release studies of Ketoprofen niosome formulation. J Chem Pharm Res 2:79–82
Azeem A, Jain N, Iqbal Z, Ahmad FJ, Aqil M, Talegaonkar S (2008) Feasibility of pro-niosomes based transdermal delivery of frusemide: formulation, optimization and pharmacotechnical evaluation. Pharm Dev Technol 13(2):155–163
Barakat HS, Darwish IA, El-Khordagui LK, Khalafallah NM (2009) Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev Ind Pharm 35:631–637
Barry BW (2001) Novel mechanisms and devices to enable successful transdermal drug. Eur J Pharm Sci 14:101–114
Benson HA (2006) Transfersomes for transdermal drug delivery. Expert Opin Drug Deliv 3:727–737
Bettley FR (1965) The influence of detergents and surfactants on epidermal permeability. Br J Dermatol 77:98–100
Bharti N, Loona S, Khan MMU (2012) Pro-niosomes: a recent advancement in nanotechnology as a drug carrier. Int J Pharm Sci Rev Res 12:67–75
Biju SS, Telegaonar S, Mishra PR, Khar RK (2006) Vesicular system: an overview. Indian J Pharm Sci 68:141–153
Biswal S, Murthy PN, Sahu J, Sahoo P, Amir F (2008) Vesicles of non- ionic surfactants (niosomes) and drug delivery potential. Int J Pharm Sci Nanotechnol 1:1–8
Brown MB, Martin GP, Jones SA, Akomeah FK (2006) Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13(3):175–187
Carafa M, Santucci E, Lucania G (2002) Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int J Pharm 231(1):21–32
Chandra A, Sharma PK (2008) Pro-niosome based drug delivery system of piroxicam. Afr J Pharm Pharmacol 2(9):184–190
Chien YW (1991) Transdermal systemic drug delivery. Recent development and future prospects. STP Pharma Sci 1:5–23
Chien YW, Keshary PR, Huang YC, Sarpotdar PP (1983) Comparative controlled skin permeation of nitroglycerin from marketed transdermal delivery systems. J Pharm Sci 72:968–970
Choi MJ, Maibach HI (2005) Liposomes and niosomes, as topical drug delivery systems. Skin Pharmacol Physiol 18:209–219
Davis AF, Hadgraft J (1991) Effect of supersaturation on membrane transport: I. Hydrocortisone acetam. Int J Pharm 76:1–8
Dubey A, Prabhu P, Kamath J (2012) Nano structured lipid carriers: a novel topical drug delivery system. Int J PharmTech Res 4(2):705–714
El-Laithy HM, Shoukry O, Mahran LG (2011) Novel sugar esters pro-niosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm 77(1):43–55
El-Menshawe SF, Hussein AK (2013) Formulation and evaluation of meloxicam niosomes as vesicular carriers for enhanced skin delivery. Pharm Dev Technol 18(4):779–786
Fang JY, Hong CT, Chiu WT et al (2001a) Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm 219:61–72
Fang JY, Yu SY, Wu PC, Huang YB (2001b) In-vitro skin permeation of estradiol from various pro-niosome formulations. Int J Pharm 215:91–99
Florence AT (1993) Nonionic surfactant vesicles. Preparation and characterization. In: Gregoriadis G (ed) Liposome technology, vol 2. CRC Press, Boca Raton, pp 157–176
Flynn GL (1990) Physicochemical determinants of skin absorption. In: Gerrity TR, Henry CJ (eds) Principles of route-to-route extrapolation for risk assessment. Elsevier, Amsterdam, pp 93–127
Gross K, Kircik L, Kricorian G (2007) 5% 5-Fluorouracil cream for the treatment of small superficial basal cell carcinoma: efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol Surg 33:433–440
Gupta RR, Jain SK, Varshney M (2005) AOT water-in-oil microemulsions as a penetration enhancer in transdermal drug delivery of 5-fluorouracil. Colloids Surf B Biointerfaces 41:25–32
Gupta A, Prajapati SK, Balamurugan M, Singh M, Bhatia D (2007) Design and development of a proniosomal transdermal drug delivery system for captopril. Trop J Pharm Res 6(2):687–693
Gupta M, Vaidya B, Mishra N, Vyas SP (2011) Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif Cells Blood Substit Immobil Biotechnol 39:376–384
Guy RH, Hadgraft J (1985) Transdermal drug delivery: the ground rules are emerging. Pharm Int 6:112–116
Guy RH, Hadgraft J (1989) Selection of drug candidates for transdermal drug delivery. In: Hadgraft J, Guy RH (eds) Transdermal drug delivery. Marcel Dekker, New York, pp 59–81
Hagedorn-Leweke U, Lippold BC (1995) Absorption of sunscreens and other compounds through human skin in vivo: derivation of a method to predict maximum fluxes. Pharm Res 12:1354–1360
Hamishehkar H, Rahimpour Y, Kouhsoltani M (2013) Niosomes as a propitious carrier for topical drug delivery. Expert Opin Drug Deliv 10(2):261–272
Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghe G (1979) Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmet Sci 1:303–314
Higaki K, Nakayama K, Suyama T, Amnuaikit C, Ogawara K, Kimura T (2005) Enhancement of topical delivery of drugs via direct penetration by reducing blood flow rate in skin. Int J Pharm 288(2):227–233
Hofland HEJ, Bouwstra JA, Ponec M, Bodde HE, Spies F, Verhoef JC, Junginger HE (1991) Interactions of non-ionic surfactant vesicles with cultured keratinocytes and human skin in vitro. A survey of toxicological aspects and ultrastructural changes in stratum corneum. J Control Release 16:155–167
Hofland HEJ, van der Geest R, Bodde HE, Junginger HE, Bouwstra JA (1994) Estradiol permeation from nonionic surfactant vesicles through human stratum corneum in vitro. Pharm Res 11:659–664
Hu C, Rhodes DG (1999) Pro-niosomes: a novel drug carrier preparation. Int J Pharm 185:23–35
Inayat BP, Mallikarjuna SC (2009) Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res 8(2):173–179
Indhu PK, Garg A, Anil KS, Aggarwal D (2004) Vesicular system in ocular drug delivery. Indian J Pharm Sci 269:1–14
Jain S, Singh P, Mishra V, Vyas SP (2005) Mannosylated niosomes as adjuvant–carrier system for oral genetic immunization against Hepatitis B. Immunol Lett 101:41–49
Jayaraman CS, Ramachandran C, Weiner N (1996) Topical delivery of erythromycin from various formulations: an in vivo hairless mouse study. J Pharm Sci 85(10):1082–1084
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D (2012) Physicochemical properties and skin permeation of span 60/tween 60 niosomes of ellagic acid. Int J Pharm 423(2):303–311
Kakkar R, Rao R, Dahiya NK, Nanda S (2011) Formulation and characterization of valsartan pro-niosomes. Maejo Int J Sci Technol 5(01):146–158
Kushla GP, Zatz JL, Millis OH, Berger RS (1993) Noninvasive assessment of anesthetic activity of Lidocaine formulations. J Pharm Sci 82:118–1122
Lawrence MJ, Chauhan S, Lawrence SM, Barlow DJ (1996) The formation, characterization and stability of non-ionic surfactant vesicles. STP Pharma Sci 1:49–60
Le VH, Lippold BC (1995) Influence of physicochemical properties of homologous esters of nicotinic acid on skin permeability and maximum flux. Int J Pharm 124:285–292
Lohumi A, Rawat S, Sarkar S, Sipai A, Yadav MV (2012) A novel drug delivery system: niosomes review. J Drug Delive Ther 2(5):129–135
Maheshwari C, Pandey RS, Chaurasiya A, Kumar A, Selvam DT, Prasad GB, Dixit VK (2011) Non-ionic surfactant vesicles mediated transcutaneous immunization against hepatitis B. Int Immunopharmacol 11:1516–1522
Manconi M, Sinico C, Valenti D, Lai F, Fadda AM (2006) Niosomes as carriers for tretinoin: III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm 311:11–19
Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi W, Manosroi J (2011) Transdermal absorption enhancement of gel containing elastic niosomes loaded with gallic acid from Terminalia chebula galls. Pharm Biol 49:553–562
Manosroi A, Ruksiriwanich W, Abe M, Manosroi W, Manosroi J (2012) Transfollicular enhancement of gel containing cationic niosomes loaded with unsaturated fatty acids in rice (Oryza sativa) bran semi-purified fraction. Eur J Pharm Biopharm 81(2):303–313
Mokhtar M, Sammour OA, Hammad MA, Megrab NA (2008) Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from pro-niosomes. Int J Pharm 361(1–2):104–111
Mura S, Pirot F, Manconi M, Falson F, Fadda AM (2007) Liposomes and niosomes as potential carriers for dermal delivery of minoxidil. J Drug Target 15:101–108
Muzzalupo R, Tavano L, Nicoletta FP, Trombino S, Cassano R, Picci N (2010) Liquid crystalline Pluronic 105 pharmacogels as drug delivery systems: preparation, characterization and in vitro transdermal release. J Drug Target 8:404–411
Nasr M, Mansour S, Mortada ND, Elshamy AA (2008) Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul 25(7):499–512
Pandey N (2011) Pro-niosomes and ethosomes: new prospect in transdermal and dermal drug delivery system Int. J Pharm Sci Res 2(8):1988–1996
Paolino D, Muzzalupo R, Ricciardi A, Celia C, Picci N, Fresta M (2007) In vitro and in vivo evaluation of Bola-surfactant containing niosomes for transdermal delivery. Biomed Microdevices 9:421–433
Paolino D, Cosco D, Muzzalupo R, Trapasso E, Picci N, Fresta M (2008) Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int J Pharm 353(1):233–242
Pfister WR, Dean S, Hsieh ST (1990) Permeation enhancers compatible with transdermal delivery systems. Part I: selection and formulation considerations. Pharm Technol X:132–140
Rubio L, Alonso C, López O, Rodríguez G, Coderch L, Notario J, de la Maza A, Parra JL (2011) Barrier function of intact and impaired skin: percutaneous penetration of caffeine and salicylic acid. Int J Dermatol 50(7):881–889
Sahin NO (2007) Niosomes as nanocarrier systems. In: Mozafari MR (ed) Nanomaterials and nanosystems for biomedical applications. Springer, Dordrecht, pp 67–81
Sankhyan A, Pawar P (2012) Recent trends in niosome as vesicular drug delivery system. J Appl Pharm Sci 02(06):20–32
Schreier H, Bouwstra J (1994) Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release 30(1):1–15
Shahiwala A, Misra A (2002) Studies in topical application of niosomally entrapped nimesulide. J Pharm Pharm Sci 5:220–225
Shamsheer Ahmad S, Sabareesh M, Patan Rafi Khan, Sai Krishna P, Sudheer B (2011) Formulation and evaluation of lisinopril dihydrate transdermal proniosomal gels. J Appl Pharm Sci 01(08):181–185
Singh BN, Singh RB, Singh J (2005) Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int J Pharm 298:98–107
Sinha VR, Kaur MP (2000) Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm 26(11):1131–1140
Sinico C, Manconi M, Peppi M, Lai F, Valenti D, Fadda AM (2005) Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle-skin interaction. J Control Release 103:123–136
Solankia AB, Parikh JR, Parikh RH, Patel MR (2010) Evaluation of different compositions of niosomes to optimize Aceclofenac transdermal delivery. Asian J Pharm Sci 5:87–95
Srikanth K, Nappinnai M, Gupta VRM, Suribabu J (2010) Niosomes: a prominent tool for transdermal drug delivery. Res J Pharm Bio Chem Sci 1:308–316
Sudhamani T, Priyadarisini N, Radhakrishna M (2010) Pro-niosomes – a promising drug carriers. Int J PharmTech Res 2:1446–1454
Tavano L, Alfano P, Muzzalupo R, Cindio B (2011) Niosomes vs microemulsions: new carriers for topical delivery of capsaicin. Colloids Surf B Biointerfaces 87:333–339
Thakur R, Anwer MK, Shams MS, Ali A, Khar RK, Shakeel F, Taha EI (2009) Proniosomal transdermal therapeutic system of losartan potassium: development and pharmacokinetic evaluation. J Drug Target 17(6):442–449
Tojo K, Keshary PR, Chien YW (1986) Drug permeation through skin from matrix-type drug delivery systems. Chem Eng J 32:57–64
Uchegbu IF, Vyas SP (1998) Nonionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 172:33–70
Udupa N (2004) Niosomes as drug carriers. In: Jain NK (ed) Controlled and novel drug delivery. CBS Publishers & Distributors, New Delhi, pp 292–303
Vanhal D, Vanrensen A, Devinger T, Junginger H, Bouwstra J (1996) Diffusion of estradiol from non-ionic surfactant vesicles through human stratum corneum in vitro. STP Pharma Sci 6:72–78
Varshosaz J, Pardakhty A, Baharanchi SM (2005) Sorbitan monopalmitate-based pro-niosomes for transdermal delivery of chlorpheniramine maleate. Drug Deliv 12(2):75–82
Venuganti VV, Perumal OP (2009) Nanosystems for dermal and transdermal drug delivery. In: Pathak Y, Thassu D (eds) Drug delivery nanoparticles formulation and characterization. Informa Healthcare USA, Inc, New York, pp 126–155
Verma S, Singh SK, Navneet S, Mathur P, Valecha V (2010) Nanoparticle vesicular systems: a versatile tool for drug delivery. J Chem Pharm Res 2:496–509
Vora B, Khopade AJ, Jain NK (1998) Pro-niosome based transdermal delivery of levonogesterol for effective contraception. J Control Release 54:149–165
Vyas SP, Khar RK (2002) Controlled drug delivery system: concept and advances. CBS Publishers and Distributors, New Delhi
Vyas SP, Khar RK (2011) Targeted and controlled drug delivery novel carrier systems. CBS Publishers and Distributors, New Delhi, pp 249–279
Williams AC, Barry BW (2004) Penetration enhancers. Adv Drug Deliv Rev. 56:603–618
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Muzzalupo, R. (2016). Niosomes and Proniosomes for Enhanced Skin Delivery. In: Dragicevic, N., Maibach, H. (eds) Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-47862-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-662-47862-2_10
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47861-5
Online ISBN: 978-3-662-47862-2
eBook Packages: MedicineMedicine (R0)