Abstract
Bioactive capability of Ti-alloy for implant application has been developed by producing thin hydroxyapatite (HAp) film on its surface. The adhesive strength between the base metal and the HAp film has to be evaluated to ensure the reliability of the implant. But the method of evaluation is not yet established because of its difficulty to cause adhesive fracture between base metal and HAp thin film. In this chapter, tensile load is applied to the HAp film on Ti-6Al-4V alloy substrate normal to its thickness by using a specimen bonded with adhesives. Surfactant is applied near the interface between Ti-6Al-4V substrate and HAp film by adhesives to avoid coverage of the interface. Thus the accuracy of this method is improved by preventing adhesive overflow from bonded surface to the side of the specimen. As a result, fracture occurs at interface between Ti-alloy and the HAp film. This method is proved to be useful for evaluating bond strength between Ti-alloy and HAp film and verifying the fracture of the adhesive layer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It is commonly recognized that about three months’ time is necessary to secure metallic implant to the bone tissue [1, 2]. Functional layer deposited on the surface of the metal-based implant improves the bone actively which can be used to shorten this period of time. Therefore, there are several methods of forming hydroxyapatite (HAp) on the surface of the metallic implant material. Hydroxyapatite layer reacts with the body fluids in the human body environment, giving bone-conducting function to metallic implant material [3–5]. However, failures due to delamination at the interface between the HAp film and the metal implant are reported [6, 7]. Therefore, to consider practical use of implant, evaluation of strength of the interface between the metal and the HAp film is extremely important. However, the evaluation methods of the bond strength of thin film have not yet been established.
The strength evaluation of the interface between the metal and the HAp film is reportedly difficult [8], because fracture does not necessarily occur at the interface between the metal and the HAp film. There is a method of assessing the strength of the interface between a substrate and a thin film of functional implant device by tensile test using adhesive [9, 10]. However, in this test, it is difficult to induce fracture at the interface between the metal and the HAp film, and the obtained interface strength is unstable. In addition, the scattering is large. In this chapter, we suggest a new testing method which is able to measure bond strength between Ti-6Al-4V alloy and HAp thin film reliably. We focus on a process of preparing specimen and evaluation of adhesive strength between Ti-alloy and HAp thin film (Fig. 7.1).
2 Materials and Specimens
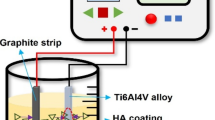
Ti-6Al-4V is used as the substrate metal. Solid bar specimens of four types in different diameters (φ4.0, φ7.0, φ10.0, and φ15.0) are used. HAp layer is coated by plasma spraying on end the bar specimen’s surface after blast treatment (Fig. 7.2) [11, 12]. Figure 7.3 shows a SEM micrograph of the surface after plasma spraying. The thickness of the HAp film is about 50 μm (HAp thin film). The spraying is repeated 20 times under the following conditions: supply voltage 68 V, spraying distance 140 mm, and HAp particles 15 g/min. To measure the interface strength between the HAp thin film and blast surface, a tensile test piece is made using an adhesive. A Ti-6Al-4V piece is bonded on the top of the HAp-coated specimen with two types of bond (EP-138 and Super Bond) by using a jig shown in Fig. 7.4. Holes of jig are made with severe tolerance to ensure the alignment of specimen. A spring is attached to the top of the jig for applying constant contact force. Also, the surfactant is used to peel off the excessive adhesive agent leaked to the side of the specimen easily after testing. This procedure is important because overflowing adhesive will cover interface between Ti-alloy and HAp film and, therefore, prevent interfacial fracture. A constant stress of 0.138 MPa was applied to the specimen. After applying the adhesive EP-138, the specimen is heat treated at 120 °C for 30 min. Super Bond is hardened within 10 min at room temperature. Figure 7.5 shows the appearance of the interface after bonding. Adhesive layers can be observed at the center of the photograph. The specimens without surfactant show overflow of adhesives from the interface, while those with surfactant do not. Therefore, surfactant protects the interface that is subjected to the examination. However, in removing the surfactant after adhesion, care should be taken.
3 Experimental Procedure of Measuring Bond Strength Between Ti-Alloy and HAp
Figure 7.6 shows bonded specimen used to measure bond strength. Tensile load is applied to this specimen under constant crosshead speed (2.5 mm/min). Care is taken to attach the specimen to testing machine to avoid bending or torsional load. If necessary, strain gauges are attached to the specimen to measure stresses within specimen. After tensile test, fracture surfaces are observed by scanning electron microscope (SEM) to confirm whether interfacial fracture occurs or not.
4 Bond Strength Between Ti-Alloy and HAp
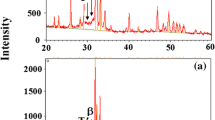
Figure 7.7 shows bond strength obtained by tensile test. Magnitude of bond strength is almost the same regardless of the diameter of the specimen. Bond strength varies depending on diameter of specimen. However, bond strength shows rather large scatter. The bond strength obtained with EP-138 is slightly higher than that of Super Bond. Scattering is larger on the diameter of φ7.0 and φ4.0. To investigate the cause of scatter, crystal structure of HAp thin film is identified by X-ray diffraction. X-ray diffraction patterns of specimens of different diameters are shown in Fig. 7.8. Crystal structure of HAp thin film is the same regardless of the specimen diameter. Therefore, HAp does not cause scattering of bond strength. Crystallinity of the HAp film will affect the bond strength. If the HAp coating has low crystallinity, interface strength value becomes low. Therefore, construction and spraying technique of HAp on substrate are important factors.
The factors affecting scatter of bond strength in terms of the method of tensile test were considered. Bond strength obtained with specimens of diameters φ4.0 and φ7.0 shows larger scatter than other diameters. Axial deviation is considered to be a reason. The axis of specimen of both sides of HAp and bond layer shift each other about 10–50 μm. This shift produces un-axial load to the interface, and interfacial fracture occurs at lower load than anticipated. This may harm the accuracy of the test. Small diameter specimens are more susceptible for this inaccuracy. Therefore, the tolerance between the jig and the specimen is an important factor in the adhesion process of the test piece.
On the other hand, adhesives protruding out on the side of the specimen near the interface tend to raise adhesion strength. Adhesives overflowing from bonded surface are considered to be a reason for dispersion. The use of surfactants prevents the adhesive protruding out near the interface between Ti-alloy and HAp film, but it is not always effective. Adhesive that remained on interface possibly suppresses interfacial fracture and affects the value of bond strength. If the same quantity of adhesive remains on the interface, the ratio between protruding adhesive and the total cross-sectional area within the interface becomes larger when the diameter of specimen is smaller. Specimens of diameters φ4.0 and φ7.0 are more susceptible to this phenomenon and may show higher adhesion strength. Thus, the adhesive suppresses interfacial failure.
It is difficult to remove all overflowing adhesives and obtain flawless alignment of bonded test pieces. Therefore, larger diameter of specimen will be suitable to minimize these effects and obtain more accurate bond strength. Thus, to determine the bond strength with smaller scatter, it is necessary to choose the specimen diameter carefully and manufacture precise tensile test jig. Because it is difficult to exclude all of these factors causing errors, a certain degree of scatters has to be accepted. Peeling off (fracture) at the interface between the HAp thin film and titanium alloy is important. It is a key to consider the test methods to accurately measure the bond strength.
5 Observation of Peeled Surface (Fracture Surface)
When fracture occurred at the interface between Ti-alloy and HAp film, HAp will be peeled off from HAp-coated surface, and Ti-alloy will appear on the surface. After tensile test, almost all of HAp is peeled off as shown in Fig. 7.9. In the previous section, the test method that can measure bond strength of Ti-alloy and HAp film correctly is verified. The result indicates that the effects of the adhesive and axial misalignment on bond strength are minimized on the method. As shown in Fig. 7.9, peeling occurs at the interface between the HAp thin film and Ti-6Al-4 V. Also, the use of the surfactant enables peeling to occur at the interface. The two photographs at the left in Fig. 7.10 show the peeled surface where surfactant was not used. It can be seen that the area peeled at the interface is smaller compared with the two photographs at the right that were obtained with specimen with surfactant. This is due to the fact that the adhesives protrude to the interface, affecting the value of bond strength, and cause scatter of the measured value.
6 Surface Treatment for Improving the Bone Compatibility
Maximum temperature of the plasma can reach 10,000 °C or higher. Therefore, the crystal structure of HAp changes due to thermal decomposition and bond strength to substrate decrease. This is caused by the formation of the amorphous calcium phosphate of low crystallinity. Low-pressure plasma spraying can prevent this phenomenon.
Spraying of HAp was performed in a vacuum chamber. Figure 7.11a shows the cross section of the layer formed by plasma-sprayed HAp on the titanium substrate. Figure 7.11b shows the cross section of two-layer coating formed with plasma-sprayed HAp after plasma spraying of titanium for enhanced adhesion of HAp layer [13]. Because the thickness of the HAp film varies from 10 to 200 μm, both adhesion and bond strength may change; testing bond strength in a unified manner has been required [14]. Other method of film formation is the high-velocity oxygen fuel (HVOF) spraying. By using oxygen gas combustion, the flight speed of the thermal spraying powder is faster than that of plasma spraying. The density of formed layer and the adhesion to the substrate are improved. There is also the cold spray method. It is a method of high-speed collision without melting the HAp particles and, therefore, holds the crystallinity of HAp. Research has been conducted such as sputtering [15] or the powder jet deposition method [16] of forming a film at room temperature by injecting particles. By using these methods, HAp-coated titanium showed good fixation to the bone. It is proven to be effective in the initial period of implant, but the peeling has been a problem in the long term. Research and development on surface modification and application techniques of bioactive performance of the metal-based materials will be actively carried out in the future.
7 Summary
-
1.
Bond strength between Ti-alloy and HAp thin film can be measured by the method based on a tensile test, which is proposed by our group.
-
2.
To obtain accurate bond strength, diameter size of specimen should be large. It minimizes the effects of adhesive overflowing and imperfect alignment.
Our method is similar to the ASTM F1147-05 [17]. However, this standard is intended for a film made in various ways. The subject of our method is a thin film that has been produced by plasma spraying. In addition, determination of the bond strength is assured by restricting a fracture within a film. In addition, there is ASTM F 1044–05 [18]. That evaluates the bond strength by shear. We also have to evaluate the bond strength in shear. However, the ASTM standards include some ambiguous factors: substrate surface, surface condition prior to coating, coating method, bonding method, heat treatment, and a fixing method to the tensile testing machine. As a result, a large error occurs in bond strength values obtained. It makes organizing the results difficult. We are planning to clarify these points. And we are considering how this method can be easily used in many fields.
References
Gruen T (1979) “Modes of failure” of cemented stem type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 141:17–27
Takayama A, Naito M, Akiyoshi Y, Kumano T, Shitama H, Shinoda A, Kiyama T, Maeyama A, Teratani T (2007) The short-term follow up study of the cementless THA using the CentPillar Stem. Hip joint 33:249–251
Neo M, Kotani S, Fujita Y, Nakamura T, Yamamuro T, Bando Y, Ohtsuki C, Kokubo T (1992) Differences in ceramic–bone interface between surface-active ceramics and resorbable ceramics: a study by scanning and transmission electron microscopy. J Biomed Mater Res 26:255–267
Hisamori N (2014) Introduction of biomaterials for beginners −1: present condition of biomaterials and its application. J Soc Mater Sci Jpn 63:417–423
Kokubo T, Miyaji F, Kim HM, Nakamura T (1996) Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc 79:1127–1129
Reis RL, Monteiro FJ, Hastings GW (1994) Stability of hydroxylapatite plasma-sprayed coated Ti-6Al-4V under cyclic bending in simulated physiological solutions. J Mater Sci Mater Med 5:457–462
Tomita N, Kitakura T, Onmori N, Ikada Y, Aoyama E (1999) Prevention of fatigue cracks in ultrahigh molecular weight polyethylene joint components by the addition of vitamin E. J Biomed Mater Res Part A 48:474–478
Toyoda M (1990) On strength of interface of bonded joints of dissimilar materials. J Mech Heterog Mater 59:34–40
Fukuda M (1992) Experimental study on the histocompatibility and bonding strength to bone of zirconia dispersed hydroxyapatite ceramics: strength to bone of zirconia dispersed hydroxyapatite ceramics. Jpn J Oral Maxillofac Surg 38:1718–1737
Inoue Y, Ono T, Noutomi A, Izuha A, Toyoda M, Tsukamoto M (1991) Applicability of stress singularity parameter for evaluating adhesive strength of coating film. (Phase 1). Adhesive strength evaluation of plasma sprayed coatings by tensile pin test: applicability of stress singularity parameter for evaluating adhesive strength of coating film (Phase 1). Quar J Jpn Weld Soc 9:167–173
Inagaki M, Kameyama T (2007) Biomaterial coatings using plasma processing. J Plasma Fusion Res 83:595–600
Heimann RB (2006) Thermal spraying of biomaterials. Surf Coat Technol 201:2012–2019
Laonapakul T, Otsuka Y, Nimkerdphol RA, Mutoh Y (2012) Acoustic emission and fatigue damage induced in plasma sprayed hydroxyapatite coating layer. J Mech Behav Biomed Mater 8:123–133
Hisamori N (2013) Interface strength evaluation method of medical metal material with thin-film coating. Mechanical Engineering Conference 2013 Japan (MECJ-13). CD-ROM F041004 (2013) Trans Jpn Soc Mech Eng
Ueda K, Narushima T, Goto T, Katsube T, Nakagawa H, Kawamura H, Taira M (2007) Evaluation of calcium phosphate coating films on titanium fabricated using RF magnetron sputtering. Mater Trans 48:307–312
Sasaki K, Kuriyagawa T (2006) Caries treatment procedure based on nano-precision mechanical manufacturing. J Jpn Soc Biomater 24:244–250
ASTM F 1147–05. Standard test method for tension testing of calcium phosphate and metallic coatings 1
ASTM F 1044–05. Standard test method for shear testing of calcium phosphate coatings and metallic coatings 1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hisamori, N. (2015). Adhesive Strength of Bioactive Surface Layer. In: Niinomi, M., Narushima, T., Nakai, M. (eds) Advances in Metallic Biomaterials. Springer Series in Biomaterials Science and Engineering, vol 4. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46842-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-46842-5_7
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46841-8
Online ISBN: 978-3-662-46842-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)