Abstract
Peanut is a good source of dietary protein. Raising protein content in peanut will not only fill the growing need for vegetable protein, but also in most cases lower oil content, which is good news to health-conscious populations. However, no attempt has been made to isolate genes related to protein content in peanut. In the present study, a total of 40 unique differentially expressed genes in developing seeds of high-protein peanut mutant (SDPM) and its normal-protein wild type (SDPW) at 46 or 49 days after flowering were isolated using Genefishing technology. Of them, 8 sequences were undescribed previously; the rest 32 were found to be significantly similar to the sequences in GenBank nr database. Three genes potentially related to protein content in peanut, viz., P2-2-2, P2-92-2 and P1-89-1-5 with high homology to thioredoxin h, arachin ahy-4 and abc transporter, respectively, were selected for further analysis. All the 3 genes validated by qRT-PCR showed differential expression between SDPM and SDPW, with relative expression ranging from 0.41–10.60. The detailed functions of the differentially expressed genes isolated from developing seeds in the present study in conditioning peanut seed protein content are yet to be validated by transgenic experiments.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The cultivated peanut, Arachis hypogaea L., as a major cash crop valued worldwide, is cultivated in more than eighty countries/regions. In western world, peanut is a main dietary protein source. In developing nations, even though most peanut produce is crushed for edible oil, the portion for food uses has been on a steady increase. Raising protein content in peanut seeds will not only fill the growing need for vegetable protein, but also in most cases lower oil content, which is good news to health-conscious populations. To lower oil content, defatting is generally useful; however, it cannot be applied to peanut for in-shell consumption.
In literature, Spanish- or Valencia-type peanut landraces/cultivars with high-protein content are not uncommon, and there are reports on heritability, heterosis and combinability for peanut protein content [1]. Recently, [2] analyzed the data from 2 environments, and concluded that protein content in peanut is conditioned by polygenes. Quantitative trait loci (QTLs) with lower than 15 % contribution to phenotypic variations have been reported from two independent research groups [3, 4]. But to the best of our knowledge, no attempt has been made to isolate genes related to this valuable trait.
The present study represented an effort aimed at isolation of candidate genes governing protein content in peanut through Genefishing technology using a large-podded peanut genotype and its high-protein chemical mutant.
2 Materials and Methods
2.1 Peanut Materials
SDPM, a peanut EMS (ethyl methane sulfonate) mutant with 28.67 % protein, developed at Shandong Peanut Research Institute (SPRI), Qingdao, China, and its wild type counterpart with 17.69 % protein (SDPW) were used in the present study. The high-protein mutant was first identified in near infra-red reflectance spectroscopy (NIRS) analysis of intact seeds [5] and further confirmed by nitrogen amount determination using an automated Kjeldahl Analyzer (model 2300 II, Foss, Sweden). A conversion factor of 5.46 was used to convert the amount of nitrogen to amount of protein [6].
2.2 Peanut Cultivation and Seed Sampling
Peanut seeds were sown in field under polythene film mulch and routine agronomic practices were followed as per the description by Wan et al. [7]. Plant population was 150,000 hills per ha, with two plants per hill. Flowers on the first and second node of the cotyledonary branches were tagged and pods were harvested 46 and 49 days after flowering (DAF). Seeds were then stored in liquid nitrogen.
2.3 RNA Isolation
Total RNA was extracted from developing seeds of SDPM and SDPW using RNAprep pure Plant Kit (Tiangen, Beijing, China) following manufacturer’s instructions. RNA concentration and integrity were determined by spectrophotometry and relative intensity of brightness of GelRed (Biotium, CA, USA) stained bands resolved on a 1.2 % agarose gel [8].
2.4 Cloning and Sequence Analysis of DEGs
Differentially expressed genes (DEGs) from developing seeds of SDPM or SDPW harvested at 46 DAF and 49 DAF were identified using GenefishingTM DEG Premix Kit (Seegene, Korea). RNase-free water was added to the mixture of 3 μg of total RNA and 2 μl of 10 μM dT-ACP1 to a total volume of 9.5 μl. The mixture was incubated at 72 °C for 10 min., cooled on ice for 2 min., centrifuged briefly, and then 4 μl of 5 × RT buffer, 5 μl of 2 mM dNTP mix, and 0.5 μl of RNase inhibitor (40 U/μ1) (Tiangen, Beijing, China) along with 200 U of M-MLV reverse transcriptase (TaKaRa, Japan) were added. Reverse transcription was conducted at 42 °C for 90 min, followed by incubation at 70 °C for 10 min to terminate the reaction. First strand cDNA products were then diluted with 80 μl of DNase-free water and directly used in subsequent isolation and analysis of differentially expressed genes from peanut using Genefishing PCR. PCR mixture (20 μl) contained 50 ng of first strand cDNA, 0.5 μM arbitrary ACP, 0.5 μM dT-ACP2 and 2 × SeeAmp ACP Master-mix. PCR program was 94 °C for 5 min, 50 °C for 3 min and 72 °C for 1 min, followed by 40 cycles of 94 °C for 40 s, 65 °C for 40 s and 72 °C for 40 s, and a final extension of 72 °C for 5 min. PCR products were separated on a 2 % agarose gel, stained with Gelred and visualized under UV light. Amplicons of interest from treated samples were cloned into a pGM-T vector (Tiangen, Beijing, China), and sequenced by Genscript Inc., Nanjing, China. Resultant sequences, after trimmed to remove poor quality reads and vector sequences, were assembled with DNAStar (DNASTAR Inc., London, UK) package. Transcript annotation and functional assignment were carried out with BLAST2GO (http://blast2go.org).
2.5 qRT-PCR
Realtime PCR primers were designed using Beacon Designer 7 (Premier Biosoft International, CA, USA) (Table 5.1). qRT-PCR was performed in a Lightcycler 2.0 PCR machine (Roche, USA) as per Tang et al. (2011), with 3 replications for each reaction. β-actin gene was utilized as an internal control. Fold changes in RNA transcripts were calculated using the 2−ΔΔCt method.
3 Results and Discussion
Of 36 primers (ACPs) provided with the Genefishing kit, 14 produced bands that could clearly differentiate SDPM and SDPW (Fig. 5.1). Cloning, sequencing and assembly of the DEGs resulted in a total of 40 unique sequences (Table 5.2). There were 8 sequences undescribed previously; the rest 32 DEGs were found to be significantly similar to the sequences in GenBank nr database, of which 12 and 20 DEGs were from SDPW and SDPM, respectively.
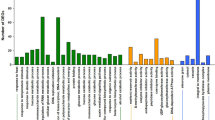
Three of the unique DEGs potentially related to protein content in peanut, viz., P2-2-2, P2-92-2 and P1-89-1-5 with high homology to thioredoxin h, arachin ahy-4 and abc transporter, respectively, were selected for further analysis. The annotation results were shown in Table 5.3. Relative expression of the 3 genes between high-protein EMS mutant (SDPM) and normal-protein wild type (SDPW) in developing seeds harvested at 46 DAF or 49 DAF was illustrated in Fig. 5.2. Notably, expression of P2-92-2 (arachin ahy-4) at 49 DAF in SDPM was 10.60 times as high as in SDPW. Expression of the same 3 genes in seeds of SDPM harvested at 49 DAF relative to the expression at 46 DAF was shown in Fig. 5.3. P2-92-2 exhibited a marked increase in expression at 49 DAF as compared with that at 46 DAF.
Relative expression of 3 genes between the high-protein EMS mutant (SDPM) and the normal-protein wild type (SDPW) in developing seeds harvested at 46DAF(46d) or 49DAF(49d), respectively. Relative expression of SDPM was computed based on the corresponding gene expression of SDPW. The error bars indicate standard deviation of mean
Through NIRS-aided selection of mutagenized populations, SPRI scientists were able to identify peanut quality mutants, providing materials for the present study [5]. In contrast to randomly selected peanut materials with different protein content, mutant and wild types have similar genetic backgrounds, thereby precluding a large number of genes unrelated to the target trait when transcriptional profiling strategy is used.
Thus far, there have been few studies on genes related to protein content in plants [9]. Two reports have shown that PEPC (encoding phosphor-enolpyruvate carboxylase) and VfAAP (encoding Vicia faba amino acid permease) were genes conditioning protein content in legume seeds. As compared with untransformed control, transgenic bean seeds with the overexpression construct of PEPC accumulated up to 20 % more protein per gram seed dry weight [10]. Overexpression of the transporter gene VfAAP in pea resulted in 43 % increase in total globulins production in seeds [11].
In the present study, through comparison of gene transcription in developing seeds of a high-protein mutant and its normal-protein wild type peanut genotype at the stage of rapid seed protein accumulation (46 DAF and 49 DAF), totally 40 DEGs were isolated using GenefishingTM technology. All the 3 DEGs further validated by qRT-PCR showed differential expression between the high-protein mutant and the peanut wild type, with relative expression ranging from 0.41–10.60. As indicated by BLAST2GO analysis, the 3 genes may have protein disulfide oxidoreductase activity, nutrient reservoir activity or ATPase/transporter activity, respectively. Further studies are still needed to validate the differential expression of the rest genes by qRT-PCR and to investigate the detailed and exact functions of confirmed DEGs through transgenic experiments.
References
Yu SL, Wang CT, Yang QL et al (2011) Peanut genetics and breeding in China. Shanghai Science and Technology Press, Shanghai, pp 18–24
Zhang XY, Han SY, Xu J et al (2011) Genetic analysis of protein using major gene plus polygene methods in peanut (Arachis hypogaea L.). Chinese J Oil Crop Sci 33(2):118–122
Liang X, Zhou G, Hong X et al (2009) Overview of research progress on peanut (Arachis hypogaea L.) host resistance to aflatoxin contamination and genomics at the guangdong academy of agricultural sciences. Peanut Sci. 36:29–34
Sarvamangala C, Gowda MVC, Varshney RK (2011) Identification of quantitative trait loci for protein content, oil content and oil quality for groundnut (Arachis hypogaea L.). Field Crops Res 122:49–59
Wang CT, Zhang JC, Tang YY et al (2013) Peanut genetic improvement. Shanghai Science and Technology Press, Shanghai, p 531
Wang CT, Tang YY, Wang XZ et al (2011) Evaluation of groundnut genotypes from China for nutritional quality. J SAT Agric. Res. vol. 9. Available at: http://ejournal.icrisat.org/Volume9/Groundnut/Evaluation.pdf. (Accessed 31 May 2013)
Wan SB (ed) (2003) China peanut cultivation science. Shanghai Science & Technology Press, Shanghai pp 408–430
Tang YY, Wang CT, Yang GP et al (2011) Identification of chilling-responsive transcripts in peanut (Arachis hypogaea L.). Electronic J. Biotechnol. 14(5). DOI: 10.2225/vol14-issue5-fulltext-5
Weber H, Sreenivasulu N, Weschke W (2010) Molecular physiology of seed maturation and seed storage protein biosynthesis. In: Pua EC, Davey MR (eds) Plant developmental biology–biotechnological perspectives, Springer, Berlin Heidelberg, pp 83–104
Rolletschek H, Borisjuk L, Radchuk R et al (2004) Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol J 2(3):211–219
Rolletschek H, Hosein F, Miranda M et al (2005) Ectopic expression of an amino acid transporter (VfAAP1) in seeds of Vicia narbonensis and pea increases storage proteins. Plant Physiol 137(4):1236–1249
Acknowledgments
We wish to express our sincere thanks to the financial support from China Agricultural Research System (CARS-14), Liaoning Natural Science Foundation Project for Fostering Talent (2014027029), Key Project of Liaoning Provincial Science and Technology Department (2011201021) and China National Science and Technology Support Project (2012BAD36B00).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Yu, S.T. et al. (2015). Isolation of Differentially Expressed Genes from Developing Seeds of a High-Protein Peanut Mutant and Its Wild Type Using GenefishingTM Technology. In: Zhang, TC., Nakajima, M. (eds) Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, vol 332. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45657-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-662-45657-6_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45656-9
Online ISBN: 978-3-662-45657-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)