Abstract
Should esophagogastric junction (EGJ) cancer be classified or managed as gastric cancer or esophageal cancer or else? The debate focused on the issue has still remained [1], though EGJ cancer is increasing worldwide, in Asia [2–5] as well as western countries [6–8]. Squamous cell carcinoma developed in EGJ region is unanimously treated as esophageal cancer. For EGJ adenocarcinoma, Siewert classification has been widely applied: type I (adenocarcinoma of the distal esophagus), tumors with an epicenter located more than 1 cm above the EGJ; type II (true cardia cancer), tumors with an epicenter located within 1 cm oral and 2 cm aboral from the EGJ; and type III (subcardial cancer), tumors with an epicenter located below 2 cm from the EGJ [9, 10]. Among Siewert classifications, Siewert type I and III tumors are usually managed like esophageal and gastric cancers, respectively. However, in 7th AJCC TNM classification, both Siewert type II and III tumors had been classified as esophageal cancer. After some discussions [11–13], in 8th version, type III was changed to gastric cancer classification, while type II still stays in esophageal classification [14–15]. There have been many papers regarding the clinicopathologic features of Siewert type II tumors to identify the pathogenesis and appropriate treatment strategy. Some papers showed that the characteristics of Siewert type II were quite similar with gastric cancers [11, 12]. However, some papers reported that there were two distinct pathways of tumorigenesis of EGJ adenocarcinoma, related or unrelated to intestinal metaplasia, gastric atrophy, and gastric acid secretion [16–17]. The etiology as well as the treatment strategy has still remained controversy, especially for Siewert type II tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Gastric Cancer or Esophageal Cancer or Else?
Should esophagogastric junction (EGJ) cancer be classified or managed as gastric cancer or esophageal cancer or else? The debate focused on the issue has still remained [1], though EGJ cancer is increasing worldwide, in Asia [2,3,4,5] as well as western countries [6,7,8]. Squamous cell carcinoma developed in EGJ region is unanimously treated as esophageal cancer. For EGJ adenocarcinoma, Siewert classification has been widely applied: type I (adenocarcinoma of the distal esophagus), tumors with an epicenter located more than 1 cm above the EGJ; type II (true cardia cancer), tumors with an epicenter located within 1 cm oral and 2 cm aboral from the EGJ; and type III (subcardial cancer), tumors with an epicenter located below 2 cm from the EGJ [9, 10]. Among Siewert classifications, Siewert type I and III tumors are usually managed like esophageal and gastric cancers, respectively. However, in 7th AJCC TNM classification, both Siewert type II and III tumors had been classified as esophageal cancer. After some discussions [11,12,13], in 8th version, type III was changed to gastric cancer classification, while type II still stays in esophageal classification [14–15]. There have been many papers regarding the clinicopathologic features of Siewert type II tumors to identify the pathogenesis and appropriate treatment strategy. Some papers showed that the characteristics of Siewert type II were quite similar with gastric cancers [11, 12]. However, some papers reported that there were two distinct pathways of tumorigenesis of EGJ adenocarcinoma, related or unrelated to intestinal metaplasia, gastric atrophy, and gastric acid secretion [16–17]. The etiology as well as the treatment strategy has still remained controversy, especially for Siewert type II tumors.
In the EGJ region, the different tissues to be potentially cancerous lesion are known to exist: esophageal gland, Barrett epithelium, cardiac gland, fundic glands, etc. Therefore, some Siewert type II tumors might have different biological features from the esophageal and gastric cancers and, if so, should be regarded as the independent disease. Investigation by cancer genetics, etc. will be undoubtedly needed in the future.
Surgical Procedures
There have been various surgical approaches for EGJ cancers: Ivor Lewis (right thoracic and abdominal), left thoracoabdominal, transhiatal, and abdominal ones. Among them, Ivor Lewis or transhiatal approaches are mainly applied to Siewert type II tumor [18]. In the former, esophagectomy through right thoracotomy with reconstruction by gastric conduit and intrathoracic anastomosis is usually performed like esophageal cancer, while in the latter, extended total gastrectomy is done like gastric cancer. Why are those quite different procedures performed to Siewert type II EGJ cancer? Recent paper described that the choice of approach has been still based on surgeon’s discretion [19]. A recent web-based worldwide questionnaire demonstrated that the majority of surgeons favor an extended gastrectomy for Siewert type II tumors (66% vs 27%) [20], while the big data based on 4996 NSQIP/SEER patients showed that esophagectomy was more frequently performed than gastrectomy (71% vs 29%) in the USA [21]. When the patients with Siewert type II tumor refer to thoracic surgeons, Ivor Lewis is likely to be indicated. Conversely, the abdominal surgeons prefer the transhiatal approach for Siewert type II tumors. The potential differences between the east and west in EGJ tumor biology were recently pointed out, and the optimal surgical approach in western countries was concluded to be Ivor Lewis [22]. That is why there have been two quite different approaches.
Several papers compared the short- and long-term outcomes between the esophagectomy and gastrectomy. No significant differences of postoperative morbidity and mortality were observed between those procedures [21, 23, 24]. As for the survival after surgery, two papers showed the better results in the esophagectomy than the gastrectomy groups [21, 23], while no difference between them was found in one report [24]. The comparison of long-term quality of life (QOL) between the two groups demonstrated the better QOL after the gastrectomy than the esophagectomy [25]. This should be considered at the decision of surgical procedures. Recently, a strong worldwide trend toward minimally invasive surgery is observed [20], and it is increasing gaining popularity over open surgery [19]. Intent to perform the minimally invasive surgery for EGJ cancers is, also, absolutely important. Laparoscopic resection of Siewert type II tumors was reported to be feasible and oncologically equivalent as compared to open procedures [26].
Lymphadenectomy
There have been many papers focusing the lymphadenectomy for EGJ cancers, to date. However, the strategy of lymphadenectomy, especially for Siewert type II tumors, has still remained controversial. The standard lymph node (LN) dissection for type II tumors has not been established, to date. However, most papers showed the significance of lymphadenectomy in the pericardial, lesser curvature and at the foot of the left gastric artery nodes (no.1, 2, 3, 7 LNs) [27,28,29]. Therefore, those LNs should be routinely dissected in all surgical cases with EGJ cancers, regardless of advanced or early stages. Optimal extent of lymph node dissection in the mediastinum for Siewert type II tumors has not been established. However, many papers showed the survival impact of lymphadenectomy in the lower mediastinum [27,28,29,30], while one paper described that mediastinal LN dissection was not essential in early Siewert type II tumors [31]. And, based on the analysis of recurrence pattern and lymph node metastasis, the complete mediastinal LN dissection was reported to be not mandatory for type II tumors arising within the stomach [32]. One paper showed that left renal vein nodal involvement (no.16a2; para-aortic LN) had a similar survival impact with the lower mediastinal and celiac axis LNs [29]. Now, multi-institutional prospective clinical trial to evaluate the significance of the lower mediastinal and no. 16a2 LN dissections for Siewert type II tumors is ongoing under the collaboration of the Japanese Gastric Cancer Association (JGCA) and the Japanese Esophageal Society (JES).
LNs along the distal portion of the stomach (no. 4, 5, 6) are located far from EGJ, though those LNs are simultaneously dissected when the total gastrectomy is performed. Many papers reported the poor prognosis and marginal therapeutic value of the Siewert type II cases with the nodal involvement in those LNs [27,28,29, 33]. The extended abdominal lymphadenectomy was suggested to improve survival because the poorer survival after D1 lymphadenectomy was shown in comparison with D1+/D2 lymphadenectomy [24]. Consistently, the nodal involvement around celiac axis (no. 9) was shown to impact the survival [29, 34].
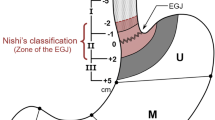
To evaluate the optimal extent of LN dissection during EGJ cancer surgery, the JGCA and the JES conducted a nationwide survey to characterize the LN spread pattern of EGJ cancer in a large cohort. That was a questionnaire-based national retrospective study, in which clinical records of 3177 patients underwent R0 resection between 2001 and 2010 at the member hospitals of the JGCA and/or the JES were collected. And, the tumors of 40 mm or less in dimension were selected since large tumors were apparently associated with poor macroscopic recognition of the anatomical EGJ. In Japan, EGJ cancers are defined as its epicenter within 2 cm proximal or distal to the EGJ according to the Japanese classification system (Nishi’ classification), regardless of histological type. Among those 3177 patients, 2601 cases were proven to be histologically adenocarcinoma. The results were summarized in the previous paper [35]. The annual number of surgical cases was observed to increase steadily since 2001, especially for adenocarcinoma, in Japan. Figures 20.1, 20.2, 20.3, and 20.4 show the rates of the dissection (red bar) and LN metastasis (blue bar) according to each LN stations of all 2418 adenocarcinoma cases, 1430 early cases, 988 advanced cases, and 234 advanced cases with its epicenter within esophagus, respectively. The numbers of LN stationed are based on the Japanese classification. The cases with neoadjuvant therapy were excluded from the analysis. No. 100–112 and 107–109 LNs are located in the lower and middle mediastinum, respectively. All figures were shown in the final report of the abovementioned study (not published but delivered to the participating hospitals). The results were quite consistent with previous reports. Nodes along the distal portion of the stomach (no. 4, 5, 6) were much less often metastatic in any stages, though those were dissected in most cases. And, survival analysis failed to show the benefit of those dissections. Lower mediastinal LN dissection might contribute to improve survival for the EGJ cancer with esophagus-predominance or esophageal invasion.
Our Surgical Procedure
When more than half stomach can be preserved, the proximal gastrectomy (PG) is chosen, because the nutritional status after the PG were shown to be better than after the total gastrectomy (TG) by many studies [36,37,38], and no survival difference was observed between those procedures [39]. Furthermore, as the previous results showed, the dissections of no. 4, 5, and 6 LNs are not necessary for the radical resection of EGJ cancers. We think that the TG should be avoided at the utmost. Usually, the stomach of 12 cm or more along the lesser curvature and 25 cm or more along the greater curvature from the pylorus ring is preserved (Fig. 20.5).
When the lower mediastinal LN dissection is considered to be beneficial, no. 110, 111, and 112 LNs are usually dissected (Video 20.1). Both side pleura are preserved, and the inferior pulmonary vein is a point of the upper margin of dissection. The reconstruction is usually done by jejunal interposition (JI) (Video 20.2). Frozen section analysis for the margin is usually submitted to confirm no cancer cells in the resection line. The jejuno-gastrostomy is created on the posterior wall of the remnant stomach by the circular stapler. That anastomotic site is at 5 cm distance from the resection line of the stomach (Fig. 20.6). The length from the esophagojejunostomy to jejuno-gastrostomy is 8 cm. Some paper recommends the 15–25 cm distance between those anastomoses [40], but our data (Fig. 20.7, not published) showed that the frequency of reflux esophagitis was lower after the short than the long JIs. When the lower mediastinal LN dissection is thought to be unnecessary, laparoscopic proximal gastrectomy is done followed by esophagogastrostomy. That anastomotic site is at the anterior wall with 5 cm distance from the resection line of the stomach (Fig. 20.8). And, several stiches between the esophagus and stomach are added to fix and prevent the reflux. Severe reflux esophagitis after PG has not been developed in our recent cases, though higher frequency of reflux esophagitis after PG was previously reported to be than TG [41].
References
Van Laethem JL, Carneiro F, Ducreux M, Messman H, Lordick F, Ilson DH, Allum WH, Haustermans K, Lepage C, Matysiak-Budnik T, Cats A, Schmiegel W, Cervantes A, Van Cutsem E, Rougier P, Seufferlein T. The multidisciplinary management of gastro-oesophageal junction tumours: European Society of Digestive Oncology (ESDO): expert discussion and report from the 16th ESMO World Congress on Gastrointestinal Cancer, Barcelona. Dig Liver Dis. 2016;48(11):1283–9. https://doi.org/10.1016/j.dld.2016.08.112. Epub 2016 Aug 20
Wang K, Yang CQ, Duan LP, Yang XS, Xia ZW, Cui RL, Jin Z, McNutt M. Changing pattern of adenocarcinoma of the esophagogastric junction in recent 10 years: experience at a large tertiary medical center in China. Tumori. 2012;98(5):568–74. https://doi.org/10.1700/1190.13196.
Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg. 2016;263(1):88–95. https://doi.org/10.1097/SLA.0000000000001148.
Hatta W, Tong D, Lee YY, Ichihara S, Uedo N, Gotoda T. Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig Endosc. 2017;29(Suppl 2):18–25. https://doi.org/10.1111/den.12808.
Koizumi S, Motoyama S, Iijima K. Is the incidence of esophageal adenocarcinoma increasing in Japan? Trends from the data of a hospital-based registration system in Akita Prefecture, Japan. J Gastroenterol. 2017; https://doi.org/10.1007/s00535-017-1412-4. [Epub ahead of print]
Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7. https://doi.org/10.1093/jnci/djn211. Epub 2008 Aug 11
Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomark Prev. 2010;19(6):1468–70. https://doi.org/10.1158/1055-9965.EPI-10-0012. Epub 2010 May 25
Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23(1):3–9. https://doi.org/10.1016/j.semradonc.2012.09.008.
Siewert JR, Hölscher AH, Becker K, Gössner W. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg. 1987;58(1):25–32. Chirurg 2003 Aug;74(8):703–8
Stein HJ, von Rahden BH, Höfler H, Siewert JR. Carcinoma of the oesophagogastric junction and Barrett’s esophagus: an almost clear oncologic model? Chirurg. 2003;74(8):703–8.
Suh YS, Han DS, Kong SH, Lee HJ, Kim YT, Kim WH, Lee KU, Yang HK. Should adenocarcinoma of the esophagogastric junction be classified as esophageal cancer? A comparative analysis according to the seventh AJCC TNM classification. Ann Surg. 2012;255(5):908–15. https://doi.org/10.1097/SLA.0b013e31824beb95.
Mullen JT, Kwak EL, Hong TS. What’s the best way to treat GE junction tumors? Approach like gastric cancer. Ann Surg Oncol. 2016;23(12):3780–5. Epub 2016 Jul 26
Adeshuko FA, Squires MH, Poultsides G, Pawlik TM, Weber SM, Schmidt C, Votanopoulos K, Fields RC, Maithel SK, Cardona K. A multi-institutional study comparing the use of the American joint committee on cancer 7th edition esophageal versus gastric staging system for gastroesophageal junction cancer in a Western population. Am Surg. 2017;83(1):82–9.
Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–30. https://doi.org/10.21037/acs.2017.03.14.
Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):304–17. https://doi.org/10.3322/caac.21399. Epub 2017 May 26
Nunobe S, Nakanishi Y, Taniguchi H, Sasako M, Sano T, Kato H, Yamagishi H, Sekine S, Shimoda T. Two distinct pathways of tumorigenesis of adenocarcinomas of the esophagogastric junction, related or unrelated to intestinal metaplasia. Pathol Int. 2007;57(6):315–21.
Horii T, Koike T, Abe Y, Kikuchi R, Unakami H, Iijima K, Imatani A, Ohara S, Shimosegawa T. Two distinct types of cancer of different origin may be mixed in gastroesophageal junction adenocarcinomas in Japan: evidence from direct evaluation of gastric acid secretion. Scand J Gastroenterol. 2011;46(6):710–9. https://doi.org/10.3109/00365521.2011.565069.. Epub 2011 Mar 30
Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP. Oesophago-gastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol. 2011;12(3):296–305. https://doi.org/10.1016/S1470-2045(10)70125-X.. Epub 2010 Nov 23
Jezerskyte E, van Berge Henegouwen MI, Cuesta MA, Gisbertz SS. Gastro-esophageal junction cancers: what is the best minimally invasive approach? J Thorac Dis. 2017;9(Suppl 8):S751–S60. https://doi.org/10.21037/jtd.2017.06.56.
Haverkamp L, Seesing MF, Ruurda JP, Boone J, V Hillegersberg R. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus. 2017;30(1):1–7. https://doi.org/10.1111/dote.12480.
Martin JT, Mahan A, Zwischenberger JB, McGrath PC, Tzeng CW. Should gastric cardia cancers be treated with esophagectomy or total gastrectomy? A comprehensive analysis of 4,996 NSQIP/SEER patients. J Am Coll Surg. 2015;220(4):510–20. https://doi.org/10.1016/j.jamcollsurg.2014.12.024. Epub 2014 Dec 29
Giacopuzzi S, Bencivenga M, Weindelmayer J, Verlato G, de Manzoni G. Western strategy for EGJ carcinoma. Gastric Cancer. 2017;20(Suppl 1):60–8. https://doi.org/10.1007/s10120-016-0685-2. Epub 2016 Dec 30
Blank S, Schmidt T, Heger P, Strowitzki MJ, Sisic L, Heger U, Nienhueser H, Haag GM, Bruckner T, Mihaljevic AL, Ott K, Büchler MW, Ulrich A. Surgical strategies in true adenocarcinoma of the esophagogastric junction (AEG II): thoracoabdominal or abdominal approach? Gastric Cancer. 2018; https://doi.org/10.1007/s10120-017-0746-1. [Epub ahead of print].
Kneuertz PJ, Hofstetter WL, Chiang YJ, Das P, Blum M, Elimova E, Mansfield P, Ajani J, Badgwell B. Long-term survival in patients with gastroesophageal junction cancer treated with preoperative therapy: do thoracic and abdominal approaches differ? Ann Surg Oncol. 2016;23(2):626–32. https://doi.org/10.1245/s10434-015-4898-0. Epub 2015 Nov 12
Fuchs H, Hölscher AH, Leers J, Bludau M, Brinkmann S, Schröder W, Alakus H, Mönig S, Gutschow CA. Long-term quality of life after surgery for adenocarcinoma of the esophagogastric junction: extended gastrectomy or transthoracic esophagectomy? Gastric Cancer. 2016;19(1):312–7. https://doi.org/10.1007/s10120-015-0466-3. Epub 2015 Jan 28
Sugita S, Kinoshita T, Kaito A, Watanabe M, Sunagawa H. Short-term outcomes after laparoscopic versus open transhiatal resection of Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2018;32(1):383–90. https://doi.org/10.1007/s00464-017-5687-6. Epub 2017 Jun 27
Yamashita H, Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg. 2011;254(2):274–80. https://doi.org/10.1097/SLA.0b013e3182263911.
Hasegawa S, Yoshikawa T, Rino Y, Oshima T, Aoyama T, Hayashi T, Sato T, Yukawa N, Kameda Y, Sasaki T, Ono H, Tsuchida K, Cho H, Kunisaki C, Masuda M, Tsuburaya A. Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann Surg Oncol. 2013;20(13):4252–9. https://doi.org/10.1245/s10434-013-3036-0. Epub 2013 Aug 14
Mine S, Sano T, Hiki N, Yamada K, Nunobe S, Yamaguchi T. Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg. 2013;100(2):261–6. https://doi.org/10.1002/bjs.8967. Epub 2012 Nov 23
Nakamura M, Iwahashi M, Nakamori M, Naka T, Ojima T, Iida T, Katsuda M, Tsuji T, Hayata K, Mastumura S, Yamaue H. Lower mediastinal lymph node metastasis is an independent survival factor of Siewert type II and III adenocarcinomas in the gastroesophageal junction. Am Surg. 2012;78(5):567–73.
Lee IS, Ahn JY, Yook JH, Kim BS. Mediastinal lymph node dissection and distal esophagectomy is not essential in early esophagogastric junction adenocarcinoma. World J Surg Oncol. 2017;15(1):28. https://doi.org/10.1186/s12957-016-1088-x.
Suh YS, Lee KG, Oh SY, Kong SH, Lee HJ, Kim WH, Yang HK. Recurrence pattern and lymph node metastasis of adenocarcinoma at the esophagogastric junction. Ann Surg Oncol. 2017;24(12):3631–9. https://doi.org/10.1245/s10434-017-6011-3. Epub 2017 Aug 21
Wang JB, Lin MQ, Li P, Xie JW, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Zheng CH, Huang CM. The prognostic relevance of parapyloric lymph node metastasis in Siewert type II/III adenocarcinoma of the esophagogastric junction. Eur J Surg Oncol. 2017;43(12):2333–40. https://doi.org/10.1016/j.ejso.2017.08.017. Epub 2017 Sep 8
Anderegg MC, Lagarde SM, Jagadesham VP, Gisbertz SS, Immanuel A, Meijer SL, Hulshof MC, Bergman JJ, van Laarhoven HW, Griffin SM, van Berge Henegouwen MI. Prognostic significance of the location of lymph node metastases in patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Ann Surg. 2016;264(5):847–53.
Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M. Japanese gastric cancer association and the Japan esophageal society. Results of a nation-wide retrospective study of lymphadenectomy for esopha-gogastric junction carcinoma. Gastric Cancer. 2017;20(Suppl1):69–83. https://doi.org/10.1007/s10120-016-0663-8. Epub 2016 Oct 28
Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, Suh YS, Kong SH, Lee KU, Yang HK. Clinical outcome of modified laparoscopy-assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper-third early gastric cancer with special references to postoperative reflux esophagitis. J Gastric Cancer. 2015;15(3):191–200. https://doi.org/10.5230/jgc.2015.15.3.191. Epub 2015 Sep 30
Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, Hisamori S, Miyazaki K, Nakayama T, Sakai Y. Superiority of laparoscopic proximal gastrectomy with hand-sewn esopha-gogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc. 2017;31(9):3664–72. https://doi.org/10.1007/s00464-016-5403-y. Epub 2017 Jan 11
Jung DH, Lee Y, Kim DW, Park YS, Ahn SH, Park DJ, Kim HH. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 2017;31(10):3961–9. https://doi.org/10.1007/s00464-017-5429-9. Epub 2017 Mar 24
Sugoor P, Shah S, Dusane R, Desouza A, Goel M, Shrikhande SV. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: total gastrectomy is not always necessary. Langenbeck’s Arch Surg. 2016;401(5):687–97. https://doi.org/10.1007/s00423-016-1422-3. Epub 2016 May 4
Tao K, Dong JH. Phase I clinical research of jejunal interposition in adenocarcinoma of the esophagogastric junction II/III proximal gastrectomy. Gastroenterol Res Pract. 2016;2016:1639654. Epub 2016 Oct 19
Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257(6):1039–46. https://doi.org/10.1097/SLA.0b013e31828c4a19.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
(MPG 453634 kb)
(MPG 476386 kb)
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
Seto, Y., Yamashita, H., Aikou, S. (2019). Surgery for EG Junction Cancer. In: Noh, S., Hyung, W. (eds) Surgery for Gastric Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45583-8_20
Download citation
DOI: https://doi.org/10.1007/978-3-662-45583-8_20
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45582-1
Online ISBN: 978-3-662-45583-8
eBook Packages: MedicineMedicine (R0)