Abstract
Esophagogastric junctional cancer is classified into three categories according to the Siewert classification, which reflects the epidemiological and biological characteristics. Therapeutic strategies have been evaluated according to the three Siewert types. There is a consensus that types I and III should be treated as esophageal cancer and gastric cancer, respectively. On the other hand, type II is often described as true cardiac cancer, which has different clinicopathological features from the other types. Thus, there is no consensus on the surgical management of type II esophagogastric junctional cancer. The optimal surgical management should focus on the principles of cancer surgery, which take into consideration oncological curability, including an appropriate resection margin, adequate lymphadenectomy, and minimization of postoperative complications. In this review, we evaluate the current relevant literature and evidence, on the surgical treatment of esophagogastric junctional cancer, focusing on type II. Esophagectomy with a thoracic approach has the advantage of ensuring a sufficient proximal resection margin and adequate mediastinal lymphadenectomy. However, the oncological benefit is offset by a high incidence of postoperative complications. Minimally invasive esophagectomy could be a possible solution to reduce complications and improve long-term outcomes. Further development of surgical treatments for Siewert type II is required to improve the outcomes. Furthermore, the surgical team should have expertise in both gastric cancer and esophageal cancer treatment, or patients should be managed with close collaboration between thoracic surgeons and gastric cancer surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
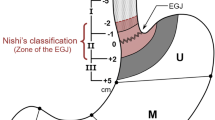
The incidence of esophagogastric junctional cancer has increased in Western countries and in Japan, together with a decrease in Helicobacter pylori infection, an increase in gastroesophageal reflux disease, and an increase in obesity. However, the definition and management of esophagogastric cancer are not simple because EGJ cancer is “a zone disease” rather than “an organ disease” with etiologically heterogeneous backgrounds with characteristics of gastric and esophageal cancers [1]. Siewert et al. [2] established a classification system for esophagogastric junctional adenocarcinoma, which classifies esophagogastric junctional cancer into three types according to the location of the epicenter of the tumor. This classification is used to determine strategy of surgical management. In this classification, Siewert type II tumor, which have an epicenter within 1 cm above and 2 cm below the esophagogastric junction, is often described as a true cardiac cancer, and there is less consensus on the surgical management in comparison to the other two types. This review introduces the previous evidence and theoretical rationale for future perspectives.
Definition of esophagogastric junctional cancer
As mentioned above, Siewert et al. [2] classified esophagogastric junctional adenocarcinoma into three types according to location of the tumor epicenter: type I, located 1–5 cm above the esophagogastric junction regardless of invasion to the esophagogastric junction; type II, invading the esophagogastric junction and located within 1 cm above and 2 cm below the esophagogastric junction; and type III, invading the esophagogastric junction and located 2–5 cm below the esophagogastric junction. Traditionally in Japan, the Nishi classification has been used for defining esophagogastric junctional cancer. This classification defines esophagogastric junctional cancer as a tumor whose epicenter is located within 2 cm above and 2 cm below the esophagogastric junction, regardless of histological type and invasion to the esophagogastric junction, which is almost equivalent to Siewert type II. The Nishi classification reflects the epidemiological and biological characteristics and does not include Siewert type I and III tumors. Siewert type I tumors generally originate in Barrett’s esophagus [3] [4], whereas Siewert type III tumors are associated with Helicobacter pylori infection [3]. In the latest edition of the UICC-TNM classification, a tumor with an epicenter ≤ 2 cm below the esophagogastric junction is staged as esophageal cancer, while a tumor with an epicenter > 2 cm below the junction is staged as gastric cancer. Thus, Siewert type I and II tumors are staged as esophageal cancer, while type III tumors are staged as gastric cancer. On the other hand, the treatment strategy is not simple. Traditionally, Siewert type I tumors have been treated as esophageal cancer, and Siewert type III tumors have been treated as gastric cancer [5,6,7,8,9,10]. However, whether type II tumors should be treated as esophageal or gastric cancer is a problematic issue.
A precise diagnosis of the tumor location is important for optimal surgical management. However, it is sometimes difficult to determine the exact location of the esophagogastric junction preoperatively [11]. It is well known that a precise diagnosis cannot be made for an advanced esophagogastric cancer forming a bulky mass.
Oncological strategy for Siewert type II esophagogastric junctional cancer
Adequate lymphadenectomy
The pathways of lymphatic flow around the esophagogastric junction are known to be complex. In a previous radioisotope study, the lymphatics of the lower esophagus were observed to flow into the lower mediastinum and upper perigastric lymph nodes [12], indicating that EGJ cancer is likely to involve both the abdominal and mediastinum lymph nodes. Thus, to improve patient prognosis, surgeons need to take this lymphatic flow into account when considering the extent of lymphadenectomy.
Many studies [13,14,15,16,17,18,19,20,21,22] have evaluated the pattern of lymph node metastasis in Siewert type II tumors. An outline of studies and rates of lymph node metastasis in each study is provided in Table 1. Figure 1 shows the location of each nodal station. The median lymph node metastatic rate for each station was visually summarized in Fig. 1b. Of the 11 studies, 9 were from Japan and 2 were from the Europe. Basically, 10 studies evaluated the rate of metastasis in each station based on a review of a clinical database, implying that the extent of lymph node resection was not consistent and was judged by each surgeon. On the other hand, in one prospective observational study, the extent of lymph node resection was defined before surgery according to the tumor location Figure 2.
Although the approach and extent of lymph node dissection differ depending on each study, the similarities among these studies are as follows. First, nodes located in the upper stomach (#1, #2, #3) had the highest rate of metastasis. Second, nodes located at the left gastric artery (#7), suprapancreatic nodes (#8a, #9, #11p), and lower mediastinal nodes (#110, #111, #112) had a relatively high rate of metastasis. Third, metastasis to nodes around the distal stomach (#4d, #5, #6, #12a) was rare. Meanwhile, nodes at the paraaortic area (16a2), middle mediastinum, upper mediastinum, and cervix showed different rates of metastasis depending on the report.
In 5 studies [15, 16, 18, 21, 22], the efficacy of lymph node resection was evaluated based on a therapeutic index, which was calculated by multiplying the frequency of metastasis to the station and the 5-year survival rate of patients with metastasis to each station, representing the priority of resection for each node. The therapeutic index in each station demonstrated in 5 studies is summarized in Table 2. In addition, we defined the priority order of nodal dissection by the ranking of the therapeutic index in each nodal station based on each study. Then, we calculated the mean priority order to determine priority of each nodal dissection. In all 5 studies, upper perigastric nodes (#1, #2, #3) and nodes located at the left gastric artery (#7) were of especially high priority. Following those nodes, the nodes of the inferior mediastinum (#110, #111, #112) and the suprapancreatic nodes (#8a, #9, #11p) could be interpreted to have the next priority. In contrast, the priority of the lymph nodes around the lower stomach (#4d, #5, #6, #12a), upper mediastinum (#106rec), and cervical lymph nodes was relatively low. Figure 3 showed the mean priority order. Top priority for nodal dissection was #1 and #2, followed by #2, #7, and #11p. Subsequent priority was #110, #16a, #9, #8a, #17, and #9. The nodes of the middle mediastinum (#107, #108) and periaortic lymph nodes (#16a2) had different priorities depending on the study and require careful interpretation. However, all 5 studies were retrospective in nature, and the extent of nodal dissection was not uniform. The presence or absence of nodal metastasis is evaluable when dissecting the corresponding lymph nodes. As lymph node dissection of the upper and middle mediastinum is difficult by a transhiatal approach, the rates of metastasis of these nodes could be underestimated unless a thoracic approach is selected for all cases.
Kurokawa et al. [23] conducted nationwide multi-center prospective study that identified the accurate rate of metastasis for each mediastinal and abdominal lymph node. Briefly, a transhiatal approach was uniformly selected for patients with esophageal involvement of < 3.0 cm, whereas a right transthoracic approach was uniformly selected for patients with esophageal involvement of > 3.0 cm. In the transthoracic approach, the nodes for which dissection was mandatory included the upper (stations 105, 106recL, 106recR) and middle mediastinal nodes (stations 107, 108, 109L, 109R), in addition to the perigastric field (stations 1, 2, 3a), the suprapancreatic field (stations 7, 8a, 9, 11p, 11d), the paraaortic field (station 16a2lat), the abdominal hiatal field (stations 19, 20), and the lower mediastinal field (stations 110, 111, 112). The results of this study are demonstrated in Table 1 (see #11). In this multi-center prospective study, stations in which metastasis was frequently observed included the perigastric nodes, suprapancreatic nodes, and lower mediastinal nodes, whereas metastasis was less frequently observed among the lower perigastric nodes, confirming the results of previous retrospective studies. On the other hand, the rate of metastasis to the nodes at the upper and middle mediastinum was 2.9% and 1.4–4.3%, respectively, when the length of esophageal invasion was ≤ 4 cm, but reached 10.7% and 7.1%, respectively, when the length was ≥ 4 cm. Based on these results, the latest edition of the JGCA Guidelines provisionally recommended the following lymph node dissection areas for EGJ cancer: upper gastric lymph nodes, suprapancreatic lymph nodes, and lower mediastinal lymph nodes when the length of esophageal invasion is ≤ 4 cm. The middle and upper mediastinal lymph nodes were additionally recommended when the length of esophageal invasion was > 4 cm. However, optimal lymph node dissection should not be based on the rate of metastasis. Long-term survival data from the Kurokawa study are expected to reveal the priority of lymph node dissection according to a therapeutic index.
Ensuring an optimal resection margin
It is generally accepted that the microscopic spread of esophageal cancer is usually far more extensive than the macroscopic boundaries of the primary tumor. In particular, direct submucosal tumor extension or intramural spread without mucosal change is common. Gao et al. [24] reported that 56% of patients with Sievert type I and II tumors had positive submucosal margins without mucosal infiltration. Adequate distance from the gross tumor margin is necessary to ensure that there are no residual tumor cells at the resection margins. Thus, many studies are conducted to identify the optimal distance of the proximal longitudinal margins (Table 3). Matiette et al. [25] reported that a proximal margin of > 8 cm is necessary, while Ito et al. demonstrated that a proximal margin of 6 cm [26] is mandatory. In both studies, the safe distance for the proximal margin was determined by examining the length from the gross tumor edge to the proximal end of the resection specimen in patients with no residual tumor cells. Meanwhile, Barabur et al. [7] investigated the correlation between the distance of the resection margin and survival, suggesting that proximal margin length of > 3.8 cm in the resected specimen was one of independent prognostic factors. Similarly, Mine et al. [27] examined the length of the proximal margin which affects the prognosis, concluding that gross proximal margin length of > 2.0 cm was an independent prognostic factor. In the 2 studies examining the prognostic impact of margin length, the recommended margin lengths were shorter than the former two studies examining margin lengths that ensured there was no residual cancer cell at the proximal margin. Since advanced EGJ cancer has a poor prognosis even when radical resection is achieved, it may be appropriate to consider the prognosis rather than the possibility of residual tumor cells at the resection margin. There are concerns regarding the determination of the optimal resection margin for patients with Sievert type II adenocarcinoma. First, the length of the esophagus resected is influenced by the methods of anastomosis and organ reconstruction, including surgical approach. The length of esophagus resected is limited in the transhiatal approach in comparison to the transthoracic approach, implying that the transthoracic approach is likely to be superior to the transhiatal approach in terms of ensuring oncological safety of the proximal margin. However, the surgical approach was determined by a variety of factors, including surgeon preference and the risk of postoperative complications. Various factors should be considered when determining the optimal resection margin. Second, the esophagus shrinks immediately after resection; thus, the length in vivo before resection is different from the length measured after resection ex vivo. Although a previous study [28] reported that the esophagus shrinks by approximately 50% from its in vivo length after resection, it is exactly unclear how long the resection margin length in vivo corresponds to the ex vivo length evaluated in previous studies. To summarize, to completely ensure that there are no residual tumor cells at the resection margin, a margin of at least 5 cm ex vivo is required. However, considering the risk of surgery and other factors that affect the prognosis, a proximal resection margin > 2.0 cm on the resected specimen is recommended as an appropriate length and has been shown to be associated with the prognosis.
Surgical strategy
Which is better a thoracic approach or a transhiatal approach?
Esophagectomy with a thoracic approach has the advantage of ensuring a sufficient proximal resection margin and adequate mediastinal lymphadenectomy. However, this requires a two-field incision and is associated with a higher degree of surgical stress in comparison to a transhiatal approach without a thoracic incision, which suggests that the thoracic approach is associated with a higher risk of fatal complications in comparison to the transhiatal approach without a thoracic incision. The transhiatal approach results in mild surgical stress and avoids fatal complications but does not provide adequate mediastinal lymphadenectomy. Hence, it has continued to be a matter of debate whether a transthoracic or transhiatal approach is more appropriate for esophagogastric junctional cancer. Hulscher et al. [29] conducted a randomized control trial comparing right thoracotomy esophagectomy with extended lymphadenectomy and transhiatal esophagectomy with limited lymphadenectomy for patients with Siewert type I and type II esophagogastric junctional cancer. In the transhiatal approach of this trial, the esophagus was bluntly resected from the neck to the abdomen followed by esophagogastrostomy performed in the neck, while en bloc mediastinal lymphadenectomy was performed in right thoracotomy extended esophagectomy. The 5-year survival rate was 34% in transhiatal esophagectomy and 36% in extended esophagectomy, suggesting that right thoracotomy with extended esophagectomy provided no significant survival benefit. However, in a subset analysis of patients with 1–8 positive lymph nodes in the resected specimen, the 5-year overall survival rate was 20% higher in patients treated with a transthoracic approach (transhiatal vs. transthoracic: 19% vs. 39%), suggesting that patients with a limited number of positive nodes seem to benefit from extended transthoracic esophagectomy. Another randomized control trial was reported from Japan. Sasako et al. [30] conducted a phase III trial to compare the left thoracoabdominal approach with the abdominal-transhiatal approach in patients with Siewert type II and III esophagogastric junctional cancer (JCOG9502). In both groups in the trial, paraaortic nodes lateral to the aorta and above the left renal vein were dissected with total D2 gastrectomy. In the left thoracoabdominal approach, radical lower mediastinal node dissection via the left thorax was performed. On the other hand, resection of the lower mediastinal nodes was limited to the peri-esophageal lymph nodes in the transhiatal approach. This trial was stopped after the first internal analysis and demonstrated that patients assigned to the left transthoracic approach did not have improved survival in comparison to those assigned to the transhiatal approach. In the final analysis, the 5-year overall survival rate of patients treated with a transhiatal approach was 52.3%, while that of patients treated with a transthoracic approach was 37.9%. The morbidity rate of patients treated with a transhiatal approach was 34%, while that of patients treated with a transthoracic approach was 49%. In particular, the incidence of postoperative pneumonia in the transthoracic approach group (13%) was significantly higher in comparison to that in the transhiatal approach group (4%). As a result, a left transthoracic approach cannot be recommended for Siewert type II and III esophagogastric junctional cancer, because a left transthoracic approach does not improve survival but does increase morbidity. A meta-analysis [31] comparing the transthoracic and transhiatal approaches confirmed the results of these 2 trials, suggesting that the thoracic approach increases postoperative complications and thereby offsets the oncological benefits of en bloc resection.
Recently, minimally invasive esophagectomy has been developing, and interest in reducing the risk of postoperative complications has increased. Some studies [32,33,34,35,36] demonstrated the superiority of minimally invasive esophagectomy to conventional open esophagectomy for decreasing postoperative morbidity. In particular, a randomized controlled trial conducted by Bare et al. [37] showed a 22% reduction in postoperative respiratory complications, suggesting that minimally invasive esophagectomy is a promising approach for the prevention of pneumonia after thoracic surgery. Since surgical safety and oncological curability are essential to the improvement of long-term outcomes, minimally invasive esophagectomy could be a possible solution to this problem.
Which procedure is best for Siewert type II?
It is widely accepted that esophagectomy by a thoracic approach is indicated for Siewert type I EGJ cancer, and total gastrectomy or proximal gastrectomy by an abdominal approach is indicated for Siewert type III EGJ cancer. On the other hand, there is no consensus on the standard surgical approach for Siewert type II tumors. As mentioned above, lymph nodes with high priority for dissection in patients with Siewert type II tumors include the upper perigastric area, suprapancreatic nodes, and paraaortic nodes; however, the priority of the lower perigastric nodes is relatively low, suggesting that total gastrectomy is unnecessary, and proximal gastrectomy or esophagectomy with gastric tube reconstruction is sufficient. Proximal gastrectomy with a transhiatal approach resecting the lower mediastinal nodes could be feasible from an oncological standpoint. However, esophagectomy via a transthoracic approach is necessary when the middle or upper mediastinal nodes should be dissected and when the transhiatal anastomosis is technically difficult due to resection of the proximal esophagus to ensure oncological safety at the resection margin. Hence, surgical teams treating patients with Siewert type II tumors need to be well-skilled in lymphadenectomy for gastric cancer and thoracic esophagectomy. In real-world clinical practice, Siewert type II tumors are treated by both thoracic and gastric surgeons, which could influence the decision to choose a surgical approach. Haverkamp et al. [38] conducted worldwide survey on preferences in the surgical treatment of EGJ cancer. This survey received responses from 435 surgeons who treat esophageal or gastric cancer, with 166 from Asia, 141 from Europe, 57 from North America, 57 from South America, and 14 from other regions. When choosing surgical procedure for type II esophagogastric junctional cancer, thoracic surgeons mainly chose esophagectomy and rarely chose gastrectomy, while gastric surgeons mainly chose gastrectomy and rarely chose esophagectomy. This result indicated that the preference of the surgical approach for Sievert type II tumors was strongly influenced by the surgeon’s specialty. Thus, the surgeon’s preference could be a problematic issue when considering whether gastrectomy or esophagectomy is the appropriate procedure for a Siewert type II tumor. The development of treatment for Siewert type II esophagogastric junctional tumors will require a surgical team with expertise in both gastric cancer and esophageal cancer treatment, or close collaboration between thoracic surgeons and gastric cancer surgeons.
No funding is available for this study. The authors declare no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
References
Giacopuzzi S, Bencivenga M, Weindelmayer J et al (2017) Western strategy for EGJ carcinoma. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 20:60–68
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459
McColl KE, Going JJ (2010) Aetiology and classification of adenocarcinoma of the gastro-oesophageal junction/cardia. Gut 59:282–284
Leers JM, DeMeester SR, Chan N et al (2009) Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. J Thorac Cardiovasc Surg 138:594–602 (discussion 601 592)
Wong CL, Law S (2019) Extent of lymphadenectomy for Barrett’s cancer. Translational gastroenterology and hepatology 4:36
Zanoni A, Verlato G, Giacopuzzi S et al (2013) Neoadjuvant concurrent chemoradiotherapy for locally advanced esophageal cancer in a single high-volume center. Ann Surg Oncol 20:1993–1999
Barbour AP, Rizk NP, Gonen M et al (2007) Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 246:1–8
de Manzoni G, Pedrazzani C, Pasini F et al (2002) Results of surgical treatment of adenocarcinoma of the gastric cardia. Ann Thorac Surg 73:1035–1040
Feith M, Stein HJ, Siewert JR (2003) Pattern of lymphatic spread of Barrett’s cancer. World J Surg 27:1052–1057
Pedrazzani C, de Manzoni G, Marrelli D et al (2007) Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J Thorac Cardiovasc Surg 134:378–385
Grotenhuis BA, Wijnhoven BP, Poley JW et al (2013) Preoperative assessment of tumor location and station-specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World J Surg 37:147–155
Cense HA, Sloof GW, Klaase JM et al (2004) Lymphatic drainage routes of the gastric cardia visualized by lymphoscintigraphy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 45:247–252
Feith M, Stein HJ, Siewert JR (2006) Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 15:751–764
Kakeji Y, Yamamoto M, Ito S et al (2012) Lymph node metastasis from cancer of the esophagogastric junction, and determination of the appropriate nodal dissection. Surg Today 42:351–358
Fujitani K, Miyashiro I, Mikata S et al (2013) Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type II adenocarcinoma of the cardia: results of a multicenter study. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 16:301–308
Goto H, Tokunaga M, Sugisawa N et al (2013) Value of splenectomy in patients with Siewert type II adenocarcinoma of the esophagogastric junction. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 16:590–595
Hasegawa S, Yoshikawa T, Rino Y et al (2013) Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann Surg Oncol 20:4252–4259
Mine S, Sano T, Hiki N et al (2013) Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg 100:261–266
Goto H, Tokunaga M, Miki Y et al (2014) The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type II and Siewert type III patients. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 18:375–381
Parry K, Haverkamp L, Bruijnen RC et al (2015) Surgical treatment of adenocarcinomas of the gastro-esophageal junction. Ann Surg Oncol 22:597–603
Yoshikawa T, Takeuchi H, Hasegawa S et al (2016) Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gas Cancer 19:143–149
Yamashita H, Seto Y, Sano T et al (2017) Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gas Cancer 20:69–83
Kurokawa Y, Takeuchi H, Doki Y et al (2021) Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg 274:120–127
Gao F, Chen J, Wang T et al (2014) Incidence of microscopically positive proximal margins in adenocarcinoma of the gastroesophageal junction. PloS one 9:e88010
Mariette C, Castel B, Balon JM et al (2003) Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol 29:588–593
Ito H, Clancy TE, Osteen RT et al (2004) Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg 199:880–886
Mine S, Sano T, Hiki N et al (2013) Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg 100:1050–1054
Siu KF, Cheung HC, Wong J (1986) Shrinkage of the esophagus after resection for carcinoma. Ann Surg 203:173–176
Hulscher JB, van Sandick JW, de Boer AG et al (2002) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347:1662–1669
Sasako M, Sano T, Yamamoto S et al (2006) Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 7:644–651
Heger P, Blank S, Gooßen K et al (2019) Thoracoabdominal versus transhiatal surgical approaches for adenocarcinoma of the esophagogastric junction-a systematic review and meta-analysis. Langenbecks Arch Surg 404:103–113
Kinjo Y, Kurita N, Nakamura F et al (2012) Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc 26:381–390
Sihag S, Wright CD, Wain JC et al (2012) Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 42:430–437
Ichikawa H, Miyata G, Miyazaki S et al (2013) Esophagectomy using a thoracoscopic approach with an open laparotomic or hand-assisted laparoscopic abdominal stage for esophageal cancer: analysis of survival and prognostic factors in 315 patients. Ann Surg 257:873–885
Lee L, Sudarshan M, Li C et al (2013) Cost-effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Ann Surg Oncol 20:3732–3739
Meng F, Li Y, Ma H et al (2014) Comparison of outcomes of open and minimally invasive esophagectomy in 183 patients with cancer. J Thorac Dis 6:1218–1224
Biere SS, van Berge Henegouwen MI, Maas KW et al (2012) Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet (London, England) 379:1887–1892
Haverkamp L, Seesing MF, Ruurda JP et al (2017) Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 30:1–7
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayashi, T., Yoshikawa, T. Optimal surgery for esophagogastric junctional cancer. Langenbecks Arch Surg 407, 1399–1407 (2022). https://doi.org/10.1007/s00423-021-02375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02375-7