Abstract
Sentinel nodes (SNs) are defined as the first draining lymph nodes from the primary tumor site, and they are thought to be the first possible site of micrometastasis along the route of lymphatic drainage from the primary lesion. Recent meta-analyses and a prospective multicenter trial of SN mapping for early gastric cancer have shown acceptable SN detection rates and accuracy of determination of lymph node status. A dual-tracer method that employs radioactive colloids and blue dyes is currently considered the most reliable method for the stable detection of SNs for early-stage gastric cancer. However the new technologies such as indocyanine green infrared or fluorescence imaging may revolutionize the SN mapping procedures in gastric cancer. For early gastric cancer, the establishment of individualized, minimally invasive treatments based on SN concept can retain the patients’ quality of life. The combination of endoscopic submucosal dissection or non-exposed endoscopic wall-inversion surgery with SN mapping and lymphatic basin dissection is expected to become a promising, ideal minimally invasive, function-preserving treatment to cure patients with cN0 early gastric cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sentinel nodes
- Gastric cancer
- Laparoscopic
- Non-exposed endoscopic wall-inversion surgery
- Lymphatic basin dissection
Introduction
In Japan, early-stage gastric cancer (cT1) is found in many asymptomatic patients due to recent advances in endoscopic diagnosis, and the population with this condition currently reaches in excess of 50% in major institutions [1]. Endoscopic submucosal dissection (ESD) has already been accepted as the most minimally invasive procedure for the resection of early gastric cancer [1]. Laparoscopic gastrectomy represents an important intermediate option between ESD and open surgery for patients with gastric cancer [2]. The technique of laparoscopic gastrectomy has shifted from partial resection to more radical procedures such as laparoscopy-assisted distal gastrectomy (LADG) with D2 lymphadenectomy, which is comparable to conventional open distal gastrectomy and can be performed in clinical practices [3, 4].
Many patients with early gastric cancer are currently treated with advanced laparoscopic gastrectomy procedures, such as LADG and laparoscopy-assisted total gastrectomy (LATG) with standard lymph node dissection in Asian countries [1,2,3,4]. LADG and LATG contribute to both better esthetics and early postoperative recovery [5]. However, patients’ quality of life (QOL) is mainly affected by late-phase complications including dumping syndrome and body weight loss resulting from oral intake disturbance due to large extent of gastric resection. Therefore, both minimal invasiveness for early-phase recovery by laparoscopic surgery and additional late-phase function-preserving gastrectomy should be carefully considered in patients indicated for these procedures.
Function-preserving gastrectomy such as partial gastrectomy, segmental gastrectomy, and proximal gastrectomy with limited lymph node dissection is known to improve postoperative late-phase function. However, a certain incidence of skip metastasis in the 2nd or 3rd compartment of regional lymph nodes remains an obstacle to the wider application of these procedures. To overcome these issues, the concept of sentinel node (SN) mapping may become a novel diagnostic tool for the identification of clinically undetectable lymph node metastasis in early gastric cancer.
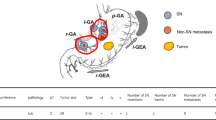
SNs are defined as the first draining lymph nodes from the primary tumor site [6, 7], and they are thought to be the first possible site of micrometastasis along the route of lymphatic drainage from the primary lesion (Fig. 19.1). The pathological status of SNs can theoretically predict the status of all regional lymph nodes. If SNs are recognizable and negative for cancer metastasis, unnecessary radical lymph node dissection could be avoided. SN navigation surgery is defined as a novel, minimally invasive surgery based on SN mapping and the SN-targeted diagnosis of nodal metastasis. SN navigation surgery can prevent unnecessary lymph node dissection, thus preventing the associated complications and improving the patient’s QOL.
Schema of gastric cancer and sentinel nodes (SN). The SN is defined as one or more lymph nodes that first receive lymphatic drainage from primary tumors. For intraoperative lymphatic mapping and SN biopsy, blue dye and/or radioisotope-labeled colloid is injected submucosally around primary tumor sites before surgery using endoscopy. Subsequently, the tracers pass through the afferent lymphatics, and blue-stained or radioactive nodes are regarded as the SN
SN mapping and biopsy were firstly applied to melanoma and breast cancer patients and were subsequently extended to patients with many other solid tumors [7,8,9]. The clinical application of SN mapping for early gastric cancer has been controversial for years. However, single institutional results, including a recent multicenter trial of SN mapping for early gastric cancer, are considered acceptable in terms of the SN detection rate and accuracy of determination of lymph node status [10, 11]. On the basis of these results, we are developing a novel, minimally invasive function-preserving gastrectomy technique combined with SN mapping.
Laparoscopic SN Mapping Procedures for Gastric Cancer
A dual-tracer method that utilizes radioactive colloids and blue or green dyes is currently considered the most reliable method for the stable detection of SNs in patients with early gastric cancer [10, 11]. An accumulation of radioactive colloids facilitates the identification of SNs even in resected specimens by using a handheld gamma probe, and the blue dye is effective for intraoperative visualization of lymphatic flow, even during laparoscopic surgery. Technetium-99 m tin colloid, technetium-99 m sulfur colloid, and technetium-99 m antimony sulfur colloid are preferentially used as radioactive tracers. Isosulfan blue and indocyanine green (ICG) are the currently preferred choices as dye tracers.
In our institution, patients with clinical T1 tumors, primary lesions less than 4 cm in diameter, and clinical N0 gastric cancer undergo SN mapping and biopsy [10, 11]. In our procedures, 2.0 ml (150 MBq) of technetium-99 m tin colloid solution is injected the day before surgery into four quadrants of the submucosal layer of the primary tumor site using an endoscopic puncture needle. Endoscopic injections to the submucosal layer facilitate accurate tracer injection rather than laparoscopic injection from the seromuscular site of the gastric wall. Technetium-99 m tin colloid with relatively large particle size accumulates in the SNs after local administration.
The blue or green dyes are injected into four quadrants of the submucosal layer of the primary site using an endoscopic puncture needle at the beginning of surgery. Blue lymphatic vessels and blue-stained nodes can be identified by laparoscopy within 15 minutes after the injection of the blue or green dyes. Simultaneously, a handheld gamma probe is used to locate the radioactive SN. Intraoperative gamma probing is feasible in laparoscopic gastrectomy using a special gamma detector introducible from trocar ports [10, 11].
For intraoperative SN sampling, the pick-up method is well established for the detection of melanoma and breast cancer. However, it is recommended that the clinical application of intraoperative SN sampling for gastric cancer should include sentinel lymphatic basin dissection, which is a sort of focused lymph node dissection involving hot and blue nodes [10, 11]. The gastric lymphatic basins were considered to be divided in the following five directions along the main arteries: left gastric artery area, right gastric artery area, left gastroepiploic artery area, right gastroepiploic artery area, and posterior gastric artery area [12].
ICG is known to have excitation and fluorescence wavelengths in the near-infrared range [13]. Till date, some investigators have used infrared ray electronic endoscopy (IREE) to demonstrate the clinical utility of intraoperative ICG infrared imaging as a new tracer for laparoscopic SN mapping [13, 14]. IREE might be a useful tool to improve visualization of ICG-stained lymphatic vessels and SNs even in the fat tissues. More recently, ICG fluorescence imaging has been developed as another promising novel technique for SN mapping [15, 16]. SN could be clearly visualized by ICG fluorescence imaging compared to the naked eye. Further studies would be needed to evaluate the clinical efficacy of ICG infrared or fluorescence imaging and to compare those with radio-guided methods in prospective studies. However these new technologies might revolutionize the SN mapping procedures in early gastric cancer.
Results of SN Mapping in Gastric Cancer
To date, more than 100 single institutional studies have demonstrated acceptable outcomes of SN mapping for early gastric cancer in terms of the SN detection rate (90–100%) and accuracy (85–100%) of determination of lymph node status; these outcomes are comparable to those of SN mapping for melanoma and breast cancer [11]. A recent large-scale meta-analysis, which included 38 relevant SN mapping studies with 2128 gastric cancer patients, demonstrated that the SN detection rate and accuracy of prediction of lymph node metastasis based on SN status were 94% and 92%, respectively [17]. They concluded that the SN concept is technically feasible for gastric cancer, especially patients with early T stage (T1), with the use of combined tracers and submucosal injection methods during the SN biopsy procedures.
Our group in Japan had conducted a multicenter prospective trial (UMIN ID: 000000476) of SN mapping using a dual-tracer method with a radioactive colloid and blue dye [10]. In the trial, SN mapping was performed between 2004 and 2008 for 397 patients with early gastric cancer at 12 comprehensive hospitals, including our institution. Eligibility criteria were that patients had cT1N0M0 or cT2N0M0 single tumor with diameter of primary lesion less than 4 cm, without any previous treatments. As results, the SN detection rate was 98%, and the accuracy of determination of metastatic status was 99% [10]. The results of that clinical trial are expected to provide us with perspectives on the future of SN navigation surgery for early gastric cancer.
Clinical Application of Laparoscopic SN Navigation Surgery in Early Gastric Cancer
The distribution of sentinel lymphatic basins and the pathological status of SNs would be useful in deciding on the minimized extent of gastric resection and in avoiding the universal application of distal or total gastrectomy with D2 dissection. Appropriate indications for laparoscopic surgeries such as partial (wedge) resection, segmental gastrectomy, pylorus-preserving gastrectomy, and proximal gastrectomy (LAPG) for cT1N0 gastric cancer could be individually determined on the basis of SN status (Fig. 19.2) [18,19,20]. Earlier recovery after surgery and preservation of QOL in the late phase can be achieved by laparoscopic limited gastrectomy with SN navigation. Our study group in Japan has currently been conducting the multicenter prospective trial (UMIN ID: 000014401) which will evaluate the function-preserving gastrectomy with SN mapping in terms of long-term survival and patients’ QOL as the next step. A Korean group has also been conducting the multicenter prospective phase III trial to elucidate the oncologic safety including long-term survival of laparoscopic stomach-preserving surgery with sentinel lymphatic basin dissection compared to a standard laparoscopic gastrectomy [21].
A combination of laparoscopic SN biopsy and endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD) for early gastric cancer is another attractive option as a novel, whole stomach-preserved, minimally invasive approach. If all SNs are pathologically negative for cancer metastasis, theoretically, EMR/ESD instead of gastrectomy may be sufficient for the curative resection of cT1 gastric cancer beyond the ESD criteria [20, 22]. However, further studies are required to verify the safety and effectiveness of combined treatments involving laparoscopic SN biopsy and EMR/ESD.
Nowadays, LADG or LAPG is frequently applied to the patients with early gastric cancer according to the results of pathological assessment of primary tumor resected by EMR/ESD in clinical practices. To date, it has not been clarified whether the SN mapping is feasible even after EMR/ESD. One of the most important issues is whether lymphatic flow from the primary tumor to the original SNs might change after EMR/ESD. In our preliminary study, however, at least the sentinel lymphatic basin is not markedly affected by previous EMR/ESD [20, 22]. Modified gastrectomy according to SN distribution and metastatic status might be feasible even for the patients who underwent EMR/ESD prior to surgery.
Non-exposed Endoscopic Wall-Inversion Surgery Plus SN Mapping
In current function-preserving surgeries such as laparoscopic local resection or segmental gastrectomy, the approach of gastrectomy is only from the outside of the stomach, in which the demarcation line of the tumor cannot be visualized at the phase of resection. Therefore, the surgeons cannot avoid a wider resection of the stomach than is desired to prevent a positive surgical margin. The recent appearance of a new technique, referred to as non-exposed endoscopic wall-inversion surgery (NEWS), is a technique of full-thickness partial resection, which can minimize the extent of gastric resection using endoscopic and laparoscopic surgery without transluminal access mainly designed to treat gastric cancer. We have been accumulating cases of NEWS with SN biopsy for early gastric cancer with the risk of lymph node metastasis in the clinical trial [23, 24].
In brief, after placing mucosal markings, ICG was injected endoscopically into the submucosa around the lesion to examine SNs (Fig. 19.3) [24]. The SN basin including hot or stained SNs was dissected, and an intraoperative pathological diagnosis confirmed that no metastasis had occurred. Subsequently, NEWS was performed for the primary lesion. Serosal markings were placed laparoscopically, submucosal injection was added endoscopically, and circumferential seromuscular incision and suturing were performed laparoscopically, with the lesion inverted toward the inside of the stomach. Finally, the circumferential mucosal incision was performed, and the lesion was retrieved perorally (Fig. 19.3).
Non-exposed endoscopic wall-inversion surgery (NEWS) with SN biopsy and sentinel lymphatic basin dissection. (a) Schema of the NEWS with sentinel lymphatic basin dissection. (b) Marking was placed around the primary tumor. (c) Indocyanine green (ICG) was endoscopically injected to the gastric submucosal layer surrounding the primary tumor. (d) Laparoscopic observation of ICG with normal light. (e) Observation of ICG with infrared ray electronic endoscopy. Infrared ray electronic endoscopy can visualize SNs and lymphatics clearly. (f) Resection of sentinel lymphatic basin. (g) Laparoscopic circumferential seromuscular incision. (h) and (i) Laparoscopic seromuscular suturing and inversion of the primary lesion
The NEWS combined with the SN biopsy can minimize not only the area of lymphadenectomy but also the extent of gastric resection as partial gastrectomy for patients with SN-negative for metastasis [22]. Furthermore, NEWS does not need intentional perforation, which enables us to apply this technique to cancers without a risk of iatrogenic dissemination. The combination of NEWS with SN biopsy is expected to become a promising, ideal minimally invasive, function-preserving surgery to cure cases of cN0 early gastric cancer.
Conclusion
For early-stage gastric cancer, for which a better prognosis can be achieved through conventional surgical approaches, the establishment of individualized, minimally invasive treatments that may retain the patients’ QOL should be the next surgical challenge. Although further studies are needed for careful validation, function-preserving gastrectomy based on SN navigation could be a promising strategy to achieve this goal.
References
Sano T, Hollowood K. Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg. 2006;95:249–55.
Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–8.
Adachi Y, Shiraishi N, Shiromizu A, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10.
Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138–42.
Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7.
Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am. 2000;80:1799–809.
Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401.
Bilchik AJ, Saha S, Wiese D, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001;19:1128–36.
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10.
Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2013;20:522–32.
Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G, Kayahara M, Ohta T, Yoh Z. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320–9.
Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A, Goto S, Otsuka K, Kato T, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. 2010;17:1787–93.
Ishikawa K, Yasuda K, Shiromizu T, Etoh T, Shiraishi N, Kitano S. Laparoscopic sentinel node navigation achieved by infrared ray electronic endoscopy system in patients with gastric cancer. Surg Endosc. 2007;21:1131–4.
Nimura H, Narimiya N, Mitsumori N, Yamazaki Y, Yanaga K, Urashima M. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg. 2004;91:575–9.
Miyashiro I, Miyoshi N, Hiratsuka M, Kishi K, Yamada T, Ohue M, Ohigashi H, Yano M, Ishikawa O, Imaoka S. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008;15:1640–3.
Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541–50.
Takeuchi H, Saikawa Y, Kitagawa Y. Laparoscopic sentinel node navigation surgery for early gastric cancer. Asian J Endosc Surg. 2009;2:13–7.
Takeuchi H, Oyama T, Kamiya S, Nakamura R, Takahashi T, Wada N, Saikawa Y, Kitagawa Y. Laparoscopy-assisted proximal gastrectomy with sentinel node mapping for early gastric cancer. World J Surg. 2011;35:2463–71.
Takeuchi H, Kitagawa Y. Sentinel node navigation surgery in patients with early gastric cancer. Dig Surg. 2013;30:104–11.
Park JY, Kim YW, Ryu KW, Nam BH, Lee YJ, Jeong SH, Park JH, Hur H, Han SU, Min JS, An JY, Hyung WJ, Cho GS, Jeong GA, Jeong O, Park YK, Jung MR, Yoon HM, Eom BW. Assessment of laparoscopic stomach preserving surgery with sentinel basin dissection versus standard gastrectomy with lymphadenectomy in early gastric cancer-A multicenter randomized phase III clinical trial (SENORITA trial) protocol. BMC Cancer. 2016;16:340.
Mayanagi S, Takeuchi H, Kamiya S, Niihara M, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y, Omori T, Nakahara T, Mukai M, Kitagawa Y. Suitability of sentinel node mapping as an index of metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol. 2014;21:2987–93.
Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y, Horii J, Uraoka T, Kitagawa Y, Yahagi N. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:440–5.
Takeuchi H, Kitagawa Y. Sentinel lymph node biopsy in gastric cancer. Cancer J. 2015;21:21–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
Takeuchi, H., Kitagawa, Y. (2019). Sentinel Node Navigation Surgery. In: Noh, S., Hyung, W. (eds) Surgery for Gastric Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45583-8_19
Download citation
DOI: https://doi.org/10.1007/978-3-662-45583-8_19
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45582-1
Online ISBN: 978-3-662-45583-8
eBook Packages: MedicineMedicine (R0)