Abstract
Where health and care provision is divided across organisation types, such as child health and palliative care, it is difficult for researchers to access comprehensive healthcare data. Integrated electronic health records offer an opportunity for research into care delivered within and across organisations. In this paper a new centralised model for accessing such data is justified using the critical success factors of an established research data provider. This validates a model that will facilitate integrated health research to inform the evidence base and systems used in clinical practice across organisations.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Research database
- Shared health records
- EPR
- Patient record access
- ResearchOne
- Multiple health providers
- Clinical system
- Database validation
1 Introduction
1.1 Summary
The multitude of health and social care organisations that may be involved in a patient’s care is frequently under-considered by research projects and in translating results into evidence-based care. Electronic health record (EHR) data is increasingly being used in research due to their widespread use in clinical practice for gathering detailed and structured data. EHRs are often non-shareable, used in only the organisation that generated them, such as a general practice or a hospital ward [1]. Such isolated records cannot comprehensively represent to the research community the health of patients who receive care in multiple settings. Alternative structures with EHR data sharing between clinicians at different health organisations can improve clinical practice and reduce errors [2, 3]. Research on records from across organisation types has enhanced healthcare through investigating clinical practice through a whole systems approach [4]. Research using integrated EHRs in England has nonetheless remained infrequent due to accessibility issues.
1.2 The Silo Issue in Health and Social Care and Research
Health and social care in many countries including the US, UK and China is delivered through multiple organisation types working with independence. These care providers struggle with an outcome of this specialisation which is often termed ‘silo working’ wherein service deliverers with different aims and professional languages gather information on separate aspects of patient care [5, 6] and store these in silos: unlinked records in closed databases. Such siloed systems often have different structures or formats that cannot be easily linked. As such these records may not be shared with other healthcare organisations of the same, or different, type. As a consequence each organisation holds partial patient records rather than the entirety of the patient’s medical history. If a patient has a general practitioner (primary physician) then they often receive summary discharge letters from other organisations, though this unstructured and delayed information flow is one-way. With closed systems it becomes difficult to share timely and pertinent information, such as diagnoses, allergies, medication and professional insights with other healthcare providers that are also intervening with and monitoring the health of the patient. This results in issues of duplication and missing data. Patient information held in such silos provides less support to patients that cross healthcare organisation types and reduces the capacity to perform longitudinal assessments [7].
This silo issue is also of relevance for the research community who consider patient health in an array of fields including health informatics, epidemiology, health economics, clinical care and medicine. Traditional data collection involves invasive, timely and resource-intensive methods such as conducting interviews and questionnaires. The increasingly routine use of EHRs in clinical practice, for example among 76 % of European general practitioners, makes EHRs an efficient source of large cohort research data [8]. This is particularly applicable in the UK where 97 % of general practices use EHRs [9]. The capacity for large EHR cohorts facilitates research on low frequency incidences or diagnoses. For example, this enabled identification of the correlation between emergency department waiting times and outcomes of mortality and readmission, by using the records of over 14.5 million emergency department attendances [10].
The silo issue impacts the timeliness and security of EHR research. Patient data dispersal in EHRs across multiple organisation types often necessitates the involvement of identifiable data for undertaking data linkage. This brings security issues and the time taken to gather and link siloed data reduces the timeliness of cross-organisation research. Further time on the part of the researchers and the data providers is often required to update the research dataset. The ethical issues surrounding the identification of relevant patients and in developing a fully informed consent mechanism remain. Nevertheless such research has successful results and was crucial in resolving the disputed link between Autism and the Measles, Mumps and Rubella vaccine [11]. Researchers, in using non-shared EHR data, face the same constraints as clinicians in not being able to view the full patient pathway in a timely, cost-effective and secure, audited manner.
QResearch has an established ten year record in supporting electronic health records research. QResearch is a UK-based not-for-profit general practice EHR research database. Over 650 practices contribute data [12]. It was developed with the aim of consolidating de-identified, siloed EHR data from a large representative cohort of general practices to provide data for ethical research purposes [13]. It has facilitated research into the development of tools for identifying patient risk of, for example, developing cardiovascular disease (CVD) and diabetes [14, 15]. Despite the importance of risk assessment in health promotion, evidence suggests that risk tools are under-used. A study of clinical practice showed that 66 % of clinicians who identify the need to perform a global CVD risk assessment fail to follow the guidelines by employing such a tool, valuing subjective assessment alone [16]. As patient risk crosses organisation boundaries it may be that the relevance of these tools is constrained by the external validity of using non-shared records data from one organisation type.
As the UK population both pay for and receive lifelong care from the National Health Service (NHS), there is a focus on efficient illness prevention. The longitudinal information already existing in EHRs is seen as a resourceful means to facilitate research to help meet this goal. The NHS Quality, Innovation, Productivity and Prevention program encourages the innovative use of existing resources [17] and the Prime Minister, in a speech made in London in December 2011, specified ‘opening up’ NHS data to research as being crucial to supporting health, the economy, and the life sciences. Subsequently in May 2012 the government’s Department of Health (DH) issued a call for more efficient EHR research. The call linked this to a move towards sharing information and delivering care across organisation boundaries in response to the increasingly complex needs of a diverse, multi-morbidity population [18]. Cross-organisation EHR research will be required in addressing this need for efficient research that is relevant across multiple organisation types.
1.3 The Integrated Solution
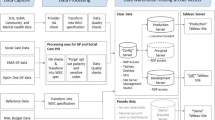
There are well established alternatives to non-integrated clinical practice. Kaiser Permanente provides comprehensive care packages to 8 million patients in America. Their shared standards result from collaboration between all care providers [19]. In the UK, SystmOne is a centrally hosted clinical system provided by TPP (The Phoenix Partnership) that enables record sharing between many of the health and care organisation types in the NHS (Fig. 1). Since 1999 its centralised database (cloud) has contained one integrated EHR per patient. From this, data is shared with the patient and across the health organisations that use SystmOne, where access rights are legitimate. Through SystmOne, 26 million patients have a shareable record (Table 1) that facilitates integrated care [20]. Both Kaiser Permanente and SystmOne exemplify long-standing alternatives that reduce the ‘silo effect’ in healthcare.
Integrated EHRs assist in cross-organisation type care management that efficiently utilises resources. Integration at Kaiser Permanente organisations contributes to the number of bed stays being 3.5 times fewer than in the NHS for 11 leading causes [21]. Benefits from EHR sharing are indicated by patient management improvements in cases that involve professionals from primary and secondary care sectors, such as are frequent in the treatment of long-term conditions. In such cases, secondary care consultants more frequently use their EHR where the patient’s general practitioner is also registered with SystmOne as the record contains updates since the previous outpatient appointment and includes advice from other specialists, recent medications and blood test results [20]. This replaces reliance upon summary letters, patient awareness or being able to telephone other organisations [22]. Comprehensive information enables all organisations to review and communicate regarding medication, ongoing treatments and appointment non-attendances [22]. Shared records also assist in prompt medicines reconciliation between care settings, which has been shown to identify errors in 38 % of prescriptions [23]. Through such means clinical systems integration delivers the benefits of an “electronic highway” envisioned by the NHS National Programme for IT [24].
The benefits that more comprehensive, timely information bring to clinical practice could also be brought to the research community. Information sharing among Kaiser Permenante organisations supports research that considers care provision across organisation types. Clinical practice has altered internationally in response to links uncovered, using Kaiser Permanente data, between hospital admissions and drugs such as rofecoxib being issued in ambulatory (primary) care settings [4, 25]. Using shared EHRs in research replaces linkage exercises that involve identifiable data and result in biased, incomplete datasets [26]. Shared EHRs enable research on the otherwise lost communications between healthcare organisations, such as referral trails. Research on cross-organisation type records can validate siloed research in a cost-effective, timely manner and inform clinical practice that occurs in these multiple settings.
1.4 Research Aim
The aim of this paper is to determine the capacity of a new ResearchOne database to facilitate cross-organisation EHR research in the UK. ResearchOne is a not-for-profit organisation with ethical approval to extract de-identified EHR data from the centralised SystmOne database into the ResearchOne database. This enables secure, audited access to anonymous records data for the research community. This access is with the purpose of developing new clinical understanding through research to improve patient care. This model must be investigated in order to justify that the ResearchOne database may bring benefits to research in the way that SystmOne does for clinical practice.
2 Method
The method was designed to assess the capacity of the ResearchOne database to support EHR research and to justify its potential benefits to integrated records research using English health data. Information regarding SystmOne and ResearchOne were determined from the ResearchOne Database Protocol and through interviews [27]. QResearch is specifically designed for EHR research in the UK and was taken as an academically established ‘standard’. A search of Web of Science, PubMed and Google Scholar identified articles published on research that used QResearch data. These and the QResearch Protocol were reviewed. The key features of QResearch were taken as critical success factors, as justified in Table 2, against which the model presented by the ResearchOne database was appraised. These factors are the headings in the following section. From this the ResearchOne database could be validated with the potential to perform to the existing standard for a research database of NHS data, in order that it can facilitate cross-organisation research.
3 Results
3.1 Data Consolidation
QResearch facilitates research on EHR data consolidated from over 650 non-integrated general practice (GP) databases [12]. The ResearchOne database can similarly hold EHR data contributed by multiple practices and so it meets this critical success factor. Moreover it can hold data from other organisation types, as it mirrors the infrastructure of SystmOne. SystmOne integrates data from multiple organisations into one centralised record per patient and so no consolidation is required in order to extract data in SystmOne from multiple organisation settings into the ResearchOne database.
Data linkages to other sources undertaken by QResearch are also feasible with ResearchOne. QResearch links GP EHR information to socio-economic, Hospital Episode Statistics (HES), disease-specific registry and death registration data [14, 31]. ResearchOne has national ethical and governance approval to undertake or request such linkage and consolidation [27]. An NHS National Institute for Health Research funded study, Improving Prevention of Vascular Events in Primary Care, has successfully piloted the capacity to link ResearchOne data to HES and Myocardial Ischaemia National Audit Project (MINAP) data.
3.2 Large Cohort of Research EHR Data
General practice involvement in QResearch has grown steadily to over 650, surpassing the original aim of 500 practices [12, 13]. SystmOne hosts patient information for over 26 million patients across England from more than 4500 organisations that may participate in ResearchOne. This includes 2100 general practices, 170 community services and 80 palliative care organisations (Table 1) that can contribute their ‘real-world’ data. Prison data recorded in SystmOne cannot be extracted into the ResearchOne database. TPP already hosts this data in SystmOne and has the data management skills and capacity to hold such a large cohort of records from multiple organisation settings in the ResearchOne database.
3.3 De-Identified EHR Data
Both the ResearchOne database and QResearch have a nationally approved governance framework under which they can hold de-identified data. Neither database can contain free text with potentially identifiable data, nor, for example, full dates of birth or death. Furthermore, given the comprehensiveness of cross-organisation type records, the ResearchOne database excludes diagnostic cases that are present in fewer than five records. QResearch requires consent from each practice in order to extract data from the practice’s database. While SystmOne is centrally hosted, ResearchOne follows this practice in requesting consent from contributing organisations, and also provides the opportunity for patients to ‘opt out’ from providing the non-identifiable data. Consent is electronically audited through SystmOne, the centralisation of which ensures that any changes will automatically update the ResearchOne database within seven days.
3.4 Representative Coverage
QResearch practices are “spread throughout the UK”, offering representative GP coverage [13]. ResearchOne has the capacity to provide an England-wide representation of cross-organisation type health and social care, through the more than 4500 invited organisations. England is divided into 433 lower tiers of local government – Local Authorities - of which over 85 % have patient representation on SystmOne. There are more than 26 million patients who have contributed to 300 million years of patient records, geographically distributed across England (Fig. 2). Over 12 million patients have registered with more than one care organisation on SystmOne at some point, and 365,000 patients have received care from five or more organisations using SystmOne. As such these records are cross-organisational. SystmOne holds 5 billion diagnostic codes, inputted by clinicians whose specialties range from ante-natal to geriatric, rehabilitation to neuropathology. This coverage crosses community, primary and secondary care. The capacity for representation also covers the indices for rurality and deprivation defined by the UK Economic and Social Data Service [32].
3.5 Ethical Research Practice
QResearch and ResearchOne are specifically designed for ethical research access with the aim of improving healthcare. The frameworks for both QResearch and the ResearchOne database have been developed with ethical and governance approval from the relevant national bodies [27, 31]. The protocol for ResearchOne was developed with input from patients, clinicians, researchers and information governance experts. Any change in either protocol is reviewed by the national boards and a database committee of patients and clinical professionals along with experts in informatics, database architecture and governance [27, 31].
A national research ethics committee has approved both the QResearch and ResearchOne database frameworks to review data requests based on the benefit to clinical practice of each project proposal [27, 31]. Research proposals submitted to QResearch are reviewed by a QResearch Scientific Committee and a national multicentre Research Ethics Committee. The ResearchOne database has a nationally approved de-identification procedure and access protocol whereby a ResearchOne Project Committee considers all data requests. This approved de-identification and project acceptance process enables project proposals to be reviewed more promptly by a specialised committee.
Ethical accessibility of both QResearch and the ResearchOne database is supported by their being not-for-profit organisations. With SystmOne data existing centrally, the cost of database maintenance is low for ResearchOne, which reduces the cost further for the research community. Remote access to the secure ResearchOne data warehouse is audited for the purpose of maintaining this ethical practice.
4 Discussion
The results of this investigation show that the ResearchOne database matches the capacity of the existing standard of a research database with EHR data. ResearchOne has been nationally approved to extract de-identified EHR data from consenting health and care organisations. There is potential for the inclusion of data from a large cohort of shared EHRs with representative coverage both geographically and demographically across England. The framework has been designed to ethically support research that delivers benefit to patient health.
4.1 Further Research Capacities
The ResearchOne database has the capacity to perform beyond the current standard in terms of cross-organisation integration. It maintains data from more organisation types in England than the current standard, and so can support more comprehensive and representative research between and within these areas of healthcare. These shared EHRs enable research on data such as communication and referral trails that isolated records cannot, even when linked, portray. The diminished likelihood of integrated records having missing or duplicated data when compared to the standard is also a benefit for research [26]. The ResearchOne database is not consolidated from multiple settings, but rather it is integrated at the point of entry. Through ResearchOne the integrated care records of more than 25 million patients have the capacity to support research, with information from over 4500 health organisations in primary, secondary and social care (Table 1). This gives ResearchOne the capacity to bring the benefits of record-sharing into the research arena.
By centrally extracting data from integrated records, the ResearchOne database moves beyond the current standard of consolidating data from isolated organisations. This extract procedure is more secure and has no potential to incorporate bias through incomplete linkage for research. This is beneficial because Bohensky et al. [26] reviewed linkage sensitivity to range between 74–98 %. Centralised extraction also does not disturb clinical practice and reduces the cost of extraction, with this saving being passed to the research community. The centralisation of SystmOne maintains an up-to-date audit of organisation consent and also enables a patient to opt out of the ResearchOne database by informing just one of their care organisations that uses SystmOne. Such centralised capacity is of relevance in ethically supporting the research community to enhance healthcare.
A further factor that assists in health research is the timeliness of the data available from ResearchOne. Timely data is required in order for research to reflect the evolving field of clinical practice and continual changes in the population and health demographics. EHRs should facilitate timely research [33]. The centralisation of SystmOne ensures that research data could reflect real-time clinical developments, without affecting SystmOne users. The ResearchOne database has a seven day update frequency though items can be extracted more frequently as required for public health monitoring. A further reason for timeliness is that consent withdrawal from the ResearchOne database results in data being removed within seven days. The timeliness of research projects is further enhanced by the streamlined data request process. Timely data provision can occur securely at minimum cost due to the centralised nature of SystmOne, which is a speedier alternative than linking data from multiple sources.
While most projects can benefit from timely access to de-identified real-world records data, some projects require identifiers. This may be to enable linkage with datasets in other sectors, such as education, or information from patient observations and interviews. With UK data, such projects require ethical approval and consent, or permission under Section 251 of the NHS Act 2006. Having de-identified data, neither the ResearchOne database nor QResearch can assist these projects. ResearchOne has experience in providing pseudonymised linkable data from records on SystmOne for such approved projects. This is a more timely mechanism for project teams than extracting data from siloed databases held in different organisations. ResearchOne can similarly support SystmOne users in joining randomised control trials or pilot studies by embedding research in the clinical system. The closeness between ResearchOne and SystmOne enables these further capabilities.
4.2 Next Steps
The capacity of ResearchOne to maintain a large cohort of de-identified EHR data from multiple organisation types depends upon organisation participation. The joining process is simple and extracts do not inconvenience SystmOne users given its centralised nature. Information about joining ResearchOne is available on SystmOne and the ResearchOne website has further information, including current project details. Organisations providing data can be assured that the ResearchOne database is maintained under the same security principles as SystmOne in an NHS-accredited data centre [27]. The aim of ResearchOne, to bring research outcomes into clinical practice, assures that it is beneficial to contribute to the ethically approved process. The success of QResearch should assist SystmOne users, some of whom may have contributed to QResearch previously, in recognising this beneficial invitation. Already organisations are participating and the count of anonymised records in the database is approaching five million. This indicates the successful realisation of the capacity of ResearchOne. As more organisations join, they will enable one of the potentially largest health databases in the world to be developed.
The aim of ResearchOne includes not only pulling data for research purposes, but pushing outcomes back into health and care. Results may be openly published, and as these can have relevance to clinical care across many organisation types, they may initiate more comprehensive innovations in care delivery. SystmOne will incorporate developments so that the clinical system continually improves the support it provides to over 145,000 users across multiple organisation types. The closeness between ResearchOne and SystmOne closes the distance between care providers and researchers so that issues and outcomes become shared. The ResearchOne Project Committee considers projects from a clinical needs driven perspective, with input from SystmOne users. Therefore a cyclical, evaluative, cloud-based model of clinical practice and research is encapsulated in the SystmOne-ResearchOne infrastructure. This can be envisioned as a global model for the future.
ResearchOne facilitates research both for validation purposes and in novel areas. Explorations will continue to compare ResearchOne data with national statistics to validate its representative coverage. Research performed on other datasets can be validated using the ResearchOne database. The impact of integrated and isolated EHR data on research may also be investigated, to explore the role of ResearchOne. ResearchOne has a structure to support different types of projects from anonymised epidemiological monitoring to randomised control trials. Research projects may use data from single or multiple care organisation types. In this way ResearchOne aims to facilitate outcomes that are of relevance across all organisations that are contributing data.
Cross-organisation records research can be used to extend clinical knowledge from one organisation type to another. Research outcomes previously based on data from one organisation type may not be pertinent to either other organisations, or when considering patients receiving treatment from multiple organisations. Research would indicate whether other organisations can see in their ‘real-world records’ the information required to utilise research outcomes. As such, the decision support from single-organisation-type research can be built on by extending investigations across organisations.
The global capacity for EHR research is continually increasing. Progression in science, particularly in the fields of security technology and machine learning, will lead to data mining of entire EHRs. The anonymisation of free text through advancements in natural language programming, and the reduction of human involvement in data analysis will open up the capacities of EHRs to ethically support research on real-world data. The number and types of organisations across which SystmOne provides integrated EHRs is rising, particularly in the acute sector, so that ResearchOne has increasing potential to represent comprehensive care in England and globally. With such future developments ResearchOne will increasingly support research that benefits healthcare.
5 Conclusions
The contribution of EHRs to research is increasing, but is hindered by the division of data across healthcare organisations as a result of the ‘silo effect’ in clinical care. Benefits in integrated care delivery have been evidenced from record-sharing. ResearchOne offers an alternative for research using this real-world shared EHR data. The model of the ResearchOne database has been critiqued using the success factors of QResearch, an established provider of EHR research data in the UK. ResearchOne meets this existing standard and brings further developments to the research community. This is particularly in terms of the timely provision of integrated, cross-organisation type data and in feeding results back into clinical care. This offers a global model for integrated evolution of innovations between clinicians, patients and research.
References
ISO/TR 20514: Health Informatics – Electronic Health Record – Definition, Scope, and Context. ISO Technical Report. www.iso.org/iso/ (2004). Accessed 21 Aug 2012
Twomey, P., Whittaker, A., Jervis, C.: Implementation of the National Service Framework for Diabetes: Initial opportunities for a PCT to facilitate practice and health community action plans – year one experience. Qual. Prim. Care 12(3), 213–218 (2004)
Ammenworth, E., Schnell-Inderst, P., Machan, C., Siebert, U.: The effect of electronic prescribing on medication errors and adverse drug events: A systematic review. J. Am. Med. Inform. Assoc. 15(5), 585–600 (2008)
Graham, D.J., Campen, D., Hui, R., Spence, M., et al.: Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case-control study. The Lancet 365(9458), 475–481 (2005)
Wilson, R., Baines, S., Cornford, J., Martin, M.: ‘Trying to do a jigsaw without the picture on the box’: Understanding the challenges of care integration in the context of single assessment for older people in England. Int. J. Integr. Care 7, 1–11 (2007)
Kawonga, M., Blaauw, D., Fonn, S.: Aligning vertical interventions to health systems: A case study of the HIV monitoring and evaluation system in South Africa. Health Res. Policy Syst. 10(2), 1–13 (2012)
Kuperman, G.J.: Health-information exchange: why are we doing it, and what are we doing? J. Am. Inf. Assoc. 18, 678–682 (2011)
Dobrey, A., Haesner, M., Hüsing, T., Korte, W.B., Meyer, I.: Benchmarking ICT use among general practitioners in Europe: final report. Empirica, Germany (2008)
Schoen, C., Osborn, R., Squires, D., Doty, M., Rasmussen, P., Pierson, R., et al.: A survey of primary care doctors in 10 countries shows progress in use of health information technology, less in other areas. Health Aff. (2012). doi:10.1377/hlthaff.2012.0884
Guttmann, A., Schull, M.J., Vermeulen, M.J., Stukel, T.A.: Association between waiting times and short term mortality and hospital admission after departure from emergency department: Population based cohort study from Ontario, Canada. Br. Med. J. 342, d2983 (2011)
Taylor, B., Miller, E., Farrington, C.P., Petropolous, M.-C.: Autism and measles, mumps, and rubella vaccine: No epidemiological evidence for a causal association. The Lancet 353, 2026–2029 (1999)
Vinogradova, Y., Coupland, C., Hippisley-Cox, J.: Exposure to bisphosphonates and risk of cancer: a protocol for nested case–control studies using the QResearch primary care database. Br. Med. J. Open 2, e000548 (2012)
Hippisley-Cox, J., Stables, D., Pringle, M.: QRESEARCH: A new general practice database for research. Inf. Prim. Care 12, 49–50 (2004)
Hippisley-Cox, J., Coupland, C., Vinogradova, Y., Robson, J., et al.: Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. Br. Med. J. 336(7659), 1475–1482 (2008)
Hippisley-Cox, J., Coupland, C., Robson, J., Sheikh, A., et al.: Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. Br. Med. J. 338, b880 (2009)
Graham, I.M., Stewart, M., Hertog, M.G.L.: Factors impeding the implementation of cardiovascular prevention guidelines: findings from a survey conducted by the European Society of Cardiology. Eur. J. Preventative Cardiol. 13(5), 839–845 (2006)
Department of Health: The NHS Quality, Innovation, Productivity and Prevention Challenge: An Introduction for Clinicians. Crown Copyright, London (2010)
Department of Health: The power of information: putting all of us in control of the health and care information we need. Crown Copyright, London (2012)
Light, D., Dixon, M.: Making the NHS more like Kaiser Permanente. Br. Med. J. 328, 763 (2004)
Stoves, J., Connolly, J., Cheung, C.K., Grange, A. et al.: Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual. Saf. Health Care 19(5), e54 PMID: 20554576 (2010)
Ham, C., York, N., Sutch, S., Shaw, R.: Hospital bed utilisation in the NHS, Kaiser permanente, and the US medicare programme: analysis of routine data. Br. Med. J. 327, 1257 (2003)
Keen, J., Denby, T.: Partnerships in the digital age. In: Glasby, J., Dickinson, H. (ed.) International Perspectives on Health and Social Care: Partnerships Working in Action, pp. 95–107. Blackwell Publishing Ltd, Sussex (2009). ISBN-10: 1444322591
Moore, P., Armitage, G., Wright, J., Dobrzanski, S., et al.: Medicines reconciliation using a shared electronic health care record. J. Patient Saf. 7(3), 148–154 (2011)
Department of Health: Making IT happen: information about the National Programme for IT. www.dh.gov.uk/en/Publicationsandstatistics/ (2003). Accessed 27 July 2012
Cheetham, T.C., Levy, G., Niu, F., Bixler, F.: Gastrointestinal safety of nonsteroidal anti-inflammatory drugs and selective cyclooxygenase-2 Inhibitors in patients on warfarin. Ann. Pharmacother. 43(11), 1765–1773 (2009)
Bohensky, M.A., Jolley, D., Sundararajan, V., Evans, S., et al.: Data Linkage: A powerful research tool with potential problems. BMC Health Serv. Res. 10(1), 346 (2010)
Crossfield, S.S.R., Bates, C.J., Parry, J.: ResearchOne Database Protocol. Copyright ResearchOne (2012)
Cohen, J.: A power primer. Psychol. Bull. 112(1), 155–159 (1992)
Lowrance, W.: Learning from experience: privacy and the secondary use of data in health research. J. Health Serv. Res. Policy 8(1), 2–7 (2003)
Wellcome Trust: Towards Consensus for Best Practice: Use of patient records from general practice for research. www.wellcome.ac.uk/GPrecords (2009). Accessed 01 Aug 2012
Hippisley-Cox, J., Stables, D.: QRESEARCH: An ethical high quality general practice database for research. Copyright QRESEARCH (2011)
Economic and Social Data Service: www.ccsr.ac.uk/esds/variables/ (2012). Accessed 28 Aug 2012
Powell, J., Buchan, I.: Electronic Health Records Should Support Clinical Research. Journal of Medical Internet Research 7(1), e4 URL: www.jmir.org/2005/1/e4/ (2005)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Crossfield, S.S.R., Clamp, S.E. (2014). Centralised Electronic Health Records Research Across Health Organisation Types. In: Fernández-Chimeno, M., et al. Biomedical Engineering Systems and Technologies. BIOSTEC 2013. Communications in Computer and Information Science, vol 452. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44485-6_27
Download citation
DOI: https://doi.org/10.1007/978-3-662-44485-6_27
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44484-9
Online ISBN: 978-3-662-44485-6
eBook Packages: Computer ScienceComputer Science (R0)