Abstract
This chapter presents a complete picture of current knowledge on useful and green bio-solvent “terpenes” obtained from aromatic plants and spices through a steam distillation procedure followed by a deterpenation process. Terpenes could be a successful substitute for petroleum solvents, such as dichloromethane, toluene, or hexane, for the extraction of natural products. This chapter provides the necessary theoretical background and some details about extraction using terpenes, the techniques, the mechanism, some applications, and environmental impacts. The main benefits are decreases in extraction times, the amount of energy used, solvents recycled, and CO2 emissions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Supercritical Fluid Extraction
- Steam Distillation
- Azeotropic Distillation
- Isoprene Unit
- Moisture Determination
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Essential Oils as Sources of Terpenes: Recovery and Composition
Essential oils are a natural complex mixture of volatile compounds synthesized by aromatic plants. Known for their medicinal properties and their fragrance, they have been used since ancient times for various purposes including medical treatments, food preservatives, and flavoring of food. According to ISO and AFNOR standards, essential oils are defined as products obtained from raw plant materials that must be isolated by physical methods such as steam distillation, water distillation, water-steam distillation, or cold pressing for citrus peel oils. Following distillation, the essential oil is physically separated from the water phase [1, 2]. They can also undergo a secondary treatment such as deterpenation or rectification intended to eliminate partially or completely a component or a group of components [3–5].
Essential oils exist almost only at the higher plants. The kinds able to synthesize the components which compose them are distributed in about 50 families, of which much is of Lamiaceae, Asteraceae, Rutaceae, Lauraceae, and Magnoliaceae. They can be obtained from various parts of an aromatic plant such as flowers, fruits, leaves, buds, seeds, twigs, bark, herbs, wood, and roots.
Essential oils are stored in specialized histological structures (glandular trichomes, secreting cells, epidermic cells, cavities, channels), often localized on or near the surface of the plant. If all the parts of the same species can contain an essential oil, the composition of this one can vary according to its localization. Thus, in the case of the bitter orange tree, the peel of the fruit provides the essential oil of bitter orange or Curaçao essence; the flower provides the neroli essence and the water distillation of sheet, branchless, and small fruits leads to the small grain bigaradier essence. The chemical compositions of these three essential oils are different.
Essential oils are liquid, volatile, soluble in usual organic solvents, liposoluble, and generally lighter than water in which it is insoluble. The quantity (yield which is generally between 0.005 and 10 %; often lower than 1 %) and quality (chemical composition) of an essential oil depend on several parameters: extraction process (steam distillation, water distillation, or water-steam distillation), operating conditions (length of distillation time, temperature, pressure, etc.), and plant material (part of the plant, environmental factors, cultivation methods, existence of chemotypes, and influence of the vegetative cycle, etc.) [6].
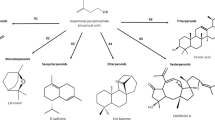
The chemical composition of essential oils is relatively complex. The number of compounds varies from oil to another and can exceed the 100 components; the major compounds can represent more than 85 % of essential oil [6]. Two types of compounds can be found in essential oils: hydrocarbon compounds known as terpenes (monoterpenes, sesquiterpenes) and oxygenated compounds or terpenoids which are functionalized terpenes (alcohols, aldehydes, ketones, esters, phenols, etc.). These compounds are classified according to the number of isoprene units (2-methyl butadiene) which constitute them. Monoterpenes, the structure of which presents two isoprene units, are natural compounds characteristic of essential oils. They can, sometimes, represent more than 90 % of the chemical composition as it is the case of citrus essential oil with more than 95 % of limonene, or turpentine oils (85 % of α-pinene) [1].
Essential oils can be recovered using a number of isolation methods. These may include the conventional or innovative methods. Steam distillation, water distillation, and water-steam distillation are the conventional methods usually used. Although these methods are simple to use, they present disadvantages such as long durations of treatment that can deteriorate the quality of extracted oils and a very significant energy consumption. The innovative technique may include use of microwaves, liquid carbon dioxide, and mainly low- or high-pressure distillation employing boiling water or hot steam. These methods are more efficient and energy saving and give a better essential oils.
Essential oils are principally used in the perfume industry; they are also used in the pharmaceutical industry, in particular in the field of external disinfectants and more generally for aromatization of the medicaments intended to be managed by oral way or as raw materials for the synthesis of active ingredients of medicaments, vitamins, etc. Essential oils also find applications in various industries such as the food industry (soft drinks, confectionery, dairy products, soups, sauces, snack bars, bakery, and in animal nutrition).
In addition, substantial quantities of essential oils are used in the preparation of toilet soaps, perfumes, cosmetics, and other home care products [7]. These last years, new aspects concerning the use of essential oils for exploitation on the production of bio-solvent have gained increasing interest. This interest was justified by several researches undertaken on the use of terpenes as green solvents for substitutions of petrochemical solvents. Essential oils constitute a safe and economically attractive renewable source of these solvents.
9.2 Terpenes: Physicochemical and Solvation Properties
The relevant properties of terpene solvent as compared to n-hexane as a solvent are listed in Table 9.1. Terpenes have similar molecular weights and structures to substitute n-hexane. Solubility parameters of solvents have been studied by means of Hansen Solubility Parameters (HSPs) [8]. The HSPs were developed by Charles M. Hansen and provide a way to describe a solvent in terms of its nonpolar, polar, and hydrogen-bonding characteristics. The HSPs work on the idea of “like dissolves like” where one molecule is defined as being “like” another if it bonds to itself in a similar way. The overall behavior of a solvent is characterized by three HSPs: δ d, the energy from dispersion bonds between molecules; δ p, the energy from dipolar intermolecular force between molecules; and δ h, the energy from hydrogen bonds between molecules. n-Hexane and terpenes have similar values of the three descriptive terms; they likely behave similarly in practice. From this point of view, the terpenes are as effective as hexane to dissolve oils.
Figure 9.1 shows the Hansen model by plotting the δ p parameter against the δ h parameter, representing the dipole and hydrogen-bonding interactions of each chemical, respectively, for lipid classes of Nannochloropsis oculata and Dunaliella salina microalgae functions of different solvents. From this figure, it is interesting to spot visually miscibility homogeneous area where it can find extraction solvents such as n-hexane, terpenes and chloroform, and microalgae lipids of interest such as triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG), and free fatty acids (FFA).
9.3 Examples of Extraction Using Terpenes
Because of the consumers’, and numerous regulation authorities, concerns with safety, environment, and health, which require a better control in the chemical and food industries, a new tendency to return towards the natural products is currently observed. Natural products, such as fruits and vegetables, spices, aromatic herbs, and medicinal plants, are complex mixtures of bioactive compounds such as lipids, proteins, vitamins, sugars, fibers, aromas, essential oils, pigments, antioxidants, etc. Extraction of these bio-compounds requires the use of petroleum solvents such as hexane, dichloromethane toluene, acetone, chloroform, etc. However, these solvents are classified as hazardous for the environment and health.
Due to these negative effects and the increasingly severe regulations aiming at the restriction of their use or their total elimination, such solvent has to be avoided as much as possible. Therefore, increasing interest was given to find alternative solvents more reliable and safer for the environment and health. In this context, several innovations towards green solvents have been developed: solvent-free technology [9, 10], use of water as alternative solvent [11], and use of ionic liquids that have low vapor pressure and less emission of COV [12, 13].
Terpenes were also investigated in this field. They are found in essential oils and oleoresins of fruit and aromatic plants and considered as renewable solvents, which have a safety impact, less hazard risks, and less environmental impact; consequently they can be a real substitution to petroleum solvents. The most commonly used terpene as solvent is probably d-limonene which represents a major by-product of the citrus fruits industry [14, 15]. Its physical properties were compared with those of hexane in order to extract fat and oil from oleaginous seeds [16, 17] or oil from rice bran [18, 19]. Limonene was also compared with toluene in the Dean-Stark procedure based on its ability to form an azeotropic mixture with water [20]. Recently, d-limonene was also used as a green solvent as a substitute of dichloromethane for carotenoid extraction especially lycopene [21].
9.3.1 Pinene: Origin, Applications, and Properties
Another monoterpene susceptible to be an interesting alternative solvent is α-pinene. It is a monoterpene hydrocarbon which represents the major constituent of turpentine oil from most conifers and a component of the wood and leaf oils obtained from leaves, bark, and wood of a wide variety of plants like rosemary, parsley, basil, yarrow, and roses [22].
Turpentine is a by-product of the wood and paper industry; its annual world production was more than 130–150,000 T/year, which makes it an abundant and cheap product. It constitutes 30 % of pine resin and is the most significant source in volume of volatile organic compounds. Its composition is generally rich in pinenes, 60 % of α-pinene and its isomer β-pinene; their respective proportions vary according to the geographical origin of the pines. Pinene was generally obtained by fractional distillation of steam-distilled wood turpentine. It is commonly used in the fragrance and flavor industry – and as an insecticide, solvent, and perfume bases as well as for camphor’s synthesis. It is completely miscible with oils and insoluble in water. These last years, several researches were carried out to test the possibilities of using pinene as a substitute of petroleum solvents for the extraction of bioactive compounds.
9.3.2 Pinene as an Alternative Solvent for Soxhlet Extraction
Oils and fats constitute a significant share of food and can have various origins: animal or vegetable. Because of its very varied composition (complex mixture of glycerides, free fatty acid, squalene, sterols, tocopherols, alkaloids, etc.), the definition of lipids was not yet clearly established. However, it is this composition which confers its taste, texture, odor and it is at the same time characteristic and particular according to its source [23]. Fats constitute a subclass of lipids; they gather the whole of fatty acids isolated in a lipidic extract [24]. These compounds, provided by food, can play an essential role in all the forms of lives in order to provide daily energy. They also intervene in certain biological mechanisms like the transport of hormones and vitamins or the integrity of the membranes of the cells [25, 26]. The interest brought to the fats is today growing, in particular because of consumers and medical authorities who require a better control of quantities and a quality of these compounds potentially absorptive in food. Consequently, dietetic and nutritional properties of these compounds, as their implications on health, are more and more controlled and require fast and effective methods of analyses.
Nowadays n-hexane is the most used solvent for extraction of oils and fats using the Soxhlet extraction [27–32]. This choice is based on its properties, namely, nonpolar, a high selectivity to fats and oils, a relatively low boiling point (69 °C), a rather low latent heat of vaporization (29.74 kJ/mol) which allows an easy evaporation, an efficient extraction, and a limited energy cost. Despite these advantages, it is ranked on top of the list of hazardous solvents and classified as harmful, irritant, and dangerous for the environment and may cause disorders of the central nervous system and fertility problems. Due to these negative effects, the possibility to use α-pinene as a substitute solvent to n-hexane for extraction of oil was investigated.
9.3.2.1 Fats and Oils from Crops
In 2013, Bertouche et al. [33] proposed to use α-pinene to extract oil of some oilseed products: peanuts, soya, sunflower, and olive. Oils were recovered using Soxhlet extraction (Fig. 9.2), according to standardized procedure [34]. The comparison of the results with that obtained with n-hexane showed that yields of α-pinene extracts were slightly higher than that of n-hexane. This difference is probably due to the polarity of the α-pinene slightly higher than that of hexane, which has as a consequence a more significant capacity for triglyceride dissolution. Gas chromatography coupled to mass spectrometry (GC-MS) and gas chromatography (GC) analyses of free fatty acid methyl ester (FAME) derivatives indicate that fatty acids extracted by both solvents are equivalent in terms of compounds identified and relative proportions. The data revealed a good agreement with literature data, and no significant differences (P > 0.05) were detected for both methods. Peanut and olive oils were characterized by strong monounsaturated fatty acid (MUFA) contents including oleic acid (C18:1) as a main component, whereas sunflower and soya oils are richer in polyunsaturated fatty acids (PUFAs) with linoleic acid (C18:2) as a principal compound.
9.3.2.2 Lipids from Microalgae
Another application using α-pinene instead of n-hexane was developed by Dejoye Tanzi et al. [35]. It concerns the extraction of oil from microalgae (Chlorella vulgaris) by means of Soxhlet extraction. In this case also, α-pinene gives better yield of oils than n-hexane, and the fatty acid composition is similar for both solvents. The main compounds are palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:3). This composition is comparable to that observed by other authors [36, 37].
α-Pinene was also used in a simultaneous distillation and extraction process (SDEP) for extraction of lipids from wet microalgae (Nannochloropsis oculata and Dunaliella salina) [38]. This procedure makes it possible to eliminate simultaneously water present in the sample followed by the extraction of oil. The innovation brought by this method is double: on one hand drying algae before extraction of oils is not anymore necessary, and on the other hand, only one green solvent (α-pinene) is used instead of drying procedure followed by petrochemical solvents – n-hexane to extract oil. Extracted lipids obtained using this new procedure and conventional Soxhlet with n-hexane have been compared in terms of total lipid content and fatty acid composition. Lipid yields for N. oculata and D. salina obtained by SDEP procedure were higher than that obtained by Soxhlet extraction. These results were in agreement with that previously reported for Chlorella vulgaris and oilseed products and that reported in the literature [16–19] and explained by the difference of polarity between the solvents used. On the other hand, in SDEP procedure the matrix is in direct contact with the boiling solvent which is not the case with the conventional Soxhlet. A higher dissolving ability of terpenes for lipids might also be pointed out by the higher temperature used to boil this solvent which could produce a lower viscosity of the analytes in the matrix and, accordingly, a better diffusion rate of the solute from the solid phase to the solvent. From a qualitative point of view, there is no significant difference in fatty acid composition obtained by the two methods using bio-based (pinene) and petroleum (hexane) solvent.
9.3.2.3 α-Pinene Recycling Capacity
In addition to the physical properties (polarity, selectivity, capacity of dissolution, toxicity, etc.), one of the parameters to be taken into account in the choice of a solvent is its capacity of recycling. In the case of the extraction by n-hexane, the solvent is separated from the extract in a vacuum rotary evaporator (Fig. 9.2). The boiling point of hexane is low (69 °C); this procedure is simple to realize. But for α-pinene, the boiling point is very high (156–158 °C); its elimination with the rotavapor requires a high vacuum that could degrade the recovered extracts with a more significant energy consumption. In order to resolve this problem in terms of energy and temperature, the recovery of oil was carried out using a Clevenger distillation of a mixture (oil + α-pinene), a method suggested by Virot et al. [18] for lipid extraction by d-limonene. This method was inspired by hydrodistillation using a Clevenger apparatus of essential oils, whose terpenes are the primary constituents (Fig. 9.2). This process, based on the principle of an azeotropic distillation with water, allows the extraction of compounds at a temperature lower than 100 °C (97–98 °C) at atmospheric pressure and even lower if reduced pressure is applied regardless of the high boiling point (150–300 °C) of terpenes. The recycling rate of α-pinene by this method, which is close to 90 %, is significantly higher than that of n-hexane (50 %), which constitutes an additional advantage for its use for the extraction of oils and fatty acids.
9.3.3 Pinene as an Alternative Solvent for Dean-Stark Distillation of “In Situ” Water
Oven drying is the most common method used for moisture determination which represents a key step in food analysis. However, for a sample containing volatile compounds, the distillation method is the most suitable method. Several methods have been developed, and nowadays, the reference method for moisture determination in food products containing volatile compounds is the Dean-Stark distillation [39]. The principle of this method consists of an azeotropic distillation between water and petroleum solvents: toluene or xylene. However, these solvents are flammable and dangerous fire risk. They are toxic by ingestion, inhalation, and skin absorption and have detrimental health effects, especially on the nervous system, on the liver, and on the auditory function [40, 41]. Consequently, they are to be avoided as much as possible. In this context, Bertouche et al. [42] investigate the possibility to use α-pinene instead of toluene in the Dean-Stark procedure for moisture determination in food products (Fig. 9.3). The results of the moisture determination of all investigated matrices (coriander and caraway seeds, onion, garlic, carrot, leek, olive, and oregano) show that the values obtained with the two solvents are comparable and were not statistically different.
In order to confirm the effectiveness of pinene, the kinetic distillation for both toluene and pinene for moisture determination of carvi seeds was followed. As shown in Fig. 9.4, the kinetics were similar for the two solvents, and only small variations could be observed in the beginning of the water recovery with pinene which is delayed for 4 min. These variations can be explained by the difference in boiling point of the solvents. Indeed, the boiling point of α-pinene (BP = 154 °C) is higher than toluene (111 °C), as the mean time boiling point of azeotropic pinene/water (BP = 97–98 °C) is also higher than azeotropic toluene/water mixture (84 °C); consequently, the beginning of the water recovery is delayed for pinene. However, when the distillation started the water recovery was faster when using α-pinene. Indeed 40 min provides the water content comparable to those obtained after 105 min with toluene. This reduction in the processing time represents a profit of more than 60 % in terms of time and thus in consumption of energy. These results were in agreement with those cited in the literature for limonene [20], from moisture content point of view, reproducibility of results, and kinetics of distillation. Thus, α-pinene is as effective as limonene and can be used like green solvent for the determination of the water content of food products to replace toluene.
9.3.4 Pinene as an Alternative Solvent for Extraction of Carotenoids from By-Products
Carotenoids are orange-red pigments belonging to the chemical family of terpenoids. They are formed by polymerization of isoprene units to an aliphatic or alicyclic structure. This group of compounds can be synthesized by a great number of plants, algae, and bacteria and present very interesting antioxidant properties and potential beneficial health properties such as prevention of cancer [43], cardiovascular diseases [44], or macular degeneration [45]. They are used as food additives, cosmetic colorants, and antioxidants in the pharmaceutical industry. Due to these properties and an increase in demand for natural products, the interest carried to these compounds is increasing.
Extraction of carotenoids is usually achieved by organic solvents generating great yields of extraction. However, these solvents are harmful and generate problems of health and a great amount of waste of questionable environmental disposal. Consequently, alternative extraction methods using green solvents are under research. In this context, the use of vegetable oils for carotenoids extraction using canola, soybean, and olive oil as cosolvents has been successfully performed by supercritical fluid extraction, resulting in a yield two to four times higher [46, 47]. Carotenoids extraction was also performed using sunflower oil as extraction media in an ultrasound-assisted extraction (UAE) [48]. This original procedure was compared with conventional solvent extraction (CSE) using hexane as a solvent. The results showed that the UAE using sunflower as a solvent gives a β-carotene yield of 334.75 mg/L, in only 20 min, while CSE using hexane as a solvent gives a similar yield (321.35 mg/L) in 60 min. Limonene is another green biodegradable solvent that has been suggested as a good alternative to organic solvent for carotenoid extraction from matrices such as tomatoes [21] and microalgae [49].
Besides vegetable oils and limonene, α-pinene was also used as an alternative to hexane for carotenoid extraction. In this context, a study was performed in order to optimize the β-carotene extraction from dried ground carrot by maceration in α-pinene (Fig. 9.5). The response surface methodology using a face-centered central composite design (CCD) was carried out. The parameters chosen for optimization were temperature (ranging from 20 to 40 °C) and solid-to-solvent ratio (10-30 %). The optimal yield obtained with \( \boldsymbol{\upalpha} \)-pinene was equal to 8.67 %, and the optimum conditions of β-carotene extraction obtained by statistical analysis of CCD results were 23 % for a solid-to-solvent ratio and 40 °C for temperature. For comparison, extraction of β-carotene with hexane in the optimal conditions was performed. The yield obtained (8.21 %) is similar to that obtained by \( \boldsymbol{\upalpha} \)-pinene. We can assess that \( \boldsymbol{\upalpha} \)-pinene may be an interesting sustainable way to replace petroleum-origin solvents for carotenoid extraction.
References
AFNOR (2000) Huiles essentielles, monographie relative aux huiles essentielles. Edition, Paris
Pharmacopée Européenne (1996) Conseil de l’Europe. Maisonneuve S.A. Editions, Sainte Ruffine
Diaz S, Espinosa S, Brignole EA (2005) Citrus oil deterpenation with supercritical fluids. Optimal process and solvent cycle design. J Supercrit Fluids 35:49–61
Arce A, Pobudkowska A, Rordíguez O, Soto A (2007) Citrus essential oil terpenless by extraction using 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid: effect of the temperature. Chem Eng J 133:213–218
Danielski L, Brunner G, Schwänke C, Zetzl C, Hense H, Donoso JPM (2008) Deterpenation of mandarin (Citrus reticulata) peel oils by means of countercurrent multistage extraction and adsorption/desorption with supercritical CO2. J Supercrit Fluids 44:315–324
Naves YR (1974) Technologie et Chimie des parfums Naturels. Masson & Cie, Paris
Raeissi S, Diaz S, Espinosa S, Peters CJ, Brignole EA (2008) Ethane as an alternative solvent for supercritical extraction of orange peel oils. J Supercrit Fluids 45:306–313
Hansen CM (1967) The three dimensional solubility parameter – key to paint component affinities II. – dyes, emulsifiers, mutual solubility and compatibility, and pigments. J Paint Technol 39:505–510
Michel T, Destandau E, Elfakir C (2011) Evaluation of a simple and promising method for extraction of antioxidants from sea buckthorn (Hippophaërhamnoides L.) berries: pressurised solvent-free microwave assisted extraction. Food Chem 126:1380–1386
Khan ZH, Abert Vian M, Maingonnat JF, Chemat F (2009) Clean recovery of antioxidant flavonoids from onions: optimising solvent free microwave extraction method. J Chromatogr A 1216:5077–5085
Kronholm J, Hartonen K, Riekkola ML (2007) Analytical extractions with water at elevated temperatures and pressures. Trends Anal Chem 26:396–412
Leveque J, Cravotto G (2006) Microwave, power ultrasound and ionic liquids, a new synergy in green chemistry. Chimia 8:313–320
Scammells P, Scott J, Singer R (2005) Ionic liquids: the neglected issues. Aust J Chem 58:155–169
Njoroge SM, Koaze H, Karanja PN, Sawamura M (2005) Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis). Flav Fragr J 20:80–85
Mira B, Blasco M, Berna A, Subirats S (1999) Supercritical CO2 extraction of essential oil from orange peel. Effect of operation conditions on the extract composition. J Supercrit Fluids 14:95–104
Virot M, Tomao V, Ginies C, Visinoni F, Chemat F (2008) Green procedure with a green solvent for fats and oils’ determination Microwave–integrated Soxhlet using limonene followed by microwave Clevenger distillation. J Chromatogr A 1196–1197:147–152
Virot M, Tomao V, Ginies C, Chemat F (2008) Total lipid extraction of food using d-limonene as an alternative to n-hexane. Chromatographia 68:311–313
Mamidipally PK, Liu SX (2004) First approach on rice bran oil extraction using limonene. Eur J Lipid Sci Technol 106:122–125
Liu SX, Mamidipally PK (2005) Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem 82:209–215
Veillet S, Tomao V, Ruiz K, Chemat F (2010) Green procedure using limonene in the Dean-Stark apparatus for moisture determination in food products. Anal Chim Acta 674:49–52
Chemat-Djenni Z, Ferhat MA, Tomao V, Chemat F (2010) Carotenoid extraction from tomato using a green solvent resulting from orange processing waste. J Essent Oil Bear Plants 13:139–147
Fernandez X, Chemat F (2012) La chimie des huiles essentielles – Tradition et innovation. Vuibert, Paris
Carrasco-Pancorbo A, Navas-Iglesias N, Cuadros-Rodríguez L (2009) From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: modern lipid analysis. Trends Anal Chem 28(3):263–278
Luque-García JL, Luque de Castro MD (2004) Ultrasound-assisted soxhlet extraction: an expeditive approach for solid sample treatment. Application to the extraction of total fat from oleaginous seeds. J Chromatogr A 1034:237–242
Desvergne B, Michalik L, Wahli W (2006) Transcriptional regulation of metabolism. Physiol Rev 86:465–514
Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM (2008) High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res 49:199–205
Luque-García JL, Luque de Castro MD (2003) Extraction of polychlorinated biphenyls from soils by automated focused microwave-assisted Soxhlet extraction. J Chromatogr A 998(1–2):21–29
Priego-Capote F, Luque de Castro MD (2005) Focused microwave-assisted Soxhlet extraction: a convincing alternative for total fat isolation from bakery products. Talanta 65(1):81–86
Virot M, Tomao V, Colnagui G, Visinoni F, Chemat F (2007) New microwave-integrated Soxhlet extraction: an advantageous tool for the extraction of lipids from food products. J Chromatogr A 1174(1–2):138–144
Pu W, Zhang Q, Wang Y, Wang T, Li X, Ding L, Jiang G (2010) Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal Chim Acta 663(1):43–48
Go-Woon J, Hee-Moon K, Byung-Soo C (2011) Characterization of wheat bran oil obtained by supercritical carbon dioxide and hexane extraction. J Ind Eng Chem 18(1):360–363
Eikani M, Fereshteh G, Homami S (2012) Extraction of pomegranate (Punica granatum L.) seed oil using superheated hexane. Food and Bioprod Process 90(1):32–36
Bertouche S, Tomao V, Hellal A, Boutekedjiret C, Chemat F (2013) First approach on edible oil determination in oilseeds products using α-pinene. J Essent Oil Res 25(6):439–443
ISO 659–1988 (E) (1988) Graines oléagineuses, détermination de la teneur en huile. International Organization for Standardization (ISO), Genève
Dejoye Tanzi C, Abert Vian M, Chemat F (2013) New procedure for extraction of algal lipids from wet biomass: a green clean and scalable process. Bioresour Technol 134:271–275
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella. Biochem Syst Ecol 35:281–285
Plaza M, Santoyo S, Jaime L, Avalo B, Cifuentes A, Reglero G, García-Blairsy G, Señoráns FJ, Ibáñez E (2011) Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT- Food Sci Technol 46:245–253
Dejoye Tanzi C, Abert Vian M, Ginies C, El maataoui M, Chemat F (2012) Terpenes as green solvents for extraction of oil from microalgae. Molecules 17:8196–8205
AOCS Official Method Ja 2a-46 (1993) American Oil Chemist’ Society, Champaign
Hass U, Lund SP, Hougaard KS, Simonsen L (1999) Developmental neurotoxicity after toluene inhalation exposure in rats. Neurotoxicol Teratol 21:349–357
Mc Williams ML, Chen GD, Fechter LD (2000) Low-level toluene alters the auditory function in guinea pigs. Toxicol Appl Pharmacol 1(167):18–29
Bertouche S, Tomao V, Ruiz K, Hellal A, Boutekedjiret C, Chemat F (2012) First approach on moisture determination in food products using α-pinene as an alternative solvent for dean–stark distillation. Food Chem 134:602–605
Andre CM, Larondelle Y, Evers D (2010) Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr Nutr Food Sci 6:2–12
Riccioni G, Mancini B, Di Ilio E, Bucciarelli T, D’Orazio N (2008) Titre. Eur Rev Med Pharmacol Sci 12:183–190
Snodderly M (1995) Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 62:1448S–1461S
Krichnavaruk S, Shotipruk A, Goto M, Pavasant P (2008) Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Biores Technol 99:5556–5560
Sun M, Temelli F (2006) Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J Supercrit Fluids 37:397–408
Li Y, Fabiano-Tixier AS, Tomao V, Cravotto G, Chemat F (2013) Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason Sonochem 20:12–18
Castro-Puyana M, Herrero M, Urreta I, Mendiola JA, Cifuentes A, Ibáñez E, Suárez-Alvarez S (2013) Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 405:4607–4616
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Boutekedjiret, C., Vian, M.A., Chemat, F. (2014). Terpenes as Green Solvents for Natural Products Extraction. In: Chemat, F., Vian, M. (eds) Alternative Solvents for Natural Products Extraction. Green Chemistry and Sustainable Technology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43628-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-43628-8_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43627-1
Online ISBN: 978-3-662-43628-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)