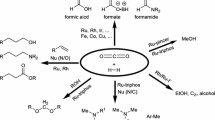
Abstract
The use of carbon dioxide as C1 source to produce chemicals and fuels can be the basis of green and sustainable chemical industries. In this regard in the last decade, significant progress has been achieved in the hydrogenation of carbon dioxide and bicarbonate using homogeneous catalysis. For example, new catalyst systems have been reported, therefore showing high activity even under mild conditions. Furthermore, for the first time catalysts based on iron and cobalt were shown to yield TON being in the same range as many noble-metal-based complexes. In addition, interesting concepts for the catalyst recycling and the formic acid product separation have been presented as well as systems capable of hydrogen storage. Very recently, promising systems for the converting of CO2 to methanol with H2 have been developed opening new ways to a future methanol economy. Further reduction to CH4 with molecular hydrogen is still not known, but related reductions of carbon dioxide using boranes or hydrosilylation reagents can gain an interesting insight into the mechanism of such processes.
This chapter summarizes the recent developments in the reduction of carbon dioxide with molecular hydrogen and other hydride reagents starting from 2005.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In today’s chemical industry, most of the product chains are based on fossil resources like petroleum, coal, or natural gas. Although it is known that these resources are limited, the major part is “just” burned as fuels, and CO2 is emitted into the atmosphere with incalculable risks for climate change [1].
Therefore, new concepts for the future have to be developed to meet the increasing demand of energy and materials by ensuring environmental safety. Using the abundant greenhouse gas CO2 as C1 building block to produce chemicals and fuels would close the anthropogenic carbon cycle and save natural resources [2].
However, the thermodynamic stability and the low reactivity of CO2 require highly active catalysts and high energy starting materials. Nature shows that such a transformation is practicable. By using photosynthesis, the biological reduction of CO2 with sunlight and water to organic carbohydrates, 385∙109 t of CO2 is fixed annually net [3]. Therefore, enzymes and different kind of mechanism have been evolved and optimized in billions of years by nature. Biochemical studies on active sites of these enzymes can be of benefit for understanding and developing new synthetic catalysts capable of CO2 activation [4].
In the last decades, chemists have followed this dream and pioneering work toward catalytic transformation of CO2 has been reported [5–7]. Especially, the reduction of CO2 to products of higher hydrogen content is of great interest. For example, direct hydrogenation of CO2 to MeOH with green, nonfossil-based hydrogen is discussed to be a key step for building up a methanol economy as MeOH can be converted into fuels as well as valuable chemicals such as olefins and aromatics [6, 8, 9].
Despite all improvements in the last years, the catalyst costs of homogeneous catalysts for hydrogenation of CO2 are still too high for industrial applications and only few heterogeneous processes are established [10, 11]. However, in the last 10 years significant progress was observed that makes it more likely that industrial processes based on homogeneous catalysis will be set up, too.
This chapter summarizes recent developments in this field starting at 2005 including hydrogen and other hydride reagents. Earlier findings have been well presented by Jessop, Leitner, and others [12–15].
Before 2005, active catalyst systems have been developed to give HCOOH and formamides and their mechanism have been intensively studied. However, the separation of HCOOH from base and catalyst as well as the use of CO2 as hydrogen storage material have been neglected—but are hot topics in this decade. Following the principles of Green Chemistry, new systems have been developed in the last years using green solvents, ambient conditions, bio-inspired catalysts, as well as non-precious metals [16]. As the combustion of fossil fuels is one of the major reasons for increasing CO2 amount in the atmosphere, the synthesis of fuels out of CO2 using clean, nonfossil-based H2 is highly desirable [17]. Therefore, reducing CO2 to MeOH or CH4 is of great interest, and promising catalytic systems have been reported recently for gaining MeOH. Homogeneous catalysts capable of hydrogenating CO2 to CH4 with H2 are still unreported, but interesting activities have been achieved using a combination of boranes and silanes.
2 Hydrogenation of CO2/HCO3 − to Formic Acid Derivatives
The direct hydrogenation of CO2 to HCOOH is an endergonic reaction. Therefore, base is often applied to drive the thermodynamics by proton transfer. Available products are formates, formic acid–base adducts, as well as alkyl formates in the presence of alcohols and formamides by using primary or secondary amines (Eq. 4.1). Catalytic systems reported before 2005 are usually noble-metal systems with mono- or bisphosphine ligands as well as a few examples of bipyridine-based ligand structures. The best catalyst presented until 2005 is the (PMe3)4RuCl(OAc) catalyst from Jessop which hydrogenates CO2 at 50 °C and 200 bar of H2/CO2 with high TON of 32,000 and an astonishing TOF of 95,000 h−1 [18].
Since then, new ligand structures have been developed and applied successfully in the hydrogenation of CO2 to formic acid derivatives. This section gives an overview of recent results subdivided by ligand classes.
2.1 Using Mono- and Bidentate Phosphine Ligands
As mentioned above, the Ru trimethylphosphine catalyst system [18] was one of the most active ones until 2005. Regarding the high interest in understanding the mechanism, several computational and analytical studies have been reported identifying possible reaction species (Scheme 4.1). All of them have in common that a Ru dihydride species 1 with four phosphine ligands exists. The dissociation of one phosphine ligand leads to cis-Ru(H)2(PMe3)3. Here, Sakaki started his investigations with DFT and MP4(SDQ) methods to figure out differences between this real catalyst and the model catalyst cis-Ru(H)2(PH3)3 [19]. As the PMe3 group is more donating than its analog, the trialkyl phosphine ligand showed a stronger trans-influence than PH3. Both, strong donating ligands and polar solvents, made the CO2 insertion into the metal-H bond more favorable. This step is proposed to be the rate-determining step in the hydrogenation of CO2 with 2 in absence of water. The catalytic cycle can be described in the following steps: CO2 insertion into Ru(II)-H,isomerization of the ruthenium(II)-η 1-formate intermediate 3, six-centered σ-bondmetathesis of the η 1-formate with a dihydrogen molecule 4, and dissociation of formic acid to give again the dihydride species (Scheme 4.1, phosphine dissociation mechanism (a), phosphine groups are drawn as P for clarity).
Taking into account that water was observed to accelerate the catalytic system, the group of Sakaki presented a second computational study using cis-Ru(H)2(PMe3)3(H2O)2 as catalytic intermediate [20]. They figured out that aqua ligands can accelerate the nucleophilic attack of the hydride ligand on the CO2 molecule by forming hydrogen bonds (6). The resulting ruthenium(II)-η 1-formate 3 isomerizes fast in the presence of water which suppresses the deinsertion of CO2. The rate-determining step now is believed to be the coordination of the H2 molecule to the ruthenium(II)-η 2-formate complex. The last step, the heterolytic H2 cleavage, is facilitated again by water molecules (7). However, Jessop’s NMR results have been inconsistent with such a phosphine dissociation mechanism. For this reason, he proposed a different cycle based on the unsaturated, cationic ruthenium species [(PMe3)4RuH]+ 8 [21]. This mechanism also explains the necessity of the added base. The base on the one hand traps the formic acid and on the other hand helps to form the active catalytic species by abstracting a hydride ligand leading from 1 to 8.
Most recently, our group synthesized benzoyl- and naphthoyl-substituted phosphines which are stable to air and moisture [22]. The related Ru complexes showed good activity in the bicarbonate reduction with H2 at 80 °C (TON up to 1,600). CO2 as well as carbonyl compounds could be hydrogenated, too.
Using simple phosphite ligands at the Ru center such as [trans-RuCl2{P(OMe)3}4], the group of Thiel achieved good TON up to 6,630 and TOF of 1,655 h−1 under supercritical conditions [23]. This was the first time phosphite ligands have been shown to form active complexes for the hydrogenation of CO2. However, the application of the expensive base DBU in the presence of C6F5OH is a drawback and an exchange through cheaper bases is highly desirable.
Taking advantage of the water-accelerating effect, Zhao and Joó published a water-soluble [RhCl(mtppms)3] catalyst capable of CO2 hydrogenation to free formic acid in aqueous HCOONa solutions [24]. However, only low TON < 150 was observed after 20 h at 100 bar pressure (CO2/H2 – 1:1) and 50 °C. This might be due to the low basicity of the formate.
The hydrogenation of CO2 to HCOOH is reversible. Hence, many catalyst systems have been developed which efficiently and selectively decompose HCOOH to hydrogen and CO2 [25–27]. Hydrogen, ideally produced by renewable energy sources, for example, by electrolysis of water, is a potentially explosive, volatile gas which is usually stored in high pressure tanks [28–31]. By reacting it with CO2 or bicarbonate, H2 can be stored and transported easily as liquid or solid and H2 can be released easily if needed. Therefore, researchers developed different systems capable of storing hydrogen based on CO2/HCOOH or bicarbonate/formate. Both the groups of Papp and Joó [32] as well as our group [33] presented at the same time catalyst systems able of recharging H2 based on a bicarbonate/formate cycle. In comparison to CO2, bicarbonate is well soluble in water and its solutions are easy to handle and can be converted catalytically under mild conditions. As these systems work amine-free, no base evaporation is possible with the H2 stream.
Papp and Joó observed reversibility with the [RuCl2(mtppms)2]2 complex in aqueous bicarbonate solution in a sapphire NMR tube [32]. They showed three cycles of both the hydrogenation of bicarbonate to formate and the dehydrogenation of formate to bicarbonate. However, the decomposition of the formate slows down significantly at around 50 % conversion. Therefore, only half of the formate amount can be used for hydrogen storage in this system. Higher yields for the dehydrogenation of formates at even lower temperature (70 % at 40 °C) were shown by our group with a [RuCl2(benzene)]2 precursor in the presence of 4 eq of dppm as ligand [33]. In comparison to earlier studies [34], the yields for the bicarbonate reduction could be improved to 96 % at 70 °C, using 80 bar of H2 in a water-THF mixture without additional CO2. A good TON of 1,108 was achieved after 2 h. Exchanging the sodium cation through NH4 + or Li+, K+, Mg2+, and Ca2+cations, the productivity decreases and often a significant amount of CO2 is released with the H2 during the formate decomposition. Our group also demonstrated that the catalyst is capable of hydrogen storage at RT based on CO2/HCOOH∙NEt3 (Scheme 4.2). The catalyst system runs eight cycles without showing significant decrease in activity [35].
For the hydrogenation at RT, high formic acid amine ratios up to 2.31 were observed with TON up to 3,200. In addition, we could present an astonishing TON up to 800,000 for continuous H2 liberation using [RuCl2(benzene)]2 and dppe. However, it should be noted that the gravimetric hydrogen content of formic acid is limited to 4.4 % which is further reduced by the addition of amine or solvent.
To clarify possible reaction mechanisms for complexes with bidentate phosphine ligands, ab initio MTD and MD studies were performed by the group of Baiker for the dihydride catalyst [Ru(dmpe)2(H)2] 13 [36]. This study was supported by further DFT calculations as well as IR and NMR experiments [37]. The authors concluded the coordination of molecular H2 to the complex is the rate-determining step. That requires dissociation of the formate from the Ru center 14 to leave a free coordination site for the H2. The formate anion then interacts weakly with the Ru(H2)+ complex 15 (Scheme 4.3). The activation energy for the H2 adduct was found to be lower for the complex where the two hydrides are in trans position to each other (13). However, in solution more than 95 % of the cis-complex 12 was observed. The CO2 insertion is believed to happen via a concerted mechanism where the Ru-H bond breaks at the same time as the Ru-O-bond is formed. This can be imagined as a “formate ion” rotation.
Based on kinetic measurements by using stop-flow technique, Schindler’s group calculated activation parameters for a Rh catalyst involving [(dppp)2RhH] as the active species [38]. For this complex, the following mechanism was proposed: the formation of an ionic Rh(I) formate complex through insertion of CO2 into the Rh-H bond and subsequent dissociation of formate, followed by oxidative addition of H2 to give an ionic Rh(III) dihydride formate complex which under reductive elimination of formic acid yields again the neutral Rh(I) complex.
In addition to catalyst variations and mechanistic studies, different kinds of concepts have been developed to solve the problem of the formic acid separation from base and catalyst. For example, the groups of Han and Leitner presented independently two systems based on ionic liquids. That the equilibrium of CO2/H2 to HCOOH is more favored in IL than in H2O, due to the strong solvation of HCOOH in IL, was shown by Nakahara [39]. The idea of Han was to use an IL with an extra amine function 16 in combination with a ruthenium catalyst bound on silica [40]. By inserting a second amine function on the IL (17), the activity could be increased from 100 h−1 up to 920 h−1 TOF with a TON of 1,840 using 90 bar of H2 and a total pressure of 180 bar [41]. Both systems were recycled four times using the procedure described in Fig. 4.1. Addition of water accelerates the reaction, maybe due to a decreased viscosity, interaction of water in the catalytic cycle, or bicarbonate formation as the true substrate.
Leitner and co-workers were using the IL as stationary phase containing the precursors [Ru(cod)(methallyl)2] and PBu4 + mtppms− as well as additional base [42]. The formic acid extraction occurs with supercritical CO2 in a continuous-flow single process unit where scCO2 serves as mobile and extracting phase at the same time. With the base NEt3 and EMIM Cl as IL, a TON of 516 was achieved after half an hour at 50 °C and 50 bar of both CO2 and H2. By exchanging the anion of the IL through HCO2 − to give 18, increased TON and TOF were observed.
Another interesting concept for the production and separation of pure formic acid was presented by Schaub and Paciello [43]. Key to success is the skillful choice of solvents by taking advantage of the diverse solubility and miscibility of all components (Fig. 4.2).
Liquid-liquid phase process for formic acid formation and separation by Schaub and Paciello [43]
Here, in the first step, CO2 is hydrogenated with the [Ru(H)2(PnBu3)4] catalyst in the presence of NHex3 and a diol. The formed formic acid salts [NHex3∙HCOOH] are not miscible in free amine, but good soluble in the diol phase. At the same time, most of the lipophilic catalyst stays in the unpolar amine phase. To get rid of the remaining catalyst traces, the diol phase is extracted with the resulting amine of the formic acid salt cleavage. The amine is then recycled to the hydrogenation vessel, whereas the formic acid salt enriched diol phase is treated thermally under mild conditions to give pure formic acid and free NHex3 which forms again a two-phase system with the diol. Both phases are back transferred to close the process cycle.
2.2 Using Bipyridine-Based and Carbene-Type Ligands
A second ligand class known for CO2 hydrogenation is based on the bipyridine structure. In this context, the groups of Ogo and Fukuzumi started kinetic investigations of Ir(III) and Ru(II) bpy complexes in citric buffer solutions (pH = 3) to obtain pure formic acid [44]. By comparing TON in dependence of H2 or CO2 pressure, they concluded that in the case of ruthenium, the H2 coordination to the corresponding aqua complex is rate determining. This is in contrast to the iridium complexes. Here, the authors believe that the rate-determining step is the CO2 insertion into the active hydride complex.
By inserting hydroxy groups in para- or ortho-position at the pyridine rings, interesting behavior of the catalysts in dependence of the pH was reported. This ligand class shows an acid–base equilibrium between the pyridinol and pyridinolate form (Scheme 4.4) which influences the catalytic activity and water solubility.
In 2007, Himeda presented different catalysts for the CO2 hydrogenation in aqueous KOH solutions to potassium formate using Ru, Ir, and Rh half-sandwich complexes bearing DHPT or DHBP ligands [45]. Under basic conditions, the deprotonated, water-soluble form exists showing strong electronic donation properties. High TON up to 220,000 was observed for an Ir DHPT complex at 120 °C and 60 bar of each CO2 and H2 in 48 h. Lowering the pH to acidic conditions, the pyridinol form of the catalyst predominates. The catalyst becomes insoluble in water and precipitates. Now, the catalyst can be reused easily and showed maintaining activity even after four cycles. To confirm the importance of the formation of the pyridinolate species, the hydroxy groups have been methylated resulting in significantly less active complexes. Other groups in para-position like carboxy or methyl groups are inferior, too [46]. For further details see review [47].
To study the effects of the ligand structure in more detail, Himeda and Fujita synthesized new iridium half-sandwich complexes differing in position and quantity of the hydroxy groups (Scheme 4.5).
Moving the hydroxy groups from para to ortho position (19–20) resulted in a rate enhancement by a factor of 2 comparing the initial TOF after 10 min [48]. Doubling both the quantity of the hydroxy groups as well as the amount of Ir centers 21, another increase in the reactivity in order of 2.5 was observed for the hydrogenation of CO2. Surprisingly, the dinuclear complex 22 showed similar activity as the mononuclear one with four hydroxy groups 21. To explain these results, DFT studies and deuteration experiments have been performed [49]. The computational study identified the water-assisted heterolysis of H2 to be the rate-determining step. This is in line with the NMR experiments as kinetic isotope effects of H2O, H2, and bicarbonate have only been observed for the ligands containing hydroxy groups in ortho position, not para. This is the first time a kinetic isotope effect was proved for the hydrogenation of CO2, revealing that H2O is involved in the rate-determining step. The significant increase in reactivity is believed to be caused by the high σ-donor strengths of the four O− groups and the accelerated proton transfer by forming water bridges.
Using the binuclear Ir complex 21, Himeda, Fujita, and Hull showed that their system is not only capable of CO2 hydrogenation but also can decompose HCOOH to CO2 and H2 under acidic conditions [50]. High TON of 308,000 and very good TOF of 228,000 h−1 were achieved for the decomposition at 80–90 °C in the presence of HCOOH/HCOONa (1:1). Increasing the pH to basic conditions, the same catalyst hydrogenates CO2 with TON of 153,000 and TOF of 53,800 h−1 in the presence of bicarbonate at 50 °C/80 °C. To rule out bicarbonate as only substrate, a test reaction without CO2 was run yielding only a low formate production. However, long reaction times and the necessity of adding stoichiometric amounts of base or acid to switch the reaction make it so far unpractical for industrial application.
The same concept of hydrogen storage was reported by Fukuzumi using the water-soluble, proton-switchable phenylpyrazolyl organoiridium aqua complex 23 (Scheme 4.6) [51]. Both the hydrogenation of CO2 under slightly basic conditions at RT under atmospheric pressure of H2 and CO2 and the formic acid decomposition under acidic conditions are possible. Due to KIE effects, the authors concluded that the rate-determining step is the formic acid decomposition via β-hydride elimination of the formate to release CO2. In case of the hydrogenation of bicarbonate, both the catalyst and bicarbonate are believed to be involved in the rate-determining step as the TOF raises with increasing amount of bicarbonate. A more general view on these Ir-H complexes is presented in a review [52].
Further systems capable of CO2 hydrogenation were presented by Niedner-Schatteburg, van Wüllen, and Thiel [53] using catalyst 24 and the group of Süss-Fink [54] including the arene ruthenium oxinato complex 25. However, in all of these cases only moderate TONs were achieved.
Another interesting ligand class for the activation of CO2 was discovered by Peris. A series of bis-N-heterocyclic carbene complexes with Ru 26 and Ir 27 were tested in both CO2 hydrogenation and transfer hydrogenation [55]. As these chelating NHC ligands are known for their high thermal stability, reaction temperatures up to 200 °C are tolerated. Interestingly, if the carbene position is blocked by a methyl group, a bis-abnormal coordination of the NHC ligand to the iridium center is observed [56]. This complex 28 is even more active and the highest known TON of 1,320 was reported for transfer hydrogenation of CO2 using iPrOH in aqueous KOH solutions.
2.3 Using Pincer Ligands
For more than 30 years, pincer-type ligands have been known [57]. The high stability of this complexes are due to the strong coordination between the tridentate ligand and the metal center. At the same time, they offer interesting redox chemistry as these noninnocent ligands are directly involved in the reaction mechanism. This enabled new and unknown reactivities. Catalytic applications of these ligand-metal cooperations are, for example, arylation and coupling reactions, alkane dehydrogenations, hydroaminations, and a bunch of hydrogenation reactions [58–61].
Recently, the first example of CO2 hydrogenation to formate with this ligand class was presented by Nozaki. This system is highly efficient and was highlighted in 2010 [62]. In fact, Nozaki’s Ir(III)(H)3-pincer complex 29 achieved astonishing TON up to 3,500,000 in 48 h at 120 °C and TOF of 150,000 h−1 after 2 h at 200 °C using each 30 bar of CO2 and H2 in an aqueous KOH solution of 1 M [63]. The proposed mechanism is presented in Scheme 4.7. The first step is the CO2 insertion into Ir-H bond of 29 forming complex 30 with the formato group in cis position to the N atom. After dissociation of the formate, the Ar-CH2-PiPr2 moiety of the ligand is deprotonated followed by dearomatization of the pyridine ring. To this amido iridium dihydride complex 35, a hydrogen molecule coordinates which is then heterolytically cleaved to give the full-aromatized Ir(H)3 29 back. Yang reported a different pathway by studying computationally PNP-pincer complexes with Ir(H)3 and Fe/Co(H)2(CO) [64]. He claimed that the OH−-triggered H2 cleavage (37) is more favorable than Nozaki’s aromatization/dearomatization mechanism as well as Ahlquist’s suggestions based on DFT studies [65]. In response Nozaki and Morokuma presented their own computational study for the CO2 hydrogenation with complex 29 [66]. As a result, both pathways, the aromatization/dearomatization reaction as well as the hydrogenolysis suggested by Yang, are possible as their transition state energies are very similar. Deeper insight in the mechanism was provided by Hazari who examined the trans-influence of various ligands for insertion of CO2 into the Ir-H bond of 29 [67]. The strongest trans-effect was observed by the hydride ligand which is believed to weaken the Ir-H bond by increasing the nucleophilicity of this hydride making the CO2 insertion more favorable.
Other non-considered mechanisms were pointed out by Milstein’s group. They proposed a double deprotonation by methylating a Ni(II) PNP complex where the negative charge is delocalized in the amido-type backbone [68] as well as a methylated Co(I)-pincer complex which lost a H radical by homolytic C-H bond cleavage [69]. Here, the unpaired electron is delocalized in the ligand backbone and evidence for the remaining aromaticity of the pyridine ring was detected. The abstraction of the H radical is believed to be caused by the solvent as an increase of the reaction rate was observed in aliphatic solvents.
2.4 Using Non-precious Metals
Despite increasing scientific interest in the hydrogenation of CO2, the use of bio-relevant metals such as iron, manganese, or cobalt as catalysts has scarcely been investigated compared to noble-metal-based systems. Before 2005, only a few examples were reported yielding higher TON than 10 [70]. However, long reaction times and harsh conditions are needed.
In 2011, Yang predicted computationally an iron-pincer complex to be capable of CO2 hydrogenation [64]. In the same year, Milstein presented an analog Fe complex 38 (Scheme 4.8) not only active in CO2 hydrogenation but also in the bicarbonate reduction to formate [71]. In aqueous solutions, bicarbonate can be converted to the corresponding formate at ambient pressure and 80 °C with a moderate yield 30 % and a TON of 320. Using CO2, even higher TON up to 600 was achieved. Based on IR and NMR experiments, a similar mechanism as for the Ir(H)3-pincer complex 29 is proposed.
Whereas mono-, bi-, and tridentate phosphine ligands are well investigated for noble metals in the catalytic hydrogenation of CO2, tetradentate ligands have not been presented at all. The application of the tetradentate phosphine ligand P(CH2CH2PPh2)3 in combination with Fe(BF4)2∙6H2O in MeOH led to the highly active complex 39 [72]. With this complex, our group showed that bicarbonate can be reduced to formate in high yields (88 %) and TON (up to 610) at 80 °C and 60 bar of H2. Testing a variety of bidentate and tridentate ligands which failed all in generating an active catalyst in situ, it seemed to be that iron needs a very well-defined environment. Even other iron sources instead of Fe(BF4)2∙6H2O gave significant lower yield. Replacing bicarbonate by CO2, methyl formate was produced in the presence of NEt3 and the corresponding formamides were observed by using dialkyl amines as base yielding TON up to 730.
Changing the metal from Fe to Co, slightly higher yields were observed for the bicarbonate reduction and higher temperature was tolerated. In addition, lower H2 pressure is applicable [73]. TON up to 3,900 for sodium formate and 1,300 for DMF could be detected. By synthesizing Co monohydride and Co dihydrogen complexes and testing them in the catalytic hydrogenation of bicarbonate, the monohydride complex was excluded as possible active species, whereas the Co dihydrogen complex was active. These results were in correlation with the performed NMR studies.
By synthesizing the phenyl-bridged analog of the tetradentate phosphine ligand mentioned above and combining it with Fe(BF4)2∙6H2O, the most active and stable iron catalyst 40 known for the hydrogenation of bicarbonate and CO2 was developed [74]. The crystal structure of 40 exhibited an unusual Fe-F bond, where the F− originally came from the BF4 − anion. Both the in situ system and the well-defined complex 40 showed the same activity, resulting in TON up to one order of magnitude higher than the previously reported Fe systems for the bicarbonate reduction (TON 7,550). With higher catalyst loading, the reaction reached its equilibrium already after 5 h with TOF up to 770 h−1. Furthermore, the hydrogenation of CO2 in the presence of base gave formic acid, methyl formate, and formamides, the latter in TON up to 5,100. NMR studies indicated that a Bianchini-type complex [75] has been formed being in equilibrium with a Fe-hydride complex where the dihydrogen ligand has been exchanged by a solvent or a CO2 molecule. Through base-assisted heterolytic hydrogen cleavage, an active dihydride Fe complex was proposed to be formed into which CO2 inserts to give the hydride formate complex. Dissociation of the formate followed by the coordination of a dihydrogen molecule led back to the Bianchini-type complex and HCOOH∙base adduct.
Very recently, the group of Linehan published NMR experiments where Co(I)(dmpe)2H shows very high TOF up to 74,000 h−1 for the hydrogenation of CO2 to formate [76]. However, by using other bases than the expensive and very unusual Verkade’s base, the activity dropped down dramatically. Even with much higher catalyst loading and the use of the expensive, in CO2 hydrogenation successfully tested base DBU, almost no activity was observed. A scale-up of this process is therefore very unlikely.
2.5 Using Other Hydride Reagents
Parallel to the hydrogenation of CO2, catalysts for the reduction of CO2 to silyl formate with Hsilanes have been reported. The most recent systems are listed in Table 4.1.
In case of the Ru2Cl5(MeCN)7, the catalyst could be recycled 10 times, resulting in an overall TON up to 4,600 [77]. The same group achieved with the similar catalyst cis-[RuCl2(MeCN)4] and MePhSiH high TOF of 3,400 h−1 yielding 96 % of silyl formate at 85 °C [78]. The highest TONs were achieved by the copper (1,2-bis(diisopropylphosphino)benzene) complex [79] with a very good TON of 70,000 after 24 h at 1 atm of CO2 pressure.
From a mechanistic point of view, two different pathways are proposed depending on how CO2 inserts. For the systems [79] and [80], in the first step, the silane is activated on the metal center resulting in the formation of a metal-hydride complex and the transfer of the silyl cation to an anionic ligand (Cl− or triflate). In the second step, the silyl cation activates CO2 and subsequent silyl formate is reductively eliminated (outer-sphere mechanism).
The other systems [79, 81–83] are believed to follow a different mechanism. Here, the active catalytic species is formed by the reaction of the precursor and silane to yield the corresponding metal-hydride complex. Into this metal-H bond inserts a CO2 molecule to build up the metal formate complex. In the last step, this formate complex reacts with silane to regenerate the metal-H species and release silyl formate.
By adding water to silyl formate, formic acid is formed. This transformation is not really competitive with the reduction of CO2 to formic acid using molecular hydrogen as for the latter even more active catalysts systems are known. However, using other nucleophiles, such as amines or Grignard reagents, offers new synthetic ways to a number of synthesis products (Scheme 4.9). For example, benzhydrol was formed in 77 % yield by reacting silyl formate with two equivalents of PhMgBr [84].
A stoichiometric metal-free reduction of CO2 with trialkyl silanes and stoichiometric amounts of trityl borate was shown by the group of Müller [85]. They observed different products depending on the solvent applied. In PhCl, disilylated formic acid or disilyl methyl oxonium is formed. By quenching these intermediates with water, HCOOH and MeOH are obtained. In contrast, in PhH, the transformation of benzylic cations gives silyl ester or benzoic acid.
An example for a hydroboration of CO2 to formate was presented by Labinger and Bercaw [86] using trialkyl boranes in the presence of Rh and Ni dmpe complexes to form formate-borane adducts. However, closing the catalytic cycle through breaking the formate-borane adducts failed.
In 2013, the group of Shintani and Nozaki presented a copper-NHC-catalyzed hydroboration of CO2 to formic acid by quenching the reaction mixture with HCl [87]. This way, yields up to 87 % of formic acid have been observed. By adding primary or secondary amines to the reaction mixture instead of quenching with HCl, they isolated the corresponding formamides in good to very good yields (81–98 %). This N-formylation is also possible in one step by reacting CO2 with pinacolborane in the presence of amine. However, the yields decreased slightly (e.g., from 84 to 71 % for p-anisidine).
3 Hydrogenation of CO2 to MeOH
Searching for alternative sources of fuels and energy storage mediums, MeOH is considered for several good reasons. First, it can be dehydrogenated to give H2 and, therefore, can serve as hydrogen storage carrier [88, 89]. Second, methanol has a high energy content of 22.7 MJ kg−1, making it suitable for energy storage (CH4: 24.3 MJ kg−1) [6]. It can be used as liquid fuel (blended with gasoline or directly in direct methanol fuel cells, DMFC [90]) or can be converted to gasoline (MTG process). Last but not least, MeOH is a valuable feedstock as it can be transformed into ethylene or propylene in the MTO (methanol-to-olefins) process or to aromatics in the MTA (methanol-to-aromatics) process using zeolites. Therefore, MeOH covers all important basic chemicals for a wide range of product chains [6, 91]. The advantage of such a MeOH economy in comparison to the often discussed H2-based technology is that MeOH as a liquid can be used analogous to petroleum and therefore can be transported and stored easily in the maintaining infrastructure. In addition, MeOH is highly H2 enriched (12.6 w%) in comparison to many other H2 storage devices.
The most sustainable way for producing MeOH out of CO2 and water combines the electrolysis of water to hydrogen and the subsequent hydrogenation of CO2 to MeOH. The cost of this process will strongly depend on the electricity price for supplying the needed hydrogen amount. However, even today, this process can be competitive regarding the highly increased prices of gasoline in European countries [8]. The first commercial CO2-to-renewable-methanol plant based on geothermal sources was established in 2012 in Iceland, producing annually 3,500 t of MeOH, and is planned to be expanded [9]. Industrial applications of homogeneous catalyst systems in this field are still far away from reality, but the first recent examples of the homogeneous catalyzed hydrogenation of carbon dioxide in the last 3 years are promising.
3.1 Catalytic Reduction with Hydrogen
One of the first concepts for the hydrogenation of CO2 to MeOH (Eq. 4.2) was presented by the group of Sanford [92]. They set up a cascade by using three different catalysts: Ru(PMe3)4Cl(OAc) for the hydrogenation of CO2 to formic acid, Sc(OTf)3 as catalyst for the esterification of the formic acid to methyl formate, and finally the Ru PNN-pincer complex 42 which converted methyl formate to methanol. Key to success was the separation of the last catalyst from the second one to avoid deactivation. Therefore, methyl formate is transferred by a temperature ramp to an outer vessel containing the pincer complex 42. The overall TON of 21 for the transformation of 13CO2 to MeOH is low, but showed that this reaction is achievable by homogeneous catalysis. As one reason for this low activity, Sanford recognized an inhibition of the Ru PNN-pincer complex 42 by CO2 [93]. Two products were observed under CO2 atmosphere (Scheme 4.10). The kinetic product 43 is observed immediately under 1 atm of CO2 by forming a C-C bond between the CO2 and the phosphine arm. This process is reversible. By simply keeping the complex longer under CO2 atmosphere or heating the solution, the irreversible product 44 is formed at the amine arm of the pincer complex and single crystals could be grown from the solution.
Milstein observed a similar phenomenon with a Ru PNP ligand with two phosphine arms [94]. Here, only a reversible addition of CO2 to the phosphine arm was found confirming Sanford’s results. Calculations reveal no solvent effect on the TS energies, indicating a concerted mechanism and the absence of a charge separation like Ru+ COO−. Another example of CO2-ligand interactions was shown by Oro and Langer [95] using an Ir(I) dppm catalyst. Here, CO2 inserts into the C-H bond of the bisphosphine ligand to give the binuclear complex [IrCl(dppm)(H){(Ph2P)2C-COOH}]2.
The first one-catalyst system for the production of methanol was reported by Klankermayer and Leitner [96]. Using the Ru catalyst 45 in the presence of 1 eq HNTf2, MeOH is produced with a TON of 221 in a THF/EtOH mixture at 20 bar of CO2 and 60 bar of H2 (Scheme 4.11). They propose a cationic Ru hydrido dihydrogen complex to be the active species.
In addition to the systems mentioned above, a few concepts for the indirect conversion of CO2 to MeOH were developed, reducing substrates which can be produced from CO2 (Scheme 4.12). Pioneering work has been published by Milstein. Using the Ru-pincer complexes 42 or 46, alkyl formates, organic carbonates, and carbamates [97] as well as challenging ureas [98] were converted under mild conditions in quantitative yield, often for the first time. Key to success is the unique behavior of the pincer complex being involved in the mechanism by metal-ligand cooperation. This work was also highlighted by Dixneuf [99] and Choudhury [100]. Notably, the activities need to be improved for industrial application and the economy of the process will also depend on H2 cost.
One year later, Yang presented a DFT study on the dimethyl carbonate reduction to MeOH with the Ru NNP-pincer complex 42. In addition, an analog Fe-pincer complex was proposed [101]. The suggested mechanism runs via three catalytic cycles, each with a direct hydride and ligand proton transfers. The cascade intermediates are methyl formate, followed by formaldehyde and subsequently MeOH as the product. The overall rate-determining step was found to be the formation of the second MeOH molecule from methyl formate in the second cycle.
However, it should be kept in mind that the reduction of dimethyl carbonate to methanol is uneconomic as the starting material with 1,000 US/t is much more expensive than MeOH (400 US/t) [102]. Therefore, Ding et al. developed another indirect route for the MeOH production [102]. The idea is based on the omega process where ethylene carbonate, synthesized from CO2 and ethylene oxide, is hydrolyzed to ethylene glycol and CO2 (Scheme 4.13).
Instead of hydrolyzing the organic carbonate, this intermediate can be hydrogenated with the Ru PNP-pincer complex 47 in the presence of KOtBu to give ethylene glycol and MeOH. This opens a nice way to get two bulk chemicals at the same time from reacting CO2 with ethylene oxide. With TON up to 87,000 and TOF of 1,200 h−1, high activity could be observed at almost quantitative yields. In addition, deuterated methanol could be obtained in 99 % yield by deuteration of the sterically hindered tetramethyl ethylene carbonate with D2. Furthermore, the catalyst is able to depolymerize the widely used material poly(propylene carbonate) by hydrogenating it to MeOH and the corresponding diol. Therefore, a recycling of this material to valuable products is possible [103].
An unusual way to gain methanol is the formic acid disproportionation (Eq. 4.3). Here, formic acid is both substrate and hydrogen source. In a HCOOH/water mixture at 60 °C, the molecular iridium catalyst [Cp*Ir(bpy)(H2O)](OTf)2 converts formic acid to methanol with a selectivity up to 12 % [104]. The selectivity increases with higher HCOOH concentration and lower temperature. Additional H2 suppresses the competing formate decomposition to CO2 and H2. As a side product methyl formate was observed through HCOOH esterification. Deuteration experiments indicate that formaldehyde is an intermediate of the catalytic cycle which is then hydrogenated to MeOH by HCOOH releasing CO2.
An interesting approach is the use of frustrated Lewis pairs which are capable of heterolytic H2 activation [105]. Ashley and O’Hare applied TMP + B(C6F5)3, forming a unique formato borate complex [TMPH]-[HCO2B(C6F5)3] with CO2 under H2 atmosphere (1–2 atm). After 6 days at 160 °C, H3COB(C6F5)2 was formed and 24 % yield of methanol was observed after distillation. However, the rendering of the catalytic cycle is still unachieved [106].
3.2 Catalytic Reduction with Other Hydride Reagents
There is a bunch of catalysts capable of reducing CO2 using other hydride reagents such as aminoboranes, Hboranes and Hsilanes. For example, the group of Stephan presented a 1:2 mixture of PMes3 and AlX3 reacting with CO2 to the corresponding phosphonium-carboxylate adduct. This intermediate then dehydrogenates aminoboranes at RT to give phosphonium methoxy aluminate in less than 15 min. Quenching with H2O leads to 37–51 % yield of methanol [107].
The first catalytic metal-free hydrosilylation of CO2 to MeOH was reported in 2009 by Zhang and Ying [108]. Using the NHC catalyst 49 under CO2 atmosphere, first imidazolium carboxylate is formed which reacted then with diphenylsilane at RT to give CH3OSiMe3. Final quenching with water gives MeOH in 90 % yield based on the used Hsilane amount with TON of 1,840 and TOF of 25 h−1. Therefore, it is competitive with known Ru catalysts lacking their typical drawbacks of moisture and air sensitivity. This system is even active under dried air. The first catalytic hydroboration of CO2 to the methoxide level was reported by Guan, applying the nickel catalyst 48 [109] in the presence of HBcat. Hydrolysis gives MeOH with TON and TOF of 495 h−1 (based on B-H).
Comparing both systems by studying the mechanism quantum mechanically, Wang proposed that the most favorable pathway for both contains analog, experimentally detected intermediates like formoxy, acetal, and methoxide species [110, 111]. In addition, he predicted formaldehyde to be another intermediate in the catalytic cycle (Scheme 4.14). Both catalysts accelerate the hydride transfer in comparison to uncatalyzed reactions, but the mechanism of the hydride transfer is different. In the Ni/borane system, the Ni catalyst 48 acts as a shuttle, transferring the hydride from the HBcat to the C-O bonds. In the first step, CO2 inserts into the [Ni]-H bond (50) forming Ni-formate. This complex reacts with catecholborane HBcat (51) to give formyl borate and [Ni]-H catalyst 48. The catalyst then activates this product by forming the acetal species. The hydride transfer to [Ni] by a second HBcat results in formaldehyde and di(boryl)ether production. Formaldehyde then inserts into the [Ni]-H, yielding the corresponding methoxide complex. A third HBcat closes the catalytic cycle by reforming the [Ni]-H catalyst and gaining the final product CH3OBcat.
In contrast, the NHC catalyst 49 “only” activates the Si-H bond by pushing electron density to the H atoms and simplifies this way the direct hydride transfer from Hsilane to the C-O bond (52 top). However, the NHC catalyst 49 can react with CO2 to the corresponding imidazolium carboxylate, too. This activated CO2 adduct can be attacked more easily by Hsilanes than unbounded CO2 (52 bottom).
It is noteworthy that the CO2 reduction catalyst and the hydride source must match for achieving high activity. Exchanging the hydride source for both systems, the reduction of CO2 to MeOH failed. The reasons are a high kinetic barrier for the reaction of the Ni-formate with silanes and the formation of a thermodynamic stable intermediate between NHC and Hboranes.
Further examples of a catalytic hydroboration with Ru catalysts were presented by Sabo-Etienne using [RuH2(H2)2(PCy3)2] in combination with pinacolborane [112, 113] and by the group of Stephan applying the precursor [Ru(PPh3)Cl2] and the ligand N((CH2)2NHPiPr2)3 in the presence of HBpin [114].
The first metal-free organocatalyst for hydroboration of CO2 to MeOH was published by Maron and Fontaine [115]. The organocatalyst 1-Bcat-2-PPh2-C6H4 reduces CO2 with 3 eq of BH3∙SMe2 to give subsequent MeOH with TON >3,000 and TOF >970 h−1.
4 Other Homogeneous Catalytic Reductions of CO2 Using Hydride Reagents
Beside methanol, the full reduction of CO2 to methane is an attractive and actual goal for homogeneous catalysis. However, so far homogeneous catalysts capable of hydrogenating CO2 to methane with molecular H2 are still unknown (Eq. 4.4). In the meantime, some efforts have been undertaken using less benign reductants such as silanes.
Interestingly, Musgrave’s ab initio studies claim that even in the absence of a metal, the reduction of CO2 with ammonia boranes via simultaneous hydride and proton transfer should be possible involving the intermediates HCOOH and hydrated formaldehyde to give finally methanol [116]. Unfortunately, ammonia boranes decompose easily in the presence of acid to H2 and NH2BH2. Unless this problem will be solved, the reduction will stop at the HCOOH step. The group of Wehmschulte showed that strong Lewis acids such as [Et2Al]+ can catalyze the hydrosilylation of CO2 to methane. However, after three cycles the activity decreased, likely caused by side reactions with the solvent C6D6 [117].
Combining borane complexes or additional borane with hydrosilylation reagents was the key to success for improved catalytic turnover numbers. All active systems have in common that different kind of metal complexes or frustrated Lewis pairs (FLP) are needed to activate the CO2 to form the corresponding formate complexes. The following reduction steps are believed to be catalyzed by species [R3Si-H∙∙∙BR3] involving the intermediates bis(silyl)acetal, methyl silyl ether, and finally methane. Active co-catalysts are presented in Scheme 4.15.
Activities and selectivities were low for the systems using 53 [118], 54 [119], and 55 [120], whereas a good TON of 2,156 was achieved in16 h by Turculet using the Pt-pincer borane complex 56 with Et3SiH at 65 °C and 1 atm of CO2 [121]. A high TON of 8,300 after 72 h at RT was published by Brookhart applying the Ir+ PCP-pincer complex 57 [122] in the presence of Me2PhSiH.
Finally, it is worth mentioning that carbon dioxide can be used as methylation reagent for amines in the presence of silanes. Our group [123] as well as the group of Cantat [124] realized the reduction of CO2 with a simultaneous C-N bond formation. With this method it is possible to methylate primary amines with CO2 as C1 feedstock.
5 Concluding Remarks
In the last decade, several improved organometallic catalysts for the hydrogenation of CO2 to formic acid derivatives have been reported. Many of them show good activity even under ambient conditions. In addition, interesting concepts have been developed, combining both formic acid product separation and catalyst recycling. However, for industrial applications the activities and long-term stabilities of the catalysts still have to be improved. Meanwhile, also catalyst systems have been presented for hydrogen storage using carbon dioxide.
While non-precious metals such as Fe and Co were rarely known for the homogeneous CO2 hydrogenation before 2009, much progress was achieved in this field in the last 3 years.
Very recently, also first promising systems capable of converting CO2 to methanol became available opening new ways to a future methanol economy. On the other hand, the homogeneously catalyzed reduction of carbon dioxide to CH4 using molecular hydrogen is still not known and more efforts are needed in this area. At this point it should be mentioned that related reductions of carbon dioxide using boranes or hydrosilylation reagents are interesting in order to understand the mechanism of such processes. Nevertheless, it should be clear that they will not be of preparative use due to price, sensitivity of the reagents, and waste generated.
What will be the future challenges in homogeneous CO2 reductions? Obviously, for industrial implementations even better catalysts are needed. With regard to basic science, the hydrogenation of carbon dioxide with simultaneous C-C bond formation represents a grand challenge. As an example, the low temperature and selective Fischer-Tropsch reaction can be considered. Furthermore, reducing carbon dioxide directly to Co is of both industrial and academic interest. Such transformations would allow using carbon dioxide instead of toxic carbon monoxide. The key tools to achieve these goals will be catalysis and organometallic chemistry.
Abbreviations
- Bcat:
-
catecholborane
- Bpin:
-
pinacolborane
- bpy:
-
bipyridine
- cat.:
-
catalyst
- Cp*:
-
pentamethylcyclopentadienyl
- Cy:
-
cyclohexyl group
- DBU:
-
1,8-diazabicycloundec-7-ene
- DFT:
-
density functional theory
- DHBP:
-
4,4′-dihydroxy-2,2′-bipyridine
- DHPT:
-
4,7-dihydroxy-1,10-phenanthroline
- DMFC:
-
direct methanol fuel cells
- dmpe:
-
1,3-bis(dimethylphophino)ethane
- dppm:
-
1,2-bis(diphenylphosphino)methane
- dppp:
-
1,3-bis(diphenylphosphino)propane
- EMIMCl:
-
1-ethyl-3-methylimidazolium chloride
- FLP:
-
frustrated Lewis pairs
- hex:
-
hexyl group
- IL:
-
ionic liquid
- iPr:
-
isopropyl group
- IR:
-
infrared spectroscopy
- KIE:
-
kinetic isotope effect
- MD:
-
molecular dynamics
- Me:
-
methyl group
- Mes:
-
mesityl groups
- MTA:
-
methanol-to-aromatics
- MTD:
-
metadynamics
- MTG:
-
methanol-to-gasoline
- MTO:
-
methanol-to-olefins
- mtppms:
-
sodium diphenylphosphinobenzene-3-sulfonate
- NHC:
-
N-heterocyclic carbene
- NMR:
-
nuclear magnetic resonance spectroscopy
- OAc:
-
acetoxy group
- OTf:
-
triflate group
- Ph:
-
phenyl group
- PNP:
-
pincer ligand (P donor atoms on the side arms N ~ in the middle); analogue PNN PCP
- RT:
-
room temperature
- SDQ:
-
single doubles quadruples
- tBu:
-
tert-butyl group
- TOF:
-
turnover frequency
- TON:
-
turnover number
- TMP:
-
tetramethylpiperidine
- TS:
-
transition state
- Verkade’s base:
-
2,8,9-triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo-[3, 3, 3]undecane
- X:
-
halide anions such as Cl− Br−
References
Cokoja M, Bruckmeier C, Rieger B, Herrmann WA, Kühn FE (2011) Umwandlung von Kohlendioxid mit Übergangsmetall-Homogenkatalysatoren: eine molekulare Lösung für ein globales Problem? Angew Chem 123(37):8662–8690. doi:10.1002/ange.201102010
Peters M, Köhler B, Kuckshinrichs W, Leitner W, Markewitz P, Müller TE (2011) Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem 4(9):1216–1240. doi:10.1002/cssc.201000447
Geider RJ, Delucia EH, Falkowski PG, Finzi AC, Grime JP, Grace J, Kana TM, La Roche J, Long SP, Osborne BA, Platt T, Prentice IC, Raven JA, Schlesinger WH, Smetacek V, Stuart V, Sathyendranath S, Thomas RB, Vogelmann TC, Williams P, Woodward FI (2001) Primary productivity of planet earth: biological determinants and physical constraints in terrestrial and aquatic habitats. Glob Chang Biol 7(8):849–882. doi:10.1046/j.1365-2486.2001.00448.x
Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, Dupuis M, Ferry JG, Fujita E, Hille R, Kenis PJA, Kerfeld CA, Morris RH, Peden CHF, Portis AR, Ragsdale SW, Rauchfuss TB, Reek JNH, Seefeldt LC, Thauer RK, Waldrop GL (2013) Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem Rev. doi:10.1021/cr300463y
Mikkelsen M, Jorgensen M, Krebs FC (2010) The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ Sci 3(1):43–81
Bertau M, Offermanns H, Menges G, Keim W, Effenberger FX (2010) Methanol findet zu wenig Beachtung als Kraftstoff und Chemierohstoff der Zukunft [Methanol needs more attention as a fuel and raw material for the future]. Chem Ing Tech 82(12):2055–2058. doi:10.1002/cite.201000159
Sakakura T, Choi J-C, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107(6):2365–2387. doi:10.1021/cr068357u
Olah GA, Prakash GKS, Goeppert A (2011) Anthropogenic chemical carbon cycle for a sustainable future. J Am Chem Soc 133(33):12881–12898. doi:10.1021/ja202642y
Olah GA (2013) Towards oil independence through renewable methanol chemistry. Angew Chem Int Ed 52(1):104–107. doi:10.1002/anie.201204995
Haggin J (1994) New processes target methanol production, off-gas cleaning. Chem Eng News 72:29–31
Goehna H, Koenig P (1994) Producing methanol from CO2. ChemTech 24:36–39
Jessop PG, Ikariya T, Noyori R (1995) Homogeneous hydrogenation of carbon dioxide. Chem Rev 95(2):259–272. doi:10.1021/cr00034a001
Leitner W (1995) Carbon dioxide as a raw material: the synthesis of formic acid and its derivatives from CO2. Angew Chem Int Ed 34(20):2207–2221. doi:10.1002/anie.199522071
Jessop PG, Joó F, Tai C-C (2004) Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord Chem Rev 248(21–24):2425–2442. doi:10.1016/j.ccr.2004.05.019
de Vries JG, Elsevier CJ, Jessop PG (2007) 17 homogeneous hydrogenation of carbon dioxide. Handb Homogen Hydrogen 1:489–511
Rothenberg G (2008) Introduction. In: Catalysis. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–38. doi:10.1002/9783527621866.ch1
Olah GA (2005) Beyond oil and gas: the methanol economy. Angew Chem Int Ed 44(18):2636–2639. doi:10.1002/anie.200462121
Munshi P, Main AD, Linehan JC, Tai C-C, Jessop PG (2002) Hydrogenation of carbon dioxide catalyzed by ruthenium trimethylphosphine complexes: the accelerating effect of certain alcohols and amines. J Am Chem Soc 124(27):7963–7971. doi:10.1021/ja0167856
Y-y O, Matsunaga T, Nakao Y, Sato H, Sakaki S (2005) Ruthenium(II)-catalyzed hydrogenation of carbon dioxide to formic acid. Theoretical study of real catalyst, ligand effects, and solvation effects. J Am Chem Soc 127(11):4021–4032. doi:10.1021/ja043697n
Y-y O, Nakao Y, Sato H, Sakaki S (2006) Ruthenium(II)-catalyzed hydrogenation of carbon dioxide to formic acid. Theoretical study of significant acceleration by water molecules. Organometallics 25(14):3352–3363. doi:10.1021/om060307s
Getty AD, Tai C-C, Linehan JC, Jessop PG, Olmstead MM, Rheingold AL (2009) Hydrogenation of carbon dioxide catalyzed by ruthenium trimethylphosphine complexes: a mechanistic investigation using high-pressure NMR spectroscopy. Organometallics 28(18):5466–5477. doi:10.1021/om900128s
Gowrisankar S, Federsel C, Neumann H, Ziebart C, Jackstell R, Spannenberg A, Beller M (2013) Synthesis of stable phosphomide ligands and their use in Ru-catalyzed hydrogenations of bicarbonate and related substrates. ChemSusChem 6(1):85–91. doi:10.1002/cssc.201200732
Muller K, Sun Y, Thiel WR (2013) Ruthenium(II)–phosphite complexes as catalysts for the hydrogenation of carbon dioxide. ChemCatChem 5(6):1340–1343. doi:10.1002/cctc.201200818
Zhao G, Joó F (2011) Free formic acid by hydrogenation of carbon dioxide in sodium formate solutions. Catal Commun 14(1):74–76. doi:10.1016/j.catcom.2011.07.017
Johnson TC, Morris DJ, Wills M (2010) Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem Soc Rev 39(1):81–88
Grasemann M, Laurenczy G (2012) Formic acid as a hydrogen source – recent developments and future trends. Energy Environ Sci 5(8):8171–8181. doi:10.1039/C2EE21928J
Loges B, Boddien A, Gärtner F, Junge H, Beller M (2010) Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials. Top Catal 53:902–914
Laurenczy G, Dalebrook AF, Gan W, Grasemann M, Moret S (2013) Hydrogen storage – beyond conventional methods. Chem Commun. doi:10.1039/C3CC43836H
Graetz J (2009) New approaches to hydrogen storage. Chem Soc Rev 38(1):73–82. doi:10.1039/B718842K
Jena P (2011) Materials for hydrogen storage: past, present, and future. J Phys Chem Lett 2(3):206–211. doi:10.1021/jz1015372
Eberle U, Felderhoff M, Schüth F (2009) Chemical and physical solutions for hydrogen storage. Angew Chem Int Ed 48(36):6608–6630. doi:10.1002/anie.200806293
Papp G, Csorba J, Laurenczy G, Joó F (2011) A charge/discharge device for chemical hydrogen storage and generation. Angew Chem 123(44):10617–10619. doi:10.1002/ange.201104951
Boddien A, Gärtner F, Federsel C, Sponholz P, Mellmann D, Jackstell R, Junge H, Beller M (2011) Kohlenstoffdioxid-neutrale Wasserstoffspeicherung basierend auf Bicarbonaten und Formiaten. Angew Chem 123:6535–6538. doi:10.1002/ange.201101995
Federsel C, Jackstell R, Boddien A, Laurenczy G, Beller M (2010) Ruthenium-catalyzed hydrogenation of bicarbonate in water. ChemSusChem 3(9):1048–1050. doi:10.1002/cssc.201000151
Boddien A, Federsel C, Sponholz P, Mellmann D, Jackstell R, Junge H, Laurenczy G, Beller M (2012) Towards the development of a hydrogen battery. Energy Environ Sci 5(10):8907–8911. doi:10.1039/c2ee22043a
Urakawa A, Iannuzzi M, Hutter J, Baiker A (2007) Towards a rational design of ruthenium CO2 hydrogenation catalysts by ab initio metadynamics. Chem Eur J 13(24):6828–6840. doi:10.1002/chem.200700254
Urakawa A, Jutz F, Laurenczy G, Baiker A (2007) Carbon dioxide hydrogenation catalyzed by a ruthenium dihydride: a DFT and high-pressure spectroscopic investigation. Chem Eur J 13(14):3886–3899. doi:10.1002/chem.200601339
Dietrich J, Schindler S (2008) Kinetic studies on the hydrogenation of carbon dioxide to formic acid using a rhodium complex as catalyst. Z Anorg Allg Chem 634(14):2487–2494. doi:10.1002/zaac.200800352
Yasaka Y, Wakai C, Matubayasi N, Nakahara M (2010) Controlling the equilibrium of formic acid with hydrogen and carbon dioxide using ionic liquid. J Phys Chem A 114(10):3510–3515. doi:10.1021/jp908174s
Zhang Z, Xie Y, Li W, Hu S, Song J, Jiang T, Han B (2008) Hydrogenation of carbon dioxide is promoted by a task-specific ionic liquid. Angew Chem Int Ed 47(6):1127–1129. doi:10.1002/anie.200704487
Zhang Z, Hu S, Song J, Li W, Yang G, Han B (2009) Hydrogenation of CO2 to formic acid promoted by a diamine-functionalized ionic liquid. ChemSusChem 2(3):234–238. doi:10.1002/cssc.200800252
Wesselbaum S, Hintermair U, Leitner W (2012) Continuous-flow hydrogenation of carbon dioxide to pure formic acid using an integrated scCO2 process with immobilized catalyst and base. Angew Chem 124:8713–8716. doi:10.1002/ange.201203185
Schaub T, Paciello RA (2011) A process for the synthesis of formic acid by CO2 hydrogenation: thermodynamic aspects and the role of CO. Angew Chem Int Ed 50(32):7278–7282. doi:10.1002/anie.201101292
Ogo S, Kabe R, Hayashi H, Harada R, Fukuzumi S (2006) Mechanistic investigation of CO2 hydrogenation by Ru(II) and Ir(III) aqua complexes under acidic conditions: two catalytic systems differing in the nature of the rate determining step. Dalton Trans 39:4657–4663. doi:10.1039/b607993h
Himeda Y, Onozawa-Komatsuzaki N, Sugihara H, Kasuga K (2007) Simultaneous tuning of activity and water solubility of complex catalysts by acid–base equilibrium of ligands for conversion of carbon dioxide. Organometallics 26(3):702–712. doi:10.1021/om060899e712
Himeda Y, Miyazawa S, Hirose T (2011) Interconversion between formic acid and H2/CO2 using rhodium and ruthenium catalysts for CO2 fixation and H2 storage. ChemSusChem 4(4):487–493. doi:10.1002/cssc.201000327
Himeda Y (2007) Conversion of CO2 into formate by homogeneously catalyzed hydrogenation in water: tuning catalytic activity and water solubility through the acid–base equilibrium of the ligand. Eur J Inorg Chem 25:3927–3941. doi:10.1002/ejic.200700494
Wang W-H, Hull JF, Muckerman JT, Fujita E, Himeda Y (2012) Second-coordination-sphere and electronic effects enhance iridium(III)-catalyzed homogeneous hydrogenation of carbon dioxide in water near ambient temperature and pressure. Energy Environ Sci 5:7923–7926
Wang W-H, Muckerman JT, Fujita E, Himeda Y (2013) Mechanistic insight through factors controlling effective hydrogenation of CO2 catalyzed by bioinspired proton-responsive iridium(III) complexes. ACS Catal 3(5):856–860. doi:10.1021/cs400172j
Hull JF, Himeda Y, Wang W-H, Hashiguchi B, Periana R, Szalda DJ, Muckerman JT, Fujita E (2012) Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat Chem 4(5):383–388
Maenaka Y, Suenobu T, Fukuzumi S (2012) Catalytic interconversion between hydrogen and formic acid at ambient temperature and pressure. Energy Environ Sci 5(6):7360–7367. doi:10.1039/c2ee03315a
Fukuzumi S, Suenobu T (2013) Hydrogen storage and evolution catalysed by metal hydride complexes. Dalton Trans 42(1):18–28. doi:10.1039/c2dt31823g
Muller K, Sun Y, Heimermann A, Menges F, Niedner-Schatteburg G, van Wüllen C, Thiel WR (2013) Structure–reactivity relationships in the hydrogenation of carbon dioxide with ruthenium complexes bearing pyridinylazolato ligands. Chem Eur J 19:7825–7834. doi:10.1002/chem.201204199
Thai T-T, Therrien B, Suss-Fink G (2009) Arene ruthenium oxinato complexes: synthesis, molecular structure and catalytic activity for the hydrogenation of carbon dioxide in aqueous solution. J Organomet Chem 694(25):3973–3981. doi:10.1016/j.jorganchem.2009.09.008
Sanz S, Azua A, Peris E (2010) ‘([small eta]6-arene)Ru(bis-NHC)’ complexes for the reduction of CO2 to formate with hydrogen and by transfer hydrogenation with iPrOH. Dalton Trans 39(27):6339–6343. doi:10.1039/C003220D
Azua A, Sanz S, Peris E (2011) Water-soluble IrIII N-heterocyclic carbene based catalysts for the reduction of CO2 to formate by transfer hydrogenation and the deuteration of aryl amines in water. Chem Eur J 17(14):3963–3967. doi:10.1002/chem.201002907
Albrecht M, van Koten G (2001) Platinum group organometallics based on “pincer” complexes: sensors, switches, and catalysts. Angew Chem Int Ed 40(20):3750–3781. doi:10.1002/1521-3773(20011015)40:20<3750::aid-anie3750>3.0.co;2-6
van der Boom ME, Milstein D (2003) Cyclometalated phosphine-based pincer complexes: mechanistic insight in catalysis, coordination, and bond activation. Chem Rev 103(5):1759–1792. doi:10.1021/cr960118r
Grützmacher H (2008) Cooperating ligands in catalysis. Angew Chem Int Ed 47(10):1814–1818. doi:10.1002/anie.200704654
van der Vlugt JI, Reek JNH (2009) Neutral tridentate PNP ligands and their hybrid analogues: versatile non-innocent scaffolds for homogeneous catalysis. Angew Chem Int Ed 48(47):8832–8846. doi:10.1002/anie.200903193
Musa S, Shaposhnikov I, Cohen S, Gelman D (2011) Ligand–metal cooperation in PCP pincer complexes: rational design and catalytic activity in acceptorless dehydrogenation of alcohols. Angew Chem 123(15):3595–3599. doi:10.1002/ange.201007367
Federsel C, Jackstell R, Beller M (2010) State-of-the-art catalysts for hydrogenation of carbon dioxide. Angew Chem Int Ed 49(36):6254–6257. doi:10.1002/anie.201000533
Nozaki K, Tanaka R, Yamashita M (2009) Catalytic hydrogenation of carbon dioxide using Ir(III) – pincer complexes. J Am Chem Soc 131(40):14168–14169. doi:10.1021/ja903574e
Yang X (2011) Hydrogenation of carbon dioxide catalyzed by PNP pincer iridium, iron, and cobalt complexes: a computational design of base metal catalysts. ACS Catal 1:849–854. doi:10.1021/cs2000329
Ahlquist MSG (2010) Iridium catalyzed hydrogenation of CO2 under basic conditions-mechanistic insight from theory. J Mol Catal A Chem 324(1–2):3–8. doi:10.1016/j.molcata.2010.02.018
Tanaka R, Yamashita M, Chung LW, Morokuma K, Nozaki K (2011) Mechanistic studies on the reversible hydrogenation of carbon dioxide catalyzed by an Ir-PNP complex. Organometallics 30(24):6742–6750. doi:10.1021/om2010172
Schmeier TJ, Dobereiner GE, Crabtree RH, Hazari N (2011) Secondary coordination sphere interactions facilitate the insertion step in an iridium(III) CO2 reduction catalyst. J Am Chem Soc 133(24):9274–9277. doi:10.1021/ja2035514
Vogt M, Rivada-Wheelaghan O, Iron MA, Leitus G, Diskin-Posner Y, Shimon LJW, Ben-David Y, Milstein D (2013) Anionic Nickel(II) complexes with doubly deprotonated PNP pincer-type ligands and their reactivity toward CO2. Organometallics 32(1):300–308. doi:10.1021/om3010838
Khaskin E, Diskin-Posner Y, Weiner L, Leitus G, Milstein D (2013) Formal loss of an H radical by a cobalt complex via metal-ligand cooperation. Chem Commun 49(27):2771–2773. doi:10.1039/C3CC39049G
Tai C-C, Chang T, Roller B, Jessop PG (2003) High-pressure combinatorial screening of homogeneous catalysts: hydrogenation of carbon dioxide. Inorg Chem 42(23):7340–7341. doi:10.1021/ic034881x
Langer R, Diskin-Posner Y, Leitus G, Shimon LJW, Ben-David Y, Milstein D (2011) Low-pressure hydrogenation of carbon dioxide catalyzed by an iron pincer complex exhibiting noble metal activity. Angew Chem Int Ed 50(42):9948–9952. doi:10.1002/anie.201104542
Federsel C, Boddien A, Jackstell R, Jennerjahn R, Dyson PJ, Scopelliti R, Laurenczy G, Beller M (2010) A well-defined iron catalyst for the reduction of bicarbonates and carbon dioxide to formates, alkyl formates, and formamides. Angew Chem Int Ed 49(50):9777–9780. doi:10.1002/anie.201004263
Federsel C, Ziebart C, Jackstell R, Baumann W, Beller M (2012) Catalytic hydrogenation of carbon dioxide and bicarbonates with a well-defined cobalt dihydrogen complex. Chem Eur J 18(1):72–75. doi:10.1002/chem.201101343
Ziebart C, Federsel C, Anbarasan P, Jackstell R, Baumann W, Spannenberg A, Beller M (2012) Well-defined iron catalyst for improved hydrogenation of carbon dioxide and bicarbonate. J Am Chem Soc 134(51):20701–20704. doi:10.1021/ja307924a
Bianchini C, Peruzzini M, Zanobini F (1988) An exceptionally stable cis-(hydride)([eta]2-dihydrogen) complex of iron. J Organomet Chem 354(2):C19–C22. doi:10.1016/0022-328x(88)87057-8
Jeletic MS, Mock MT, Appel AM, Linehan JC (2013) A cobalt-based catalyst for the hydrogenation of CO2 under ambient conditions. J Am Chem Soc. doi:10.1021/ja406601v
Jansen A, Pitter S (2004) Homogeneously catalysed reduction of carbon dioxide with silanes: a study on solvent and ligand effects and catalyst recycling. J Mol Catal A Chem 217(1–2):41–45, http://dx.doi.org/10.1016/j.molcata.2004.03.041
Deglmann P, Ember E, Hofmann P, Pitter S, Walter O (2007) Experimental and theoretical investigations on the catalytic hydrosilylation of carbon dioxide with ruthenium nitrile complexes. Chem Eur J 13(10):2864–2879. doi:10.1002/chem.200600396
Motokura K, Kashiwame D, Takahashi N, Miyaji A, Baba T (2013) Highly active and selective catalysis of copper diphosphine complexes for the transformation of carbon dioxide into silyl formate. Chem Eur J 19(30):10030–10037. doi:10.1002/chem.201300935
Lalrempuia R, Iglesias M, Polo V, Sanz Miguel PJ, Fernández-Alvarez FJ, Pérez-Torrente JJ, Oro LA (2012) Effective fixation of CO2 by iridium-catalyzed hydrosilylation. Angew Chem Int Ed 51(51):12824–12827. doi:10.1002/anie.201206165
Sattler W, Parkin G (2012) Zinc catalysts for on-demand hydrogen generation and carbon dioxide functionalization. J Am Chem Soc 134(42):17462–17465. doi:10.1021/ja308500s
Motokura K, Kashiwame D, Miyaji A, Baba T (2012) Copper-catalyzed formic acid synthesis from CO2 with hydrosilanes and H2O. Org Lett 14(10):2642–2645. doi:10.1021/ol301034j
Zhang L, Cheng J, Hou Z (2013) Highly efficient catalytic hydrosilylation of carbon dioxide by an N-heterocyclic carbene copper catalyst. Chem Commun 49(42):4782–4784. doi:10.1039/C3CC41838C
Itagaki S, Yamaguchi K, Mizuno N (2013) Catalytic synthesis of silyl formates with 1 atm of CO2 and their utilization for synthesis of formyl compounds and formic acid. J Mol Catal A Chem 366:347–352, http://dx.doi.org/10.1016/j.molcata.2012.10.014
Schäfer A, Saak W, Haase D, Müller T (2012) Silyl cation mediated conversion of CO2 into benzoic acid, formic acid, and methanol. Angew Chem Int Ed 51(12):2981–2984. doi:10.1002/anie.201107958
Miller AJM, Labinger JA, Bercaw JE (2011) Trialkylborane-assisted CO2 reduction by late transition metal hydrides. Organometallics 30(16):4308–4314. doi:10.1021/om200364w
Shintani R, Nozaki K (2013) Copper-catalyzed hydroboration of carbon dioxide. Organometallics 32(8):2459–2462. doi:10.1021/om400175h
Stephan DW (2013) Catalysis: a step closer to a methanol economy. Nature 495(7439):54–55
Nielsen M, Alberico E, Baumann W, Drexler H-J, Junge H, Gladiali S, Beller M (2013) Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 495(7439):85–89
(a) Surumpudi S, Narayanan SR, Vamos E, Frank HA, Halpert G, Prakash GKS, Olah GA, US Patent 6,248,460, 2001; (b) Surumpudi S, Narayanan SR, Vamos E, Frank HA, Halpert G, Olah GA, Prakash GKS, US Patent 6,444,343, 2002
Chang CD (1997) In: Ertl G, Knözinger H, Weitkamp J (eds) Handbook of heterogeneous catalysis. VCH, Weinheim, p 1894 and references therein
Huff CA, Sanford MS (2011) Cascade catalysis for the homogeneous hydrogenation of CO2 to methanol. J Am Chem Soc 133(45):18122–18125. doi:10.1021/ja208760j
Huff CA, Kampf JW, Sanford MS (2012) Role of a noninnocent pincer ligand in the activation of CO2 at (PNN)Ru(H)(CO). Organometallics 31(13):4643–4645. doi:10.1021/om300403b
Vogt M, Gargir M, Iron MA, Diskin-Posner Y, Ben-David Y, Milstein D (2012) A new mode of activation of CO2 by metal–ligand cooperation with reversible C–C and M–O bond formation at ambient temperature. Chem Eur J 18(30):9194–9197. doi:10.1002/chem.201201730
Langer J, Fabra MJ, Garcia-Orduna P, Lahoz FJ, Oro LA (2008) A C-H activation-CO2-carboxylation reaction sequence mediated by an ‘Iridium(dppm)’ species. Formation of the anionic ligand (Ph2P)2C-COOH. Chem Commun 39:4822–4824
Wesselbaum S, vom Stein T, Klankermayer J, Leitner W (2012) Hydrogenation of carbon dioxide to methanol by using a homogeneous ruthenium–phosphine catalyst. Angew Chem 124:7617–7620. doi:10.1002/ange.201202320
Balaraman E, Gunanathan C, Zhang J, Shimon LJW, Milstein D (2011) Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO2 and CO. Nat Chem 3(8):609–614
Balaraman E, Ben-David Y, Milstein D (2011) Unprecedented catalytic hydrogenation of urea derivatives to amines and methanol. Angew Chem Int Ed 50(49):11702–11705. doi:10.1002/anie.201106612
Dixneuf PH (2011) Bifunctional catalysis: a bridge from CO2 to methanol. Nat Chem 3(8):578–579
Choudhury J (2012) New strategies for CO2-to-methanol conversion. ChemCatChem 4:609–611. doi:10.1002/cctc.201100495
Yang X (2012) Metal hydride and ligand proton transfer mechanism for the hydrogenation of dimethyl carbonate to methanol catalyzed by a pincer ruthenium complex. ACS Catal 2(6):964–970. doi:10.1021/cs3000683
Han Z, Rong L, Wu J, Zhang L, Wang Z, Ding K (2012) Catalytic hydrogenation of cyclic carbonates: a practical approach from CO2 and epoxides to methanol and diols. Angew Chem Int Ed 51(52):13041–13045. doi:10.1002/anie.201207781
Li Y, Junge K, Beller M (2013) Improving the efficiency of the hydrogenation of carbonates and carbon dioxide to methanol. ChemCatChem 5(5):1072–1074. doi:10.1002/cctc.201300013
Miller AJM, Heinekey DM, Mayer JM, Goldberg KI (2013) Catalytic disproportionation of formic acid to generate methanol. Angew Chem 125(14):4073–4076. doi:10.1002/ange.201208470
Stephan DW, Erker G (2010) Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew Chem Int Ed 49(1):46–76. doi:10.1002/anie.200903708
Ashley AE, Thompson AL, O’Hare D (2009) Non-metal-mediated homogeneous hydrogenation of CO2 to CH3OH. Angew Chem Int Ed 48(52):9839–9843. doi:10.1002/anie.200905466
Ménard G, Stephan DW (2010) Room temperature reduction of CO2 to methanol by Al-based frustrated Lewis pairs and ammonia borane. J Am Chem Soc 132(6):1796–1797. doi:10.1021/ja9104792
Riduan SN, Zhang Y, Ying JY (2009) Conversion of carbon dioxide into methanol with silanes over N-heterocyclic carbene catalysts. Angew Chem Int Ed 48(18):3322–3325. doi:10.1002/anie.200806058
Chakraborty S, Zhang J, Krause JA, Guan H (2010) An efficient nickel catalyst for the reduction of carbon dioxide with a borane. J Am Chem Soc 132(26):8872–8873. doi:10.1021/ja103982t
Huang F, Lu G, Zhao L, Li H, Wang Z-X (2010) The catalytic role of N-heterocyclic carbene in a metal-free conversion of carbon dioxide into methanol: a computational mechanism study. J Am Chem Soc 132(35):12388–12396. doi:10.1021/ja103531z
Huang F, Zhang C, Jiang J, Wang Z-X, Guan H (2011) How does the nickel pincer complex catalyze the conversion of CO2 to a methanol derivative? A computational mechanistic study. Inorg Chem 50(8):3816–3825. doi:10.1021/ic200221a
Bontemps S, Vendier L, Sabo-Etienne S (2012) Borane-mediated carbon dioxide reduction at ruthenium: formation of C1 and C2 compounds. Angew Chem Int Ed 51(7):1671–1674. doi:10.1002/anie.201107352
Bontemps S, Sabo-Etienne S (2013) Trapping formaldehyde in the homogeneous catalytic reduction of carbon dioxide. Angew Chem Int Ed. doi:10.1002/anie.201304025
Sgro MJ, Stephan DW (2012) Frustrated Lewis pair inspired carbon dioxide reduction by a ruthenium tris(aminophosphine) complex. Angew Chem 124(45):11505–11507. doi:10.1002/ange.201205741
Courtemanche M-A, Légaré M-A, Maron L, Fontaine F-G (2013) A highly active phosphine–borane organocatalyst for the reduction of CO2 to methanol using hydroboranes. J Am Chem Soc 135(25):9326–9329. doi:10.1021/ja404585p
Zimmerman PM, Zhang Z, Musgrave CB (2010) Simultaneous two-hydrogen transfer as a mechanism for efficient CO2 reduction. Inorg Chem 49(19):8724–8728. doi:10.1021/ic100454z
Khandelwal M, Wehmschulte RJ (2012) Deoxygenative reduction of carbon dioxide to methane, toluene, and diphenylmethane with [Et2Al]+ as catalyst. Angew Chem Int Ed 51(29):7323–7326. doi:10.1002/anie.201201282
Matsuo T, Kawaguchi H (2006) From carbon dioxide to methane: homogeneous reduction of carbon dioxide with hydrosilanes catalyzed by zirconium – borane complexes. J Am Chem Soc 128(38):12362–12363. doi:10.1021/ja0647250
Berkefeld A, Piers WE, Parvez M (2010) Tandem frustrated Lewis pair/Tris(pentafluorophenyl)borane-catalyzed deoxygenative hydrosilylation of carbon dioxide. J Am Chem Soc 132(31):10660–10661. doi:10.1021/ja105320c
Berkefeld A, Piers WE, Parvez M, Castro L, Maron L, Eisenstein O (2013) Decamethylscandocinium-hydrido-(perfluorophenyl)borate: fixation and tandem tris(perfluorophenyl)borane catalysed deoxygenative hydrosilation of carbon dioxide. Chem Sci 4(5):2152–2162. doi:10.1039/C3SC50145K
Mitton SJ, Turculet L (2012) Mild reduction of carbon dioxide to methane with tertiary silanes catalyzed by platinum and palladium silyl pincer complexes. Chem Eur J 18(48):15258–15262. doi:10.1002/chem.201203226
Park S, Bézier D, Brookhart M (2012) An efficient iridium catalyst for reduction of carbon dioxide to methane with trialkylsilanes. J Am Chem Soc 134(28):11404–11407. doi:10.1021/ja305318c
Li Y, Fang X, Junge K, Beller M (2013) A general catalytic methylation of amines using carbon dioxide. Angew Chem Int Ed. doi:10.1002/anie.201301349
Jacquet O, Frogneux X, Das Neves Gomes C, Cantat T (2013) CO2 as a C1-building block for the catalytic methylation of amines. Chem Sci 4(5):2127–2131. doi:10.1039/C3SC22240C
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ziebart, C., Beller, M. (2014). Hydrogenation and Related Reductions of Carbon Dioxide with Molecular Catalysts. In: Bhanage, B., Arai, M. (eds) Transformation and Utilization of Carbon Dioxide. Green Chemistry and Sustainable Technology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-44988-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-44988-8_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-44987-1
Online ISBN: 978-3-642-44988-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)