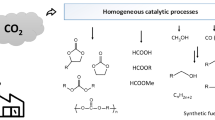
Abstract
Efficient chemical transformations of carbon dioxide into value-added chemicals are of growing importance in academic and industrial laboratories. In this respect, the reduction of carbon dioxide to formic acid, methanol etc., offers interesting possibilities. Herein, we describe the recent developments in carbon dioxide reductions mainly focusing on the use of defined organometallic catalysts and in some cases organocatalysts are also included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the beginning of the industrial revolution, the concentration of carbon dioxide in the atmosphere has been significantly increased because of burning fossil resources to generate energy supply. This increase is generally considered to be the main reason for global warming [1]. Although the chemical use of carbon dioxide cannot solve the problem of global warming alone, it’s utilization in synthesis offers interesting opportunities for the chemical industry and organic synthesis [2]. In general, carbon dioxide is a favourable C1 feedstock due to its abundance, availability, low toxicity and recyclability. Recent advances in organometallic catalysis provide effective means for the chemical transformation of CO2 and its incorporation into valuable organic compounds [3–8]. Notably, a variety of products including urea, cyclic carbonates and salicylic acid are produced already on industrial scale. In addition, direct carboxylation of Ar–X (X = Cl, Br, OPiv, B(OR)2 or H) and hydrocarboxylation of carbon–carbon multiple bonds offer interesting opportunities although these areas are still far away from practical applications.
While in biological systems the reduction of carbon dioxide is associated with photosynthesis, artificial reductions ideally make use of hydrogen. Several excellent reviews in this area have been published by Jessop and Leitner in 1995 [9, 10] and 2004 [11] and recently in 2011 by Gong [12]. Mechanistic perspectives and applications using homogeneous (Jessop and Leitner) and heterogeneous (Gong) catalysts were covered. Thus, the main subject of this review will be on advancements, which have been made since then.
2 Products from CO2 Reduction
2.1 From Carbon Dioxide to Formic Acid and Its Derivatives
2.1.1 Using Hydrogen as Reductant
The first homogeneously catalysed hydrogenation of carbon dioxide to formic acid was reported in 1976 [13]. Since then, especially in the last two decades, intensive studies followed. Normally, bases were added to thermodynamically drive the reaction from carbon dioxide to formic acid because the gas phase reaction has a positive ΔG value (Scheme 1). In the presence of inorganic base, formates are produced as final products, while in the presence of primary and secondary amine bases, formamides are obtained. Nowadays, tertiary amines are often used and proved to be the most active additive. In the presence of tertiary amines, an acid/amine ratio (AAR) normally in the range of 1.3 to 2.7 is obtained. On the other hand, the reduction of carbon dioxide into formates in aqueous solution is more favourable, in which case HCO3 − or CO3 2− are the actual substrates.
Initial investigations of carbon dioxide reduction revealed ruthenium complexes to be most active. For example, Hashimoto and Inoue found that H2Ru(PPh3)4 gave the best yield for the carbon dioxide reduction among different transition metal complexes [9]. Further improvement was disclosed by Noyori and co-workers who used RuII catalysts (RuCl2[P(CH3)3]4) in supercritical CO2 (scCO2) in the presence of triethylamine (NEt3) to give a TON of 7,200 [14, 15]. The increased catalyst efficiency was believed to be a result of the higher miscibility of H2 in scCO2 compared with other previously used solvents. This strategy was further improved, and catalyst TON values up to 28,500 and TOF 95,000 h−1 by using [RuCl(OAc)(P(CH3)3)4] were reported [16]. In this latter work, Jessop was also able to obtain methyl formate with a TON of 3,500 and dimethylformamide (DMF) with 420,000, respectively. In 2007, Himeda and co-workers [17] reported the use of [IrIIICp*] as efficient catalyst for the hydrogenation of carbon dioxide; formic acid was obtained with a TON of 222,000. In 2009, Nozaki and co-workers [18] used a defined iridium–pincer trihydride complex [IrIIIPNP] 1 for the hydrogenation of carbon dioxide (Scheme 2) and reported the highest TON value of 3,500,000 and TOF of 150,000 h−1 in aqueous KOH generating potassium formate as the final product.
It is also worth mentioning that in 2010 the group of Fachinetti [19] reported the production of HCOOH/NEt3 adducts by CO2 hydrogenation using [RuCl2(P(CH3)3)4] precursor at 40°C and 120 bar pressure in neat trimethylamine. In their work, the goal was to prevent HCOOH decomposition and to use the resulting adducts as hydrogen transfer reagents.
From all these examples, we can conclude that still today second- and third-row transition metals of groups eight through ten constitute metals of choice for carbon dioxide hydrogenation under mild conditions.
Besides hydrogenation of CO2, the biologically relevant reduction of carbonates and bicarbonates to formates constitutes interesting reactions (Scheme 3). However, relatively less work has been reported on such transformations. Notably, in 2003 Joó and co-workers reported [RuCl2(mTPPMS)22] as active catalyst (TON = 108) using an aqueous bicarbonate solution [20]. Later on, our group published an improved system based on [RuCl2(benzene)]2 and dppm for the hydrogenation of bicarbonates in water [21]. By applying dppm as ligand, a TON of 2,473 and a yield of 55% could be achieved. At that time, this constituted the highest TON recorded for the hydrogenation of bicarbonates without the addition of CO2.
In 2013, novel acyl-phosphine ligands such as 4 (Fig. 1) were designed and used in the presence of Ru(Me-allyl)2(COD). Using this system, the TON for the Ru-catalysed hydrogenation of bicarbonates was improved to 9,128 [22].
The hydrogenation of bicarbonates to formates offers the possibility to reversibly store hydrogen. In such a “hydrogen battery”, the hydrogen can be released on demand in the presence of suitable catalyst under mild conditions. Performing several hydrogenation/dehydrogenation cycles, this concept was demonstrated by our group in early 2011 by using the previously investigated RuII/dppm catalyst (Scheme 4) [23]. Notably, the group of Laurency developed a similar concept and used [RuCl2(mTPPMS)2]2 as catalyst for hydrogenation/dehydrogenation. Their system showed good stability and no significant drop of activity was observed after several cycles [24].
Another H2-storage system was also developed by our group using HCO2H as the storage material (Scheme 5) [25]. In this respect, the work of Himeda and co-workers describing the hydrogenation of carbon dioxide and decomposition of formic acid using half-sandwich iridium complex-4.4′-dihydroxy-2,2′-bipyridine (DHBP) 5 is of interest, too (Fig. 2) [26]. In this latter case, the hydroxyl group of the ligand is crucial for the high activity. The same positive ligand effect was also observed later in the ruthenium and rhodium analogues [27, 28]. More recently, Himeda and Fujita were able to synthesize a dinuclear [Cp*Ir] 6 catalyst for the CO2 hydrogenation. A TOF of 70 h−1 was achieved under very mild conditions (25°C and 1 bar).
So far, all the shown bicarbonate reductions are performed in the presence of precious metal complexes. However, also nonnoble metal-catalysed reactions have been discovered in recent years. For example, applying an iron catalyst [29], formates are obtained from bicarbonate in yield up to 88% with a TON of 610. Here, [FeH(PP3)]BF4 (PP3 = tris[(2-diphenylphosphino)ethyl]phosphine) was used as the defined catalyst. Besides, the reduction of carbon dioxide was also realized; thus, methyl formate was obtained by hydrogenation of CO2 in the presence of NEt3 and methanol with a TON = 585 and 56% yield. For the production of DMF, 75% yield and TON = 727 were obtained. In 2012, we were able to improve the productivity and activity of this iron system (TON = 7,500 and TOF = 750) via the modification of ligand structure [30].
Soon after our first report using an iron catalyst, the group of Milstein used the iron (II) pincer complex trans-[(tBu-PNP)Fe(H)2(CO)] 7 in the hydrogenation of carbon dioxide and sodium bicarbonate [31]. The reactions were run at a relatively low temperature (80°C) and pressure (6–10 bar) with TON up to 788 and TOF up to 156 h−1. Based on mechanistic studies, the reaction cycle shown in Scheme 6 was proposed. Firstly, insertion of carbon dioxide into the Fe–H bond leads to the oxygen-bound formate complex 8; the formate ligand is easily replaced by a water molecule to give the cationic complex 9. Then, in the presence of hydrogen, the dihydrogen-coordinated species 10 is formed which after heterolytic cleavage of the H2 regenerates the iron complex 7.
Regarding nonnoble metal catalysts, in 2012 our group reported a well-defined cobalt dihydrogen complex prepared from Co(BF4)2 .6H2O and tetraphos (PP3 = tris[(2-diphenylphosphino)ethyl]phosphine) for the hydrogenation of sodium bicarbonate and carbon dioxide. For the sodium formate formation, TON up to 3,877 was obtained [32]. In addition, Linehan and co-workers reported in 2013 an active cobalt system containing Co(dmpe)2H 12 (dmpe = 1,2-bis(dimethylphosphino)ethane) and Verkade’s base (2,8,9-triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane) for the hydrogenation of carbon dioxide at ambient conditions. TOF of 3,400 h−1 at room temperature and 1 bar of 1:1 CO2 and H2 were achieved. At higher pressure 20 bar, the TOF was improved to 74,000 h−1 [33]. The key to their success was the choice of a suitable base to match the pKa of the cobalt dihydrogen intermediate [Co(dmpe)2(H)2]+ 14, so the deprotonation can occur easily to form the mono-hydride active species 12 for carbon dioxide insertion (Scheme 7) [34].
Though numerous catalysts for the hydrogenation of carbon dioxide to formic acid have been investigated, for practical applications, improvements regarding the recycling of the catalysts and separation of formic acid from the resulting salts are still problematic. In this context, it is noteworthy that researchers from BASF reported the use of trihexylamine as amine additive and polar diol as solvent [35]. The key point of their finding is that the resulting formic acid trihexylamine salts are not miscible with the free amine and can be easily cleaved thermally to formic acid and amine under mild condition. The respective ammonium salts are soluble in diols, while the amine is not. Thus, a two-phase system is formed, and most of the active ruthenium species can be recycled from the amine phase. Interestingly, they also found that ruthenium carbonyl species were formed in the carbon dioxide hydrogenation reactions.
Most of the active carbon dioxide hydrogenation catalysts require basic additives [36], which inevitably produce the formate salts as final products. In order to avoid the tedious separation of formic acid from the salt mixture, one solution is to run the reaction under acidic conditions. In this respect, the work of Ogo and Fukuzumi is interesting, who firstly reported the reduction of CO2 to formic acid under acidic conditions using a water-soluble ruthenium catalyst [37, 38]. Very recently, Laurenczy and his colleagues were also able to run the direct hydrogenation of carbon dioxide into formic acid in acid buffer system (pH 2.7), which prevents the formation of formate salts in aqueous solution. In the presence of a water-soluble ruthenium catalyst [RuCl2(PTA)4] (PTA = 1,3,5-triaza-7-phosphaadamantane), 0.2 M formic acid was obtained [39]. Moreover, it was demonstrated that this catalyst can be reused multiple times.
Another way to separate formic acid from the reaction solution is to heterogenize the catalyst. Although a number of heterogeneous catalysts are known for the hydrogenation of carbon dioxide, in this review, the immobilization of molecular-defined complexes will be addressed. In this respect, Han [40] described a process using an immobilized ruthenium catalyst together with an ionic liquid (IL) containing a tertiary amino group as non-volatile base. After reaction, simple filtration and distillation lead to formic acid. Though the catalyst “Si”-(CH2)3NH(CSCH3)-RuCl3-PPh3 is heterogenized, at the micro level, it is homogeneous. Similar strategies were also used by Nakahara [41] and Leitner [42] independently.
2.1.2 Transfer Hydrogenation of Carbon Dioxide
Though phosphine-based catalysts were extensively investigated for carbon dioxide reduction, the use of related NHC (N-heterocyclic carbene) complexes received less attention. In 2010, Peris reported the carbon dioxide reduction to formate in the presence of several iridium NHC complexes (Scheme 8) [43].
Among the different complexes investigated, 16 performed best with a TON up to 1,800 at 80°C in the presence of 60 bars of carbon dioxide and hydrogen (1:1). Because these NHC complexes were also active in the transfer hydrogenation of C=O bonds, the transfer hydrogenation of carbon dioxide using isopropanol as hydrogen source was investigated, too. Indeed, they achieved catalyst TON up to 150. Besides, the use of other secondary alcohols such as cyclohexanol and 1-phenylethanol was also possible even though the activity was somewhat lower. Later on, the same group synthesized ruthenium complexes (η6-arene)Ru(bis-NHC) for the reduction of CO2 with hydrogen and isopropanol. At higher temperature (200°C), a maximum TON 874 was achieved for the transfer hydrogenation of carbon dioxide to formate [44]. Moreover, water-soluble carbenes (Fig. 3) were used, which led to improved catalyst TON [45]. Using the transfer hydrogenation concept, Dibenedetto and Aresta reported in 2011 the [RuCl2(PPh3)3]-catalysed reduction of carbon dioxide using aqueous glycerol. Though the TON was low, this concept is very interesting [46].
2.1.3 Using Hydrosilanes
Apart from hydrogen and alcohols, some hydrosilanes constitute interesting reductants which are easy to handle and readily available. Thus, the reduction of carbon dioxide to formic acid using hydrosilanes was investigated in several studies. In 2010, Baba reported the copper-catalysed formation of formic acid from carbon dioxide. Here, silyl formate is formed initially, which is then hydrolysed to formic acid (Scheme 9) [47]. At 1 bar of carbon dioxide, formic acid was obtained in 95% yield with a TON of 8,100. Interestingly, the copper catalyst performed better compared to other transition metal catalysts, e.g. ruthenium [48] and iridium. Later on, Nozaki and her colleagues also reported a similar transformation using Cu/NHC as catalyst [49]. In 2013, Garcίa reported that [(dippe)Ni(μ-H)]2 catalysed such reaction and also showed the possibility of transfer of the silyl formate to alkyl formates or formamides (Scheme 9) [50].
Using metal-free conditions, Cantat and co-workers demonstrated elegantly that simply base [51] or carbene [52] catalysed the formation of formamide from CO2 and hydrosilane. Here, the base TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) performed best in the reduction of CO2 (Scheme 10).
2.2 From Carbon Dioxide to Carbon Monoxide
2.2.1 Hydrogen as Reductant
The hydrogenation of CO2 to CO, the so-called reverse-water–gas-shift reaction, is equivalent to the hydrogenation of CO2 to formic acid and subsequent dehydration (Scheme 11). Indeed, Khan [53] reported the carbon dioxide reduction to formic acid which was decomposed to CO and hydrogen. However, it should be noted that formic acid is not always the reaction intermediate for CO production.
In 1993, Sasaki and Tominaga reported the hydrogenation of CO2 to CO using Ru3(CO)12 as catalyst. In the presence of KI as additive, methanol and methane were also observed as products [54]. The latter two products are assumed to arise from the hydrogenation of in situ formed CO. Soon after, the same group improved this reaction by using [PPN]Cl (bis(triphenylphosphine)iminium chloride) as additive. Here, improved catalyst TON of 80 was achieved at 160°C after 5 h [55]. The formation of formic acid as intermediate was ruled out by mechanistic studies. Instead, a chloride-assisted deprotonation of ruthenium hydride species was proposed as shown in Scheme 12.
Interestingly, 20 years after the original work, the same group reported the synthesis of a series of mononuclear Ru halogen carbonyl complexes, [PPN][RuX3(CO)3] (X = Cl−, Br−, and I−), which showed similar productivity in the RWGS reactions [56].
In order to drive the reaction more towards CO, strategies to remove/use the CO or water generated from the reaction system have been developed. In this respect, Sasaki’s group reported that in the presence of ethylene oxide (which absorbs the water to give ethylene glycol), CO2 is readily hydrogenated to CO in 71% yield (Scheme 13) [57].
Here, ethylene oxide simply acts as dehydration agent to remove the water in the RWGS equilibrium. Moreover, the subsequent use of CO to produce value-added chemicals has been investigated as well [58]. Thus, in 2000, Tominaga and Sasaki combined carbon dioxide reduction and alkene carbonylation in a one-pot manner to produce alcohols from CO2, hydrogen and alkenes [59]. At 140°C, cyclohexylmethanol is obtained in 88% yield directly from cyclohexene in the presence of LiCl and H4Ru4(CO)12 (Scheme 14). Four years later, the same group reported a detailed study on the above-mentioned reaction. They found that the catalytic activity of ruthenium complex is strongly affected by the anionic species of the added salts. The reaction rate increased in the order of I− < Br− < Cl−, which is also the order of their proton affinities [60]. In order to improve the selectivity, more recently, additives like [Bmim][X] (Bmim:1-butyl-3-methylimidazolium) as well as different ruthenium clusters [61] such as H4Ru4(CO)12 and Ru3(CO)12, [Ru(CO)3Cl2]2 and ligands were added to the reaction. Interestingly, improved results (higher TON and selectivity) were obtained when bulky monodentate phosphite ligands were used [62].
2.2.2 Reductants Other than H2
Based on our experience in hydrogen-borrowing reactions, we further developed the transfer hydrogenation of carbon dioxide to CO using alcohols and its direct use in alkene carbonylation reactions (Scheme 15) [63]. In such carbonylations which make use of RWGS reactions, water is produced as a side product and is not involved in the following alkene carbonylation reactions. Notably, Leitner and co-workers reported a related rhodium-catalysed hydrocarboxylation of alkenes with carbon dioxide and hydrogen to produce carboxylic acids [64].
Besides dihydrogen and alcohols, there are also other reductants reported for the reduction of carbon dioxide to CO. For example, in 2005, Sadighi reported the use of diboron compounds as CO2 deoxygenation reagents. More specifically, organocopper (I) complexes stabilized by NHC ligands reacted with pinB–pinB (bis(pinacolato)-diboron) to form the (IPr)Cu(Bpin) which reacted with CO2 under atmospheric pressure to produce the (IPr)Cu(OBpin) and release CO at the same time. The active species can be regenerated by reaction of (IPr)Cu(OBpin) with pinB–pinB (Scheme 16) [65].
In 2014, Lindhardt and Skrydstrup reported the mild and selective reduction of CO2 to CO using disilane as deoxygenation reagent in the presence of catalytic amount of CsF at room temperature (Scheme 17). In this context also the work of Stephan and co-workers is noteworthy, which demonstrated the catalytic reduction of CO2 to CO with phosphine as reductant through an in situ generated carbodiphosphorane and zinc (II) [66].
2.2.3 Photocatalysed CO2 Reduction to CO
Inspired by plants’ efficiency in converting carbon dioxide to carbohydrates and other organic matter through photosynthesis, chemists have explored the possibilities of directly reducing CO2 to CO by photocatalytic means [67]. Because CO2 does not absorb either visible or UV radiation in the wavelengths of 200–700 nm, this process normally requires a suitable photocatalyst (PS: photosensitizer) to absorb UV–vis radiation and then transfer an electron to CO2. Hence, the reduction process begins with excitation of the photosensitizer (PS). In the case of organometallic PS in general, a transfer of an electron from the metal centre onto the coordinated ligands takes place. Back-electron transfer from the ligands to the metal after the charge transfer is prevented by including an electron-donating species to quench the exciting state of the photocatalyst, thus forming the one electron-reduced (OER) intermediate for further CO2 reduction processes.
Already in 1982, seminal work by Lehn and co-workers led to the development of the first photocatalytic CO2 reductions. Here, CoCl2 was used as the catalytic site for CO2 reduction with [Ru(bpy)3]Cl2 as photosensitizer (Scheme 18) [68]. Apart from CO, hydrogen was produced as a side product via proton reduction. Though the turnover number of (CO + H2) was comparably low (32) based on [Ru(bpy)3]Cl2, the simultaneous reduction of CO2 and H2O produces syngas, which is of interest, too. Clearly, this work represents an early step in the development of chemical systems capable of artificial photosynthesis and solar energy conversion. Later on, the same research group found that rhenium-based complexes [Re(bipy)(CO)3X] (X = Cl−, Br−) acting as both photosensitizer and catalyst are more efficient and selective catalysts for CO2 reduction [69]. It was proposed that the dissociation of the ligand X− from the unstable 19-electron complex by OER is a key step in the photocatalysed reduction of CO2.
Additionally, Fujita and co-workers identified the binuclear [Re-C(O)O-Re] moiety as a key intermediate of the two-electron reduction process from CO2 to CO [70]. In 2008, an efficient photocatalytic system was successfully developed by the group of Ishitani using mixed catalysts with fac-[Re(bpy)(CO)3(CH3CN)]+ and fac-[Re-4,4′-(MeO)2bpy(CO)3P(OEt)3]+ as photocatalysts [71]. Despite the improved activity of rhenium poly-pyridine complexes, a major problem with these photocatalysts is the lack of an extended absorption into the visible region. To solve this problem, Ishitani, Bian and co-workers presented the use of covalently linked hetero-nuclear Ru and Re multimetallic complexes in the photocatalytic reduction of CO2. The enhancement of the photocatalytic response to light in the visible region was achieved due to intramolecular electron transfer from the OER Ru species to the Re moiety. The binuclear complex Ru–Re, tri-nuclear complex RuRe2 and tetra-nuclear complex RuRe3 all furnished higher TON for the reduction of CO2 to CO (Fig. 4) [72, 73].
In recent years, the synthesis of new Re complexes for CO2 reduction to CO continued to attract attention. As an example, Hadadzadeh [74] reported the synthesis of a dinuclear Re(I) complex in 2014 (Fig. 5), [ReCl(CO)3(l-tptzH)Re(CO)3] 24, (tptzH = 2,4,6-tri(pyridine-2-yl)-2H-1,3,5-triazine-1-ide), for the CO2 reduction with a TON of 17.
Apart from Re and Re–Ru dinuclear complexes, in 2013, Sato and Ishitani [75] developed the mononuclear iridium (III) complex [Ir(tpy)(ppy)Cl]+ 25 for the selective reduction of CO2 to CO under visible light at 480 nm. Interestingly, there was no extra photosensitizer needed. Here, tri-ethanolamine was used as an artificial electron donor and the proposed mechanism is shown in Scheme 19.
As shown in Fig. 6, ruthenium complexes alone also showed activity in the photocatalytic CO2 reduction. For example, in 2014 Ishida [76] reported the use of [Ru(bpy)2(CO)2](PF6)2 (bpy = 2,2′-bipyridine) 28 as catalyst and [Ru(bpy)3](PF6)2 29 as photosensitizer in the presence of 1-benzyl-1,4-dihydronicotinamide (BNAH) as an electron donor for the CO2 reduction to CO. Interestingly, the iron-based complex 32 was also reported as active catalyst by Bonin and Robert using Ir(ppy)3 or 9-CNA (9-cyanoanthracene) as photosensitizers. Here, TON up to 140 was obtained (Fig. 7) [77].
2.3 From Carbon Dioxide to Formaldehyde
Though formaldehyde was proposed as intermediate in several CO2 reduction reactions, HCHO had not been isolated or even observed until 2013 when Bontemps and Sabo-Etienne reported the evidence for formaldehyde formation through NMR studies in the course of CO2 reduction using HBpin [78]. Later on, they used amine to trap the formaldehyde to form the corresponding imine. After hydrolysis, they obtained a formalin solution, which demonstrated for the first time the production of formaldehyde from CO2 (Scheme 20) [79].
Most recently, Oestreich reported the use of tethered Ru–S complexes for the catalytic hydrosilylation of carbon dioxide to formaldehyde bis(silyl)acetal or silylated methanol [80].
2.4 From Carbon Dioxide to Methanol
The conversion of atmospheric carbon dioxide to methanol is a potential technology for the production of fuel alternatives based on renewable energy [81, 82]. Besides, methanol is used as starting material for the synthesis of various bulk chemicals, for example, ethylene and propylene [83]. In this respect, the “George Olah Carbon Dioxide to Renewable Methanol Plant” represents a demonstration plant in Iceland using a heterogeneous copper–zinc oxide–alumina catalyst for the hydrogenation of carbon dioxide [84]. Meanwhile, homogeneous catalysts able to reduce CO2 at low(er) temperature received increasing attention.
2.4.1 Using Hydrogen as Reductant
In 2011, Sanford and colleagues reported the sequential hydrogenation of CO2 to methanol using a combination of a Ru–Pincer 33 and a ruthenium–phosphine 34 pre-catalyst in the presence of Lewis acid Sc(OTf)3. The catalytic sequence involves (a) hydrogenation of carbon dioxide to formic acid by 34, (b) acid esterification to formate and (c) hydrogenation of ester to methanol by 33 (Scheme 21). The incompatibility of 33 and Sc(OTf)3 was solved by physically separating the catalysts using a vial in the autoclave. Thus, an overall catalyst turnover number of 21 was achieved.
More recently, the reduction of carbon dioxide to methanol using one single ruthenium–phosphine complex was achieved by Klankermayer and Leitner. The combination of the tridentate ligand triphos (1,1,1-tris(diphenylphosphinomethyl)ethane) and [Ru(acac)3] in the presence of MSA (methanesulfonic acid) in situ formed an active catalyst to produce methanol with a TON of 221 at 140°C, 60 bar hydrogen and 20 bar CO2 (Scheme 22) [85]. The same authors reported in 2015 a detailed mechanism via DFT study on this reduction process. Moreover, a biphasic reaction system involving H2O and 2-MTHF was developed, in which case methanol is extracted into the aqueous phase.
In addition, Milstein and co-workers reported in early 2011 an alternative route to methanol from carbon dioxide using organic carbonates or formats as substrates in the presence of Ru–pincer catalysts. Turnover number as high as 4,700 was achieved [86]. Though the process is an “efficient” way from carbon dioxide to methanol, the economic viability is problematic due to the price of the starting materials. In this respect, the work of the group of Ding is interesting, who used cyclic carbonates as starting material for the production of methanol. Excellent TON of 87,000 and TOF of up to 1,200 h−1 were accomplished [87].
2.4.2 Using Other Reductants for the Production of Methanol
The reduction of CO2-derived formic acid was studied by Miller and Goldberg [88]. Here, formic acid acted as hydrogen source and carbon substrate. The iridium complex [Cp*Ir(bpy)(H2O)] (OTf)2 35 (Cp* = pentamethylcyclopentadienyl, bpy = 2,2-bipyridine, OTf = trifluoromethanesulfonate) which is known as an active transfer hydrogenation catalyst proved to be the most active catalyst (Scheme 23). Methanol and methyl formate were observed in the solution with 2% yield of methanol. Based on isotope labelling experiments, the author proposed that the formic acid was first reduced to formaldehyde by the in situ formed [Ir–H] and the formaldehyde was further reduced to methanol. The low product yield and expensive iridium catalyst encouraged Cantat and colleagues to further develop this method. In fact, using [Ru(methylallyl)2COD] in the presence of triphos and MSA improved the yield to 50% at 150°C [89].
Interestingly, in 2014 Hong [90] from Seoul reported the transfer hydrogenation method using isopropanol hydrogen source to produce methanol indirectly from carbon dioxide (cyclic carbonates). Again, PNP–Ru(II)-type complexes were the most active catalysts. In the presence of inorganic base, TON up to 16,600 was achieved (Scheme 24).
The hydrosilylation of CO2 to methoxysilyl species is already known since 1989 using Ir(CN)(CO)(dppe) (dppe = 1,2-bis(diphenylphosphino)ethane) at 40°C though with low TON [91]. In 2010, Guan was able to demonstrate that in the presence of nickel–pincer hydride, the catalytic hydroboration of CO2 to methoxyboryl species was feasible using borane at room temperature [92]. The nickel hydride complex 36 was isolated and a mechanism was proposed (Scheme 25) [93]. Firstly, CO2 inserts into the Ni–H bond; then the subsequent cleavage of Ni–O bond with HBcat (catecholborane) releases HCOOBcat, which is reduced to formaldehyde by another HBcat. Finally, hydroboration of formaldehyde gives the product CH3OBcat.
It is well known that nucleophilic NHC (N-heterocyclic carbene) activates CO2 to form imidazolium carboxylates. Based on this work, in 2009 Zhang and Ying [94] reported the reduction of carbon dioxide with silane to silylmethoxide [95] which after hydrolysis produced methanol under ambient conditions (Scheme 26). Regarding transition metal-free reduction systems [96–99] for methanol production, the work from O’Hare and Stephan is noteworthy, who have used FLPs (frustrated Lewis pairs) as catalyst. The activation of CO2 by FLPs forms bridging carboxylate species which are reduced with ammonia borane [100] or by low pressure of hydrogen [101]. Finally, hydrolysis of CH3O–LA (LA = BX3 or AlX3) led to methanol [102].
2.5 From Carbon Dioxide to Ethanol
It is known that ethanol can be produced via CO hydrogenation using Co-, Ru- and Rh-based catalysts. However, the homogeneous hydrogenation of CO2 to ethanol is barely known. In their studies on the hydrogenation of carbon dioxide to CO, Sasaki and Tominaga found that a small amount of EtOH was formed, too. Notably, they further improved the yield of ethanol by using Co2(CO)8 as cocatalyst in the Ru3(CO)12/KI system. Mechanistic studies revealed that the ruthenium was responsible for the production of MeOH, while cobalt species catalysed methanol homologation with CO [103].
2.6 From Carbon Dioxide to Methylamines
In 2014, the market value of methylamines such as MeNH2, Me2NH and Me3N exceeded 4,000 Euro/ton. Therefore, the reductive methylation of amines with CO2 can create additional value. The methylation of amines via six-electron reduction of CO2 remained unknown until 2013 when Cantat and his colleagues reported a zinc catalyst for the methylation of amines with CO2 and hydrosilanes at low pressure [104]. Furthermore, the selective reduction of urea was possible under similar reaction conditions. Meanwhile, our group developed a Ru/phosphine catalyst system that was able to convert carbon dioxide and amines into various kinds of N-methylated products in the presence of hydrosilanes (PhSiH3). Notably, diverse functional groups were well tolerated under these conditions (Scheme 27) [105]. Very recently, Cantat also showed the possibility of using iron catalysts for this transformation albeit high catalyst loading was needed [106].
Although some silanes are considered to be waste products from the silicone industry, all these methodologies are limited by the accessibility of the hydrosilanes and an additional workup step to remove siloxane by-products. Obviously, catalytic methylations using CO2 and H2 represent a greener method with H2O as the only by-product. In this respect, it is noteworthy to mention the work from Klankermayer [107] and Beller [108] who simultaneously reported the conversion of amines into methylamines in the presence of CO2 and H2. On the one hand, Klankermayer et al. presented the use of a molecularly defined ruthenium complex [Ru(triphos)(tmm)] together with readily available organic acids as cocatalysts to afford the methylation of aryl amines in good yields. On the other hand, our group demonstrated the N-methylation of both aromatic and aliphatic amines using CO2/H2. Applying an in situ combination of Ru(acac)3, triphos and either acid additives or LiCl, the desired methylated amines were obtained (Scheme 28). Most recently, our group also developed the methylation of C–H bonds using the same system; thus, indoles, pyrroles and electron-rich arenes react with CO2 and H2 to give the corresponding methylated products [109].
2.7 From Carbon Dioxide to Methane
The reduction of carbon dioxide to methane (Scheme 29) is of substantial industrial importance; however, synthetic systems capable of reducing carbon dioxide to methane have been elusive, though carbon dioxide can be reduced to all kinds of other chemicals as mentioned above. In 1989, Vaska showed the possibility of producing methane from carbon dioxide and hydrogen in the presence of ammonia using [Ir(Cl)(CO)(PPh3)2] as catalyst; formamide was found to be the intermediate [110]. In 2006, Matsuo and Kawaguchi reported the reduction of carbon dioxide to methane with hydrosilanes as the reductant [111]. In this case, in situ formed zirconium–borane complexes were used as the catalyst. The reaction was run in an NMR tube and TON up to 225 was achieved. The structure of zirconium is shown in Fig. 8.
In 2012, Brookhart [112] reported a more efficient iridium catalyst with general structure (POCOP)Ir(H)(HSiR3) for the hydrosilylation of CO2 to methane. More specifically, they found that less bulky silanes like Me2EtSiH gave more selectively methane. TON up to 8,300 was achieved. Meanwhile, Turculet [113] reported similar reactions using platinum and palladium silyl pincer complexes. The formation of a formate ester as intermediate was observed, which was further reduced to R3SiOCH2OSiR3 and R3SiOCH3 and finally to R3SiOSiR3 and CH4 (Scheme 30).
Notably, Piers et al. discovered that frustrated Lewis pairs also act as catalysts for the reduction of CO2 to methane using boranes. Here, the key step is the activation of CO2 by FLPs (frustrated Lewis pairs) which is further reduced (Scheme 31) [114]. Besides, strong Lewis acid such as [Et2Al]+ and even [R3Si]+ showed activity in the methane production from CO2 [115, 116].
Finally, it is worth mentioning that the photoreduction of CO2 to CH4 was reported, as early as 1986. In this respect, Willner and co-workers [117] reported the use of Ru(bpz)3 2+ (bpz = tri(bipyrazine)) as photosensitizer and ruthenium metal as catalyst for the process.
3 Concluding Remarks
In the last two decades, significant developments were achieved in the area of carbon dioxide reduction. Important basic discoveries, e.g. metal-free reductions, as well as significant improvements of more practical processes took place. As an example, after several years of research work, homogeneously catalysed reduction of carbon dioxide to formic acid derivatives has become a promising tool even for industrial applications. Particularly, the production of formic acid can be achieved using iridium and ruthenium complexes with very high catalyst turnover numbers or nonnoble metal catalysts such as iron. Notably, in these cases, catalyst efficiency does not represent today the limiting factor for commercial realizations. Here, feedstock prices and process engineering technologies are crucial. In the next years, similar efficient catalytic processes are expected for methanol and methane generation. Apart from hydrogenations, most reductions using silanes or boranes constitute interesting basic studies, but will be very difficult to realize on a larger scale. In these works, the basic understanding of the individual elementary steps of the catalytic cycles is most important. In contrast, the “simple” optimization of catalyst turnover numbers using boranes or silanes is less interesting. In the future, challenging work for carbon dioxide valorization will include easily available feedstocks such as CO2 streams directly from power plants. Besides, the efficient production of C–C bonds via (photo)catalytic means constitutes a major goal for basic science.
References
Aresta M (2010) Carbon Dioxide as Chemical Feedstock
Cokoja M, Bruckmeier C, Rieger B, Herrmann WA, Kühn FE (2011) Angew Chem Int Ed 50:8510–8537
Behr A (1988) Angew Chem 100:681–698
Maeda C, Miyazaki Y, Ema T (2014) Catal Sci Technol 4:1482–1497
Huang K, Sun C-L, Shi Z-J (2011) Chem Soc Rev 40:2435–2452
Mikkelsen M, Jorgensen M, Krebs FC (2010) Energy Environ Sci 3:43–81
Sakakura T, Choi J-C, Yasuda H (2007) Chem Rev 107:2365–2387
Liu Q, Wu L, Jackstell R, Beller M (2015) Nat Commun 6:5933
Jessop PG, Ikariya T, Noyori R (1995) Chem Rev 95:259–272
Leitner W (1995) Angew Chem Int Ed 34:2207–2221
Jessop PG, Joó F, Tai C-C (2004) Coord Chem Rev 248:2425–2442
Wang W, Wang S, Ma X, Gong J (2011) Chem Soc Rev 40:3703–3727
Inoue Y, Izumida H, Sasaki Y, Hashimoto H (1976) Chem Lett 863–864
Jessop PG, Hsiao Y, Ikariya T, Noyori R (1996) J Am Chem Soc 118:344–355
Jessop PG, Ikariya T, Noyori R (1994) Nature 368:231–233
Munshi P, Main AD, Linehan JC, Tai C-C, Jessop PG (2002) J Am Chem Soc 124:7963–7971
Himeda Y, Onozawa-Komatsuzaki N, Sugihara H, Kasuga K (2007) Organometallics 26:702–712
Tanaka R, Yamashita M, Nozaki K (2009) J Am Chem Soc 131:14168–14169
Preti D, Squarcialupi S, Fachinetti G (2010) Angew Chem Int Ed 49:2581–2584
Elek J, Nádasdi L, Papp G, Laurenczy G, Joó F (2003) Appl Catal A 255:59–67
Federsel C, Jackstell R, Boddien A, Laurenczy G, Beller M (2010) ChemSusChem 3:1048–1050
Gowrisankar S, Federsel C, Neumann H, Ziebart C, Jackstell R, Spannenberg A, Beller M (2013) ChemSusChem 6:85–91
Boddien A, Gärtner F, Federsel C, Sponholz P, Mellmann D, Jackstell R, Junge H, Beller M (2011) Angew Chem Int Ed 50:6411–6414
Papp G, Csorba J, Laurenczy G, Joó F (2011) Angew Chem Int Ed 50:10433–10435
Boddien A, Federsel C, Sponholz P, Mellmann D, Jackstell R, Junge H, Laurenczy G, Beller M (2012) Energy Environ Sci 5:8907–8911
Yuichiro H (2010) J Am Chem Soc 1056:141–153
Himeda Y, Miyazawa S, Hirose T (2011) ChemSusChem 4:487–493
Hou C, Jiang J, Zhang S, Wang G, Zhang Z, Ke Z, Zhao C (2014) ACS Catal 4:2990–2997
Federsel C, Boddien A, Jackstell R, Jennerjahn R, Dyson PJ, Scopelliti R, Laurenczy G, Beller M (2010) Angew Chem Int Ed 49:9777–9780
Ziebart C, Federsel C, Anbarasan P, Jackstell R, Baumann W, Spannenberg A, Beller M (2012) J Am Chem Soc 134:20701–20704
Langer R, Diskin-Posner Y, Leitus G, Shimon LJW, Ben-David Y, Milstein D (2011) Angew Chem Int Ed 50:9948–9952
Federsel C, Ziebart C, Jackstell R, Baumann W, Beller M (2012) Chem Eur J 18:72–75
Jeletic MS, Mock MT, Appel AM, Linehan JC (2013) J Am Chem Soc 135:11533–11536
Jeletic MS, Helm ML, Hulley EB, Mock MT, Appel AM, Linehan JC (2014) ACS Catal 4:3755–3762
Schaub T, Paciello RA (2011) Angew Chem Int Ed 50:7278–7282
Drake JL, Manna CM, Byers JA (2013) Organometallics 32:6891–6894
Hayashi H, Ogo S, Fukuzumi S (2004) Chem Commun 2714–2715
Ogo S, Kabe R, Hayashi H, Harada R, Fukuzumi S (2006) Dalton Trans 4657–4663
Moret S, Dyson PJ, Laurenczy G (2014) Nat Commun 5. doi:10.1038/ncomms5017
Zhang Z, Xie Y, Li W, Hu S, Song J, Jiang T, Han B (2008) Angew Chem Int Ed 47:1127–1129
Yasaka Y, Wakai C, Matubayasi N, Nakahara M (2010) J Phys Chem C 114:3510–3515
Wesselbaum S, Hintermair U, Leitner W (2012) Angew Chem Int Ed 51:8585–8588
Sanz S, Benítez M, Peris E (2009) Organometallics 29:275–277
Sanz S, Azua A, Peris E (2010) Dalton Trans 39:6339–6343
Azua A, Sanz S, Peris E (2011) Chem Eur J 17:3963–3967
Dibenedetto A, Stufano P, Nocito F, Aresta M (2011) ChemSusChem 4:1311–1315
Motokura K, Kashiwame D, Miyaji A, Baba T (2012) Org Lett 14:2642–2645
Jansen A, Pitter S (2004) J Mol Catal A Chem 217:41–45
Shintani R, Nozaki K (2013) Organometallics 32:2459–2462
González-Sebastián L, Flores-Alamo M, Garcıa JJ (2013) Organometallics 32:7186–7194
Das Neves Gomes C, Jacquet O, Villiers C, Thuéry P, Ephritikhine M, Cantat T (2012) Angew Chem Int Ed 51:187–190
Jacquet O, Das Neves Gomes C, Ephritikhine M, Cantat T (2012) J Am Chem Soc 134:2934–2937
Khan MMT, Halligudi SB, Shukla S (1989) J Mol Catal 57:47–60
Tominaga K-I, Sasaki Y, Kawai M, Watanabe T, Saito M (1993) J Chem Soc Chem 629–631
Tominaga K-I, Sasaki Y, Hagihara K, Watanabe T, Saito M (1994) Chem Lett 23:1391–1394
Tsuchiya K, Huang J-D, Tominaga K-I (2013) ACS Catal 3:2865–2868
Tominaga K-I, Sasaki Y, Watanabe T, Saito M (1997) Energy 22:169–176
Wu L, Liu Q, Jackstell R, Beller M (2014) Angew Chem Int Ed 53:6310–6320
Tominaga K-I, Sasaki Y (2000) Catal Commun 1:1–3
Tominaga K-I, Sasaki Y (2004) J Mol Catal A Chem 220:159–165
Jääskeläinen S, Haukka M (2003) Appl Catal A 247:95–100
Liu Q, Wu L, Fleischer I, Selent D, Franke R, Jackstell R, Beller M (2014) Chem Eur J 20:6809
Wu L, Liu Q, Fleischer I, Jackstell R, Beller M (2014) Nat Commun 5:3091
Ostapowicz TG, Schmitz M, Krystof M, Klankermayer J, Leitner W (2013) Angew Chem Int Ed 52:12119–12123
Laitar DS, Müller P, Sadighi JP (2005) J Am Chem Soc 127:17196–17197
Dobrovetsky R, Stephan DW (2013) Angew Chem Int Ed 52:2516–2519
Takeda H, Ishitani O (2010) Coord Chem Rev 254:346–354
Lehn J-M, Ziessel R (1982) Proc Natl Acad Sci 79:701–704
Hawecker J, Lehn J-M, Ziessel R (1983) Chem Commun 536–538
Hayashi Y, Kita S, Brunschwig BS, Fujita E (2003) J Am Chem Soc 125:11976–11987
Takeda H, Koike K, Inoue H, Ishitani O (2008) J Am Chem Soc 130:2023–2031
Bian Z-Y, Sumi K, Furue M, Sato S, Koike K, Ishitani O (2008) Inorg Chem 47:10801–10803
Gholamkhass B, Mametsuka H, Koike K, Tanabe T, Furue M, Ishitani O (2005) Inorg Chem 44:2326–2336
Shakeri J, Hadadzadeh H, Tavakol H (2014) Polyhedron 78:112–122
Sato S, Morikawa T, Kajino T, Ishitani O (2013) Angew Chem Int Ed 52:988–992
Kuramochi Y, Kamiya M, Ishida H (2014) Inorg Chem 53:3326–3332
Bonin J, Robert M, Routier MS (2014) J Am Chem Soc 136:16768–16771
Bontemps S, Sabo-Etienne S (2013) Angew Chem Int Ed 52:10253–10255
Bontemps S, Vendier L, Sabo-Etienne S (2014) J Am Chem Soc 136:4419–4425
Metsänen TT, Oestreich M (2015) Organometallics 34:543–546
Goeppert A, Czaun M, Jones J-P, Surya Prakash GK, Olah GA (2014) Chem Soc Rev 43:7995–8048
Ganesh I (2014) Renew Sust Energ Rev 31:221–257
Cui Z-M, Liu Q, Song W-G, Wan L-J (2006) Angew Chem Int Ed 45:6512–6515
Olah GA (2013) Angew Chem Int Ed 52:104–107
Wesselbaum S, vom Stein T, Klankermayer J, Leitner W (2012) Angew Chem Int Ed 51:7499–7502
Balaraman E, Gunanathan C, Zhang J, Shimon LJW, Milstein D (2011) Nat Chem 3:609–614
Han Z, Rong L, Wu J, Zhang L, Wang Z, Ding K (2012) Angew Chem Int Ed 51:13041–13045
Miller AJM, Heinekey DM, Mayer JM, Goldberg KI (2013) Angew Chem Int Ed 52:3981–3984
Savourey S, Lefèvre G, Berthet J-C, Thuéry P, Genre C, Cantat T (2014) Angew Chem Int Ed 53:10466–10470
Kim SH, Hong SH (2014) ACS Catal 4:3630–3636
Eisenschmid TC, Eisenberg R (1989) Organometallics 8:1822–1824
Chakraborty S, Zhang J, Krause JA, Guan H (2010) J Am Chem Soc 132:8872–8873
Chakraborty S, Patel YJ, Krause JA, Guan H (2012) Polyhedron 32:30–34
Riduan SN, Zhang Y, Ying JY (2009) Angew Chem Int Ed 48:3322–3325
Huang F, Lu G, Zhao L, Li H, Wang Z-X (2010) J Am Chem Soc 132:12388–12396
Das Neves Gomes C, Blondiaux E, Thuéry P, Cantat T (2014) Chem Eur J 20:7098–7106
Anker MD, Arrowsmith M, Bellham P, Hill MS, Kociok-Kohn G, Liptrot DJ, Mahon MF, Weetman C (2014) Chem Sci 5:2826–2830
Zhang L, Cheng J, Hou Z (2013) Chem Commun 49:4782–4784
Courtemanche M-A, Légaré M-A, Maron L, Fontaine F-G (2013) J Am Chem Soc 135:9326–9329
Ménard G, Stephan DW (2010) J Am Chem Soc 132:1796–1797
Ashley AE, Thompson AL, O’Hare D (2009) Angew Chem Int Ed 48:9839–9843
Courtemanche M-A, Légaré M-A, Maron L, Fontaine F-G (2014) J Am Chem Soc 136:10708–10717
Tominaga K-I, Sasaki Y, Saito M, Hagihara K, Watanabe T (1994) J Mol Catal 89:51–55
Jacquet O, Frogneux X, Das Neves Gomes C, Cantat T (2013) Chem Sci 4:2127–2131
Li Y, Fang X, Junge K, Beller M (2013) Angew Chem Int Ed 52:9568–9571
Frogneux X, Jacquet O, Cantat T (2014) Catal Sci Technol 4:1529–1533
Beydoun K, vom Stein T, Klankermayer J, Leitner W (2013) Angew Chem Int Ed 52:9554–9557
Li Y, Sorribes I, Yan T, Junge K, Beller M (2013) Angew Chem Int Ed 52:12156–12160
Li Y, Yan T, Junge K, Beller M (2014) Angew Chem Int Ed 53:10476–10480
Vaska L, Schreiner S, Felty RA, Yu JY (1989) J Mol Catal 52:L11–L16
Matsuo T, Kawaguchi H (2006) J Am Chem Soc 128:12362–12363
Park S, Bézier D, Brookhart M (2012) J Am Chem Soc 134:11404–11407
Mitton SJ, Turculet L (2012) Chem Eur J 18:15258–15262
Berkefeld A, Piers WE, Parvez M (2010) J Am Chem Soc 132:10660–10661
Khandelwal M, Wehmschulte RJ (2012) Angew Chem Int Ed 51:7323–7326
Wen M, Huang F, Lu G, Wang Z-X (2013) Inorg Chem 52:12098–12107
Maidan R, Willner I (1986) J Am Chem Soc 108:8100–8101
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wu, L., Liu, Q., Jackstell, R., Beller, M. (2015). Recent Progress in Carbon Dioxide Reduction Using Homogeneous Catalysts. In: Lu, XB. (eds) Carbon Dioxide and Organometallics. Topics in Organometallic Chemistry, vol 53. Springer, Cham. https://doi.org/10.1007/3418_2015_109
Download citation
DOI: https://doi.org/10.1007/3418_2015_109
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22077-2
Online ISBN: 978-3-319-22078-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)