Abstract
Their conspicuous acoustic communication behaviour makes crickets excellent model systems to study the neural mechanisms underlying signal generation and auditory pattern recognition. Male singing is driven by a central pattern generator (CPG) housed in the metathoracic and anterior abdominal ganglia with rhythmically active opener and closer interneurons that can reset the chirp rhythm. Command neurons descending from the brain control the singing behaviour. Female phonotaxis is tuned towards the species-specific pattern of the male calling song and auditory orientation behaviour demonstrates a parallel organisation of pattern recognition and highly accurate steering. First order auditory processing occurs in the thorax and pattern recognition in the brain. Local auditory brain neurons are tuned to the structure of the calling song, based on fast integration of inhibitory and excitatory synaptic activity. How pattern recognition is linked to the generation of auditory steering commands still remains an open question.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
More than 2,000 species of crickets use species-specific acoustic signals for intraspecific communication (Alexander 1962). Today, their conspicuous behaviour has been the focus of ethological, biophysical and neurophysiological research for 100 years (Regen 1913) and has led to major scientific contributions to the comprehensive characterisation of an insect auditory behaviour and its neural pathway (Huber and Thorson 1985), the neural basis of bat avoidance (Nolen and Hoy 1984), neural mechanisms of selective attention (Pollack 1988; Sobel and Tank 1994) the cellular mechanisms of a corollary discharge mechanism (Poulet and Hedwig 2002, 2006), biophysical and neural mechanisms of directional hearing (Michelsen et al. 1994; Michelsen and Löhe 1995; Schöneich and Hedwig 2010) frequency processing in the afferent and central pathway (Nocke 1972; Pollack and Faulkes 1998) and recently to implementing these findings on auditory orientation in bio-inspired robots (Reeve and Webb 2003). This paper will review aspects of these advances related to the neural mechanisms underlying male singing and female phonotactic behaviour.
8.2 Male Singing Behaviour
Male crickets produce a calling, rivalry or courtship song by rhythmically rubbing a plectrum at the edge of the left front wing against a file at the underside of the right wing. The underlying opening and closing wing movements are powered by muscles driven by motoneurons in the meso- and also the prothoracic ganglion (Kutsch 1969). Only closing movements generate a sound pulse setting structures on the wings in resonant coherent oscillations (Montealegre-Z et al. 2011). A salient feature of cricket auditory behaviour is the broad variety of species-specific song patterns, encompassing pulsing, chirping and trilling calling songs (Popov et al. 1974; Fig. 8.1). In some species song structure is the only feature to discriminate between identical morphs and points towards evolutionary changes in the neural mechanisms controlling singing as decisive steps in speciation (Otte 1992).
a Male and female cricket acoustic communication behaviour; while the male sings its calling song in front of a burrow the female approaches by orienting towards the song. Modified from Roesel von Rosenhof (1749) species probably G. campestris. b Variety of calling song patterns of sympatric cricket species in Azerbaijan. (1) Pteronemobius heydeni Fish., (2) Tartarogryllus bucharicus B.-B.; (3) Tartarogryllus tartarus obscurior Uv. (4) Tartarogryllus burdigalensis Latr. (5) Oecanthus pellucens Scop. (6) Turanogryllus lateralis Fieb. (7) Gryllus bimaculatus DeGeer (8) Modicogryllus pallipalpis Farb. Modified from (Popov et al. 1974; Fig. 8.1) with permission of the Nordrhein-Westfälische Akademie der Wissenschaften und der Künste
8.2.1 Neuropharmacology and Fictive Singing Pattern
Focal electrical brain stimulation in Gryllus campestris and Gryllus bimaculatus demonstrated the importance of the brain for singing behaviour and especially that the anterior protocerebrum controls the generation of calling song (Kutsch and Huber 1989). Mircoinjection of neuroactive substances in this brain area allowed systematic testing of neuroactive substances that release singing behaviour (Otto 1978; Wenzel and Hedwig 1999). In intact tethered males with only the head capsule opened, injections of cholinergic agonists (0.5 nL, 10−3 mol/l−1) were effective to elicit singing (Fig. 8.2a). Activation of ligand coupled nicotinic ACh-recepetors with acetylcholine or nicotine had fast effects and elicited bouts of singing after a latency of 11.5 s. Injection of muscarine, which activates a second messenger cascade via muscarinic ACh receptors, led to a gradual build-up of the behaviour over 60 s with singing sequences lasting for several minutes. Subsequent injection of GABA stopped ongoing singing activity. The experiments point towards a cholinergic pathway driving the release of singing. This pathway can effectively be activated by injection of Eserine, an acetylcholine-esterase inhibitor, causing a gradual build-up of ACh in the tissue. After injection of Eserine, singing can take several minutes to start, but then may continue for several hours. For intracellular studies of the cricket CNS pharmacological brain stimulation with Eserine is an efficient way to release singing activity, even after all thoracic sensory and motor nerves are cut a fictive singing motor pattern is generated (Poulet and Hedwig 2006).
a Brain of G. bimaculatus; micro-injection sites (■) eliciting singing behaviour are located between the α-lobe and the pedunculus. b, c Fictive singing motor pattern and cricket CNS. During successive sections of abdominal connectives singing always stopped when the T3–A3 connectives were severed. Motor pattern recorded from wing nerve T2-N3A, opener and closer motoneuron activity indicated by (○) and (●). MB mushroom body, α-L alpha lobe, β-L beta lobe, Pe pedunculus, CB central body complex, PB protocerebral bridge. a From Wenzel and Hedwig (1999), b from Schöneich and Hedwig (2011), c modified from (Huber 1955; Fig. 1) with permission of John Wiley and Sons
Fictive singing motor activity can be recorded as spike activity of wing-opener and closer motoneurons in the mesothoracic nerve 3A (Fig. 8.2b) and closely resembles the timing of the normal pattern in G. bimaculatus (Schöneich and Hedwig 2011, 2012). As in normal singing the mean chirp rate ranges from 2 to 3 Hz and the mean pulse rate is about 24 Hz. The chirp rate decreases when the pulse number per chirp increases from 3 to 5. In contrast, the chirp interval always remains stable between 210 and 260 ms. Over a chirp the mean opener–closer interval is very constant and between 21 and 24 ms for different males, whereas the closer–opener interval may increase from about 19–25 ms causing a gradual increase in the pulse-period. The close similarity between fictive and normal singing motor pattern indicates that sensory feedback is not required for generating the singing pattern; it might however, be used during sound production for fine adjustments of the front wing movements (Kutsch and Huber 1989).
8.2.2 Locating the Singing CPG
As the singing wing movements are mainly controlled by motoneurons in the mesothoracic ganglion T2, it was assumed that the singing CPG is also located in the thoracic ganglia with the brain and the reproductive organs being necessary to trigger calling and courtship song (Huber 1955; Kutsch and Huber 1989). Evidence based on micro-lesions in the CNS (Hennig and Otto 1996) and on the structure of singing interneurons (Hennig 1990) gradually pointed against this hypothesis and towards a contribution of abdominal ganglia to singing pattern generation. When connectives between the abdominal ganglion A3 and the terminal ganglion TG are cut during fictive singing there is little effect on singing activity. However, singing always immediately stops when connectives between T3 and A3 are sectioned (Fig. 8.2b, c; Schöneich and Hedwig 2011). Thus, a crucial part of the singing network must be housed in A3 and current results point towards T3–A3 as the ganglia housing the network of the singing pattern generator. Beyond this descending ventilatory interneurons from the SOG have an inhibitory effect on the chirp pattern (Otto and Hennig 1993) and coordinate ventilatory and singing motor activity.
8.2.3 Unravelling the CPG Network for Singing
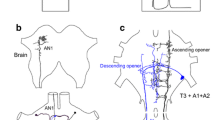
Microelectrode recordings within the singing network revealed two crucial opener interneurons (Schöneich and Hedwig 2012), which are activated just before the opener motoneurons. The metathoracic-descending-opener interneuron has dendrites running at the midline posteriorly towards the abdominal neuromeres A1 and A2 which are fused with T3 (Fig. 8.3a). Its axon projects into the abdominal nerve cord giving off characteristic anterior and posterior axonal collaterals in ganglia A3–A6. There is only one of these interneurons at each side of the CNS. Activity in this interneuron is characterised by typical rhythmic membrane potential oscillations with consecutive de- and hyperpolarisation of the dendritic membrane potential in the pulse pattern and burst of spikes generated during the depolarisation phase (Fig. 8.3b). Manipulating spike activity of this interneuron by intracellular current injection requires unusual high currents of 10–20 nA, but if successful, elicits additional sequences of open-closer activity during fictive singing that reset the chirp rhythm.
a Structure of the metathoracic opener interneuron T3–DO descending from T3 towards A6. b A burst of spikes in the interneuron precedes the opener phase of singing activity and inhibition precedes the closer activity. Depolarisation of the interneuron elicits a bout of opener–closer activity and resets the chirp pattern. c Structure of the abdominal-opener interneuron A3–AO ascending from A3 towards T1. d Activity of the interneuron is coupled to the opener activity; a depolarising current pulse elicits a sequence of opener–closer activity and resets the chirp pattern. Expected timing of undisturbed chirp pattern indicated by light grey shades; modified from Schöneich and Hedwig (2012)
An ascending opener interneuron is located in A3 where its dendrites closely match the axonal arborisation pattern of the metathoracic-opener interneuron (Fig. 8.3c). The axon of the abdominal-opener interneurons runs up to T1 with axon collaterals projecting towards the midline of the thoracic ganglia. There is a left–right pair of abdominal-opener interneurons, which may be electrically coupled by gap-junctions as dye-coupling is observed in neurobiotin staining. Also, the abdominal-opener interneuron generates rhythmic membrane potential oscillations in phase with the opener motor activity; bursts of spikes are generated during each depolarisation, which are terminated by an inhibition (Fig. 8.3d). Injection of depolarising current pulses reliably elicits complete opener–closer cycles for the duration of current injection and reliably resets the chirp pattern, as the chirp interval between the last syllable of the elicited chirp and the first syllable of the subsequently generated chirp remains constant. Injection of current pulses within a chirp has no effect. Extended current injection even drives the CPG to produce long-lasting chirps, controlled by the duration of the current pulse. Thus, activity of the abdominal-opener interneuron not only controls the chirp rhythm but also chirp duration. Activity in the abdominal-opener interneuron starts 10 ms before the opener activity in the wing nerve and 3 ms before the metathoracic-opener interneuron, and so far appears to be the first interneuron activated in the syllable cycle.
Closer interneurons have been recorded in the anterior abdominal neuromers A1–A3 (Schöneich and Hedwig 2012) but have not yet been anatomically identified (Fig. 8.4a). They spike in phase with the wing-closer motoneurons and are inhibited during the wing-opening phase. Furthermore, over the course of a singing episode they receive a gradual inhibition, shifting their membrane potential below the resting potential. Depolarising current pulses also reset the chirp pattern. Some closer interneurons receive a barrage of IPSPs before the start of any singing sequence, which are coupled to subsequent depolarisation. Also, hyperpolarising current injection in closer interneurons is reliably followed by subsequent depolarisation, which increases with the amplitude of hyperpolarisation. Such responses of the membrane potential point towards a post-inhibitory rebound mechanism in the closer interneurons, which may be crucial for singing motor pattern generation.
a An unidentified closer interneuron receives inhibition in the opener phase and is depolarised in the closer phase. b Depolarising current pulses reset the chirp pattern, but do not elicit additional singing motor activity. c A speculative model of the singing CPG network based on mutual inhibition, post-inhibitory rebound and excitatory drive from command neurons may be sufficient to generate the pulse pattern; a, b from Schöneich and Hedwig (2012)
A simple tentative circuit for the singing CPG can be based on reciprocally inhibitory coupled opener and closer interneurons and a post-inhibitory rebound mechanism, which are sufficient to generate rhythmic neuronal activity (Fig. 8.4c; Bentley 1969; Perkel and Mulloney 1974). Tonic activity of descending command neurons (see below) may drive the opener interneurons, which generate a burst of spike activity and inhibit the closer interneurons. Due to post-inhibitory rebound the closer interneurons depolarise, generating a burst of spikes and inhibiting the opener neurons during the closer cycle. The excitation from the descending command neurons starts the opener activity again and the chirp pattern continues until some intrinsic properties of the opener interneurons reduce their activity and terminate the chirp. Singing stops, when the command activity comes to an end. As the singing circuit requires no sensory feedback, just changing the intrinsic properties of the CPG neurons or the strength of synaptic coupling could be an effective way to generate species-specific singing pattern.
As the layout of the auditory pathway is highly conserved in different species of crickets and bush-crickets (Stumpner and von Helversen 2001) we may assume a similarly conservative consistency at the pattern generation side. Thus the CPG interneurons identified in G. bimaculatus may provide the blueprint for the organisation of the singing motor network and comparative studies may now demonstrate how singing circuits are adapted for species-specific signalling.
8.2.4 Brain Neurons Controlling Calling Song
Extracellular electrical stimulation of the neck connectives (Otto 1971) or of small fibre bundles in the cervical connectives is sufficient to elicit singing (Bentley 1977), and indicates the existence of descending singing command neurons (Kupfermann and Weiss 1978). Probing the crucial regions of the anterior protocerebrum with intracellular recordings demonstrated a calling song command neuron at each side of the brain (Fig. 8.5a, Hedwig 2000). The primary neurite of this neuron runs towards the dorsally located cell body and dendrites arborise in the frontal medial area of the protocerebrum. Dendrites are densely packed within a delimited neuropil area between the pedunculus and the α-lobe of the mushroom body. The axon of the neuron descends in the contralateral connective towards the thoracic ganglia; its arborisation pattern in the ventral nerve cord however is not known.
a Structure of the descending calling song command neuron in G. bimaculatus, showing dense dendritic arborisations occurring between the α-lobe and the pedunculus. ON optic nerve, OcN ocellar nerve, Dc Deutocerebrum, see Fig. 8.2 for other abbreviations. b Current injection in the interneuron in an intact tethered male elicits a spike rate of about 120 AP/s and singing, that gradually wanes after current injection. c Current injection during singing accelerates the chirp pattern and causes a reduction in the number of pulses per chirp. Singing activity monitored by wing movements and sound pattern; modified from Hedwig (2000)
This neuron fulfils the criteria set up for the characterisation of command neurons (Kupfermann and Weiss 1978). When its spike activity is driven to 100–120 AP/s by intracellular current injection into its dendrites, crickets will lift their wings and start singing (Fig. 8.5b). Singing is maintained while the tonic activity of the neuron is kept at about 120 AP/s. With the end of current injection and a decrease in the command spike activity singing ceases within a few seconds. Singing activity generally is facilitated with repetitive sequences of current injection, to the extent that even short 1 s current pulses can trigger ongoing singing, while the command neuron activity then is as low as 30–35 AP/s. Stimulation of the command neurons can alter the overall chirp rate but it has no effect on the syllable repetition rate (Fig. 8.5c; Otto 1971; Bentley 1977, Hedwig 2000). Syllable generation therefore appears to be largely independent of descending control. In intracellular stimulation experiments the command neuron always only elicited calling song, whereas in extracellular brain and connective stimulation experiments transitions between different songs occurred. Therefore, clear-cut conclusions may not yet be drawn on the control of different song patterns in crickets, however, at least in grasshoppers parallel descending pathways are required to control the full spectrum of male singing activity (Hedwig and Heinrich 1997).
8.3 Female Phonotaxis and Peripheral Auditory Pathway
Female crickets walk or fly towards singing conspecific males using acoustic cues for orientation (Regen 1913; Ulagaraj and Walker 1973). Their ears provide an excellent opportunity to study how insect hearing organs operate within the limitations set by the laws of physics (Michelsen 1992) and how the neural auditory pathway processes directional information and species-specific song patterns as the basis of phonotactic behaviour (Popov et al. 1974; Hoy 1978; Huber and Thorson 1985; Pollack 2000). A hearing organ is located in each front tibia comprising an array of 40–60 auditory afferents linearly arranged in the crista acoustica. Sound enters the auditory system via two tympanic membranes and an auditory trachea, with openings in the first thoracic segment that connects both ears. The structure (Ball et al. 1989), biophysics (Larsen et al. 1989) and afferent activity (Esch et al. 1980; Imaizumi and Pollack 2001) have been analysed in detail and appear as specialisations for directional hearing and for low and high frequency discrimination. It is still unknown which forces and structures trigger the mechano-electrical transduction process in afferent neurons and how their frequency tuning is established.
8.3.1 Temporal Tuning of Phonotaxis
Behavioural studies on phonotaxis has focussed on auditory pattern recognition and on the mechanisms of auditory orientation using arena experiments (Murphey and Zaretsky 1972; Tschuch 1977; Rheinlaender and Blätgen 1982; Stout et al. 1983), flight paradigms (Pollack and Hoy 1979) or treadmill systems (Wendler et al. 1980; Thorson et al. 1982; Doherty and Pires 1987; Hedwig and Poulet 2004) the latter of which monitor the animals’ walking speed and direction and indicate its phonotactic response (Fig. 8.6a). When exposed to acoustic patterns with systematic variations of the pulse duration and pulse interval, in all test paradigms female crickets prefer temporal patterns that match the species-specific male calling song (Fig. 8.6a). Although the pulse period may be a crucial component for phonotactic orientation in G. campestris and G. bimaculatus (Thorson et al. 1982), other parameters like pulse number and chirp rate contribute to the phonotactic response as well (Stout et al. 1983). The response to auditory patterns is not fixed, but transiently modulated by phonotaxis itself. If non-attractive chirp patterns are inserted into a sequence of normal calling song, the animals will steer towards these non-attractive chirps as well, which do not elicit phonotaxis when presented on their own (Fig. 8.6b; Poulet and Hedwig 2005). In this way phonotaxis broadens the acceptance of auditory patterns and the animals transiently tolerate distorted calling song patterns as they occur in natural environments due to sound diffraction and noise (Kostarakos and Römer 2010). The system may operate to maximise phonotactic targeting success while pursuing conspecific auditory patterns.
a Open-loop track ball system for analysing cricket phonotactic behaviour. While the tethered female walks on the trackball different sound patterns are presented from left or right and the movement of the trackball is monitored. Female phonotaxis of G. bimaculatus is tuned to the temporal pattern of the species-specific calling song. b Modulation of phonotactic tuning. Steering responses to different chirps during phonotaxis (black lines). Responses to pulse periods PP18 and PP98 (grey line) are stronger when these are inserted into a sequence of normal calling song. c Rapid steering responses to individual sound pulses occur during presentation of a split calling song pattern. d Accuracy of directional steering allows crickets to reliably steer to speaker positions 1–2° off their length axis. e The bilateral difference in tympanic membrane vibrations corresponds to 0.4 dB/° a from Hedwig (2006), b, c from Poulet and Hedwig (2005), d, e from Schöneich and Hedwig (2010)
8.3.2 Auditory Steering and Pattern Recognition
How do pattern recognition and directional steering interact during auditory orientation? Are they organised in serial or parallel pathways (Helversen and Helversen 1995)? Early trackball recordings of female orientation apparently showed that steering responses only occur after processing of complete chirps (Schildberger and Hörner 1988; Schmitz et al. 1982) and indicated a serial organisation of pattern recognition and steering. Auditory steering responses are, however, surprisingly fast when monitored with a low inertial track ball system (Fig. 8.6c; Hedwig and Poulet 2004, 2005). Females exposed to split-song paradigms of the calling song, with subsequent sound pulses presented from opposite sides, steer towards individual pulses with a latency of just 55–60 ms. As the animals do not evaluate a complete chirp to guide their orientation, auditory pattern recognition and steering have to be organised in parallel pathways; pattern recognition however is first required for fast steering responses to occur. Thus pattern recognition and steering are transiently processed in a serial manner and once recognition is established they then occur in parallel. Overall female phonotaxis is characterised by three properties: it is tuned towards the species-specific sound pattern, steering is fast and precise and steering responses are transiently modulated.
8.3.3 Directional Hearing and Orientation
Phonotactic orientation requires directional auditory sensitivity and processing. The primary auditory afferents project into the prothoracic ganglion which is the first stage of auditory processing (Esch et al. 1980). The directionality of the system appears to be tuned to the carrier frequency of the calling song (Michelsen and Löhe 1995). Due to phase shifts in the auditory trachea the cricket’s hearing system generates bilateral response differences up to 20–30 dB. Directional cues are, however, small in the frontal range of the animal as the overall gradient for directional discrimination may only be 10–12 dB over 25° and appear to be even less for small angular deviation. This led to the assumption that crickets face a frontal area of uncertainty where they cannot consistently turn towards a sound (Rheinlaender and Blätgen 1982; Larsen et al. 1989), in line with reports that the animals use a meandering walk when approaching a singing male (Schmitz et al. 1982). When phonotactically walking females were tested on an open-loop trackball with a constant alignment of their body relative to the sound signal, they showed a remarkably precise directional orientation and reliably steered towards calling songs, which were just 1° off their length axis (Fig. 8.6d, e; Schöneich and Hedwig 2010). Relating behavioural directional discrimination to bilateral response differences of tympanic membrane oscillations and auditory afferents revealed that the animals can use a slope of 0.4 dB/° interaural signal difference in the frontal ±30° for their directed phonotactic approach. In summed recordings of the afferent response this gradient is reflected in changes of response latency of 42 μs/°. Interaural amplitude and latency differences of the afferent activity are forwarded to the thoracic auditory neuropil where reciprocal inhibition by the local omega interneurons (ON1) allows for further bilateral contrast enhancement leading to highly directional responses of the ON1 neurons even at small angles close to frontal stimulation (Wiese and Eilts-Grimm 1985; Wohlers and Huber 1982).
On each side of the CNS only two auditory interneurons forward auditory activity towards the brain: interneuron AN1 is tuned in the range of 4–5 kHz the carrier frequency of the calling song and AN2 responds best to high-frequency sounds in the range of 10–15 kHz. The activity of AN1 is crucial for phonotactic steering (Schildberger and Hörner 1988), whereas AN2 triggers bat-avoidance behaviour (Nolen and Hoy 1984). When females steer towards a sound pattern presented from the left and the left AN1 (i.e., axon in the left connectives) is hyperpolarised to reduce its spike activity, then these animals alter their direction and walk towards the right (Schildberger and Hörner 1988). The conclusion from these experiments was that the animals steer towards the side where the calling song activates AN1 more strongly, by comparing bilateral AN1 activity levels in the brain. To meet the requirements of reflex-like fast steering, descending motor commands could be generated in the brain. However, as pattern recognition modulates the steering process, descending interneurons might just alter the response properties of the thoracic sensory-motor pathway involved in the control of walking (Poulet and Hedwig 2005). Overall in terms of directional hearing, crickets together with the fly Ormia ochracea (Mason et al. 2001), are among the most precise invertebrates and can easily compete with vertebrate achievements (Heffner et al. 2007).
8.3.4 Auditory Brain Neurons and Pattern Recognition
Crickets have been established as a model system for the processing and recognition of acoustic pulse patterns. No temporal filtering occurs at the level of the thoracic auditory processing, AN1 rather copies any auditory patterns presented at the species-specific carrier frequency (Schildberger et al. 1989). How is phonotaxis controlled by the brain? Different concepts on neural mechanisms underlying pattern recognition have been put forward such as feature detection via cross-correlation, autocorrelation via a delay line and a combination of high- and low-pass filters (see Weber and Thorson 1989).
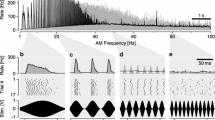
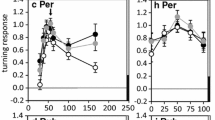
What is the evidence from brain neurons? Based on the response properties of local auditory brain neurons Schildberger (1984) concluded that G. bimaculatus the band-pass like tuning of female phonotaxis is due to specific low-pass and high-pass filter neurons, which are finally combined to result in band-pass tuned neural responses, akin to auditory filtering in the anuran brain (Rose and Capranica 1983). This concept is challenged by recent recordings from local auditory brain neurons, in which neural response towards different pulse patterns were compared with phonotactic responses to corresponding sound patterns (Kostarakos and Hedwig 2012). The brain neurons closely match the axonal arborisation pattern of AN1 in the anterior protocerebrum (Fig. 8.7a). The axonal projections of AN1 as well as the neurites of the interneurons form a ring-like arborisation pattern in the anterior ventral protocerebrum. Although the structure of the local auditory brain neurons is similar, their auditory response properties are very different and demonstrate an increasing level of tuning towards temporal patterns (Fig. 8.7b, c). When tested with different pulse periods female phonotaxis is tuned towards pulse periods of 34–42 ms. The B-LI2 neuron responds phasically with EPSPs and spikes to the individual sound pulses of all patterns. Its activity even reflects minor differences in the overall sound energy presented. Its response function, however, exhibits no tuning to the different pulse patterns and like the ascending interneuron AN1 its does not match the phonotactic behaviour. At the level of B-LI3 a more complex tuned response pattern is apparent, as the strongest excitatory response occurs to the second pulse of a chirp. Moreover, the response of B-LI3 to short and long pulse periods is about 60 % lower than the response to chirps presented at the species-specific pulse period. Tuning to the species-specific temporal pattern is best in B-LI4 neurons, which receive a mixture of inhibitory and excitatory synaptic inputs. Inhibition dominates the neuron’s response, when chirps with low or high pulse periods are presented. At the species-specific pulse period, however, the excitatory spiking response of the neuron is strongest leading to a close match between the tuning of the neural response and phonotactic behaviour.
a Structure of local auditory brain neurons in G. bimaculatus in the anterior protocerebrum, close to the axonal arborisation of the ascending interneuron AN1. b The neurons exhibit different degrees of temporal filtering when exposed to sound pattern with different pulse periods. Activity pattern in B-LI4 is shaped by inhibitory and excitatory synaptic inputs. c Whereas B-LI2 shows no sign of filtering, spike activity in B-LI3 and especially B-LI4 closely match the tuning of female phonotactic behaviour; modified from Kostarakos and Hedwig (2012)
While the temporal tuning of the local auditory brain neurons becomes sharper from B-LI2 to B-LI4, their spike response latency increases from 21 to 37 ms and their maximum response decreases from 14.3 to 3.9 AP/Chirp, respectively. This is in line with the concept of sparse coding, shifting the representation of stimulus features from a temporal activity-based code to a neuron-specific place code, which appears to be an energetically efficient way for small nervous systems to ensure a robust representation of stimulus patterns (Olshausen and Field 2004).
Thus, in the cricket brain temporal processing and tuning to the species-specific pattern seems to occur in a network of local auditory interneurons, closely matching the axonal arborisation pattern of AN1. Auditory band-pass properties encountered in other brain interneurons may just be the consequence of this processing. Like in frogs (Edwards et al. 2007; Rose et al. 2011) auditory processing appears to be based on fast pulse-by-pulse interactions of excitatory and inhibitory synaptic activity; the precise mechanisms still need to be explored. How many tuned filters for pulse patterns may crickets have? As the local auditory brain neurons are only tuned to the species-specific song pattern, it appears that they employ just one filter for pattern recognition. This is besides their ability of categorical discrimination of sounds presented with different carrier frequencies (Wyttenbach et al. 1996).
8.3.5 The Auditory-to-Motor Loop
It is not yet clear how auditory pattern recognition is linked to descending motor control for phonotaxis. Two types of motor activities are required: locomotion and auditory-induced steering. About 200 interneurons descend from the brain towards the ventral nerve cord (Staudacher 1998) some of which exhibit auditory responses in resting and especially in walking crickets (Staudacher and Schildberger 1998; Staudacher 2001; Zorovic and Hedwig 2011). However, due to response latencies and/or variability in their spike patterns the properties of these descending neurons are not sufficient to drive the fast phonotactic steering responses. As phonotactic steering occurs already to the very first pulse of a chirp with a latency of only 55–60 ms, pattern recognition cannot directly be involved in the steering process. However, pattern recognition apparently modulates auditory steering, as the animals respond to non-attractive sounds inserted in or presented immediately after a sequence of calling song (Poulet and Hedwig 2005). The underlying modulatory mechanism may be central to complete the auditory-to-motor loop. It may be elucidated by pharmacological approaches and by recording descending pre-motor brain neurons during phonotactic behaviour.
At the thoracic level the integration of phonotactic steering responses into the cricket’s walking pattern has been elucidated by identifying the motoneurons (Baden and Hedwig 2008) and by high speed video recordings (Witney and Hedwig 2011; Petrou and Webb 2012). During phonotactic walking, while exposed to the calling song, females do not couple their stepping cycle to the chirp pattern. They rather integrate phonotactic steering into the ongoing walking pattern and alter the trajectories of the legs to accommodate changes in walking direction. Although the trajectories of all legs are altered (Fig. 8.8), changes in the front leg movements appear to be most prominent for auditory steering and are accompanied by bending the first thoracic segment against the thoracic box, supporting steering towards the sound source.
a Monitoring leg movements and tarsal positions in phonotactic walking crickets with high speed video recordings. Tarsi are marked with a white spot and the angle between tarsi and the animal’s length axis is measured. b Tarsal trajectories in a cricket walking straight ahead without acoustic stimulation. Upon orienting towards a calling song pattern from the left the tarsal trajectories tilt to the left. Radius of polar-plot 1.5 cm. Modified from Witney and Hedwig (2011)
8.3.6 Conclusion: Crickets as a Neurobiological Model System for Acoustic Communication
Exploring the cricket nervous system with intracellular recordings has allowed us to establish a close link between neural pathways, identified neurons, neural mechanisms and auditory behaviour. Future experiments may draw upon the variety of song patterns in different species and provide insight into how species-specific neural networks for auditory communication were shaped during evolution and to what degree these networks develop in a sex-specific way. However, supplementary to a neurophysiological approach cricket neurobiology may advance by genetic techniques that allow expressing genetically encoded calcium indicators in the central nervous system or subset of cell lines. As first successful steps to sequence the cricket genome are being made (Danley et al. 2007) and first transgenic crickets have been created (Nakamura et al. 2010) the future looks promising.
References
Alexander RD (1962) Evolutionary change in cricket acoustical communication. Evolution 16:443–467
Baden T, Hedwig B (2008) Front leg movements and tibial motoneurons underlying auditory steering in the cricket (Gryllus bimaculatus deGeer). J Exp Biol 211: 2123–2133
Ball E, Oldfield B, Rudolph K (1989) Auditory organ structure, development, and function. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, London, pp 391–422
Bentley D (1977) Control of cricket song patterns by descending interneurons. J Comp Physiol A 116:19–38
Bentley DR (1969) Intracellular activity in cricket neurons during the generation of behaviour patterns. J Insect Physiol 15:677–684
Danley P, Mullen S, Liu F, Nene V, Quackenbush J, Shaw KL (2007) A cricket gene index: a genomic resource for studying neurobiology, speciation, and molecular evolution. BMC Genomics 8:109. doi:10.1186/1471-2164-8-109
Doherty JA, Pires A (1987) A new microcomputer-based method for measuring walking phonotaxis in field crickets (Gryllidae). J Exp Biol 130:425–432
Edwards CJ, Leary CJ, Rose GJ (2007) Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci 27:13384–13392
Esch H, Huber F, Wohlers DW (1980) Primary auditory neurons in crickets: physiology and central projections. J Comp Physiol A 137:27–38
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83:712–722
Hedwig B, Heinrich R (1997) Identified descending brain neurons control different stridulatory motor patterns in an acridid grasshopper. J Comp Physiol A 180:285–294
Hedwig B, Poulet J (2005) Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J Exp Biol 208:915–927
Hedwig B, Poulet JFA (2004) Complex auditory behaviour emerges from simple reactive steering. Nature 430:781–785
Heffner R, Koay G, Heffner H (2007) Sound-localization acuity and its relation to vision in large and small fruit-eating bats: I. Echolocating species, Phyllostomus hastatus and Carollia perspicillata. Hearing Res 234:1–9
Helversen D, Helversen O (1995) Acoustic pattern recognition and orientation in orthopteran insects: parallel or serial processing? J Comp Physiol A 177:767–774
Hennig RM (1990) Neuronal organisation of the flight motor pattern in the cricket, Teleogryllus commodus. J Comp Physiol A 167(5):629–639
Hennig R, Otto D (1996) Distributed control of song pattern generation in crickets revealed by lesions to the thoracic ganglia. Zoology 99:268–276
Hoy R (1978) Acoustic communication in crickets: a model system for the study of feature detection. Fed Proc 37:2316–2323
Huber F (1955) Sitz und Bedeutung nervӧser Zentren für Instinkthandlungen beim Männchen von Gryllus campestris L. Zeitschrift für Tierpsychologie 12:12–48
Huber F, Thorson J (1985) Cricket auditory communication. Sci Am 253:46–54
Imaizumi K, Pollack GS (2001) Neural representation of sound amplitude by functionally different auditory receptors in crickets. J Acoust Soc Am 109:1247–1260
Kostarakos K, Rӧmer H (2010) Sound transmission and directional hearing in field crickets: neurophysiological studies outdoors. J Comp Physiol A 196:1–13
Kostarakos K, Hedwig B (2012) Calling song recognition in female crickets: temporal tuning of identified brain neurons matches behaviour. J Neurophysiol 32:9601–9612
Kupfermann I, Weiss KR (1978) The command neuron concept. Behav Brain Sci 1:3–10
Kutsch W (1969) Neuromuskuläre Aktivität bei verschiedenen Verhaltensweisen von drei Grillenarten. J Comp Physiol A 63:335–378
Kutsch W, Huber F (1989) Neural basis of song production. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, London, pp 262–309
Larsen O, Kleindienst B, Michelsen A (1989) Biophysical aspects of sound perception. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, London, pp 364–390
Mason AC, Oshinsky ML, Hoy RR (2001) Hyperacute directional hearing in a microscale auditory system. Nature 410:686–690
Michelsen A (1992) Hearing and sound communication in small animals: evolutionary adaptations to the laws of physics. In: Webster DB, Fay RR, An Popper (eds) The evolutionary biology of hearing. Springer, Berlin, pp 61–77
Michelsen A, Löhe G (1995) Tuned directionality in cricket ears. Nature 375:639
Michelsen A, Popov A, Lewis B (1994) Physics of directional hearing in the cricket Gryllus bimaculatus. J Comp Physiol A 175:153–164
Montealegre-Z F, Jonsson T, Robert D (2011) Sound radiation and wing mechanics in stridulating field crickets (Orthoptera: Gryllidae). J Exp Biol 214:2105–2117
Murphey R, Zaretsky M (1972) Orientation to calling song by female crickets, Scapsipedus marginatus (Gryllidae). J Exp Biol 56:335–352
Nakamura T, Yoshizaki M, Ogawa S, Okamoto H, Shinmyo Y, Bando T, Ohuchi H, Noji S, Mito T (2010) Imaging of transgenic cricket embryos reveals cell movements consistent with a syncytial patterning mechanism. Curr Biol 20:1641–1647
Nocke H (1972) Physiological aspects of sound communication in crickets (Gryllus campestris L.). J Comp Physiol A 80:141–162
Nolen T, Hoy R (1984) Initiation of behavior by single neurons: the role of behavioral context. Science 226:992
Olshausen BA, Field DJ (2004) Sparse coding of sensory inputs. Curr Opin Neurobiol 14:481–487
Otte D (1992) Evolution of cricket songs. J Orthop Res 1:25–49
Otto D (1971) Untersuchungen zur zentralnervӧsen kontrolle der lauterzeugung von grillen. J Comp Physiol A 74:227–271
Otto D (1978) Änderungen von Gesangsparametern bei der Grille (Gryllus campestris L.) nach Injektion von Pharmaka ins Gehirn. Verh Dtsch Zool Ges 245
Otto D, Hennig R (1993) Interneurons descending from the cricket subesophageal ganglion control stridulation and ventilation. Naturwissenschaften 80:36–38
Perkel DH, Mulloney B (1974) Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185:181–183
Petrou G, Webb B (2012) Detailed tracking of body and leg movements of a freely walking female cricket during phonotaxis. J Neurosci Meth 203:56–68
Pollack G (2000) Who, what, where? recognition and localization of acoustic signals by insects. Curr Opin Neurobiol 10:763–767
Pollack G, Faulkes Z (1998) Representation of behaviorally relevant sound frequencies by auditory receptors in the cricket Teleogryllus oceanicus. J Exp Biol 201:155–163
Pollack GS (1988) Selective attention in an insect auditory neuron. J Neurosci 8:2635–2639
Pollack GS, Hoy RR (1979) Temporal pattern as a cue for species-specific calling song recognition in crickets. Science 204:429
Popov A, Shuvalov V, Svetlogorskaya I, Markovich A (1974) Acoustic behaviour and auditory system in insects. In: J. Schwartzkopff, (ed) Mechanoreception. Abh Rheinisch-Westfäl Akad Wiss, vol 53. pp 281–306
Poulet JFA, Hedwig B (2002) A corollary discharge maintains auditory sensitivity during sound production. Nature 418:872–876
Poulet JFA, Hedwig B (2006) The cellular basis of a corollary discharge. Science 311:518
Poulet JFA, Hedwig B (2005) Auditory orientation in crickets: pattern recognition controls reactive steering. Proc Nat Acad Sci 102:15665–15669
Reeve RE, Webb BH (2003) New neural circuits for robot phonotaxis. Phil Trans Roy Soc London A 361:2245–2266
Regen J (1913) Ueber die anlockung des weibchens von Gryllus campestris L. durch telephonisch übertragene Stridulationslaute des Männchens. Pflüg Archiv Europ J Physiol 155:193–200
Rheinlaender J, Blätgen G (1982) The precision of auditory lateralization in the cricket, Gryllus bimaculatus. Physiol Entomol 7:209–218
Roesel von Rosenhof AJ (1749) Insectenbelustigung zweyter Theil, welcher acht Klassen verschiedener sowohl inlaendischer als auch einiger auslaendischer Insecten enthaelt. Nuernberg, Fleischmann JJ
Rose G, Capranica RR (1983) Temporal selectivity in the central auditory system of the leopard frog. Science 219:1087–1089
Rose GJ, Leary CJ, Edwards CJ (2011) Interval-counting neurons in the anuran auditory midbrain: factors underlying diversity of interval tuning. J Comp Physiol A 197:97–108
Schildberger K (1984) Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A 155:171–185
Schildberger K, Hӧrner M (1988) The function of auditory neurons in cricket phonotaxis I. Influence of hyperpolarization of identified neurons on sound localization. J Comp Physiol A 163:621–631
Schildberger K, Huber F, Wohlers D (1989) Central auditory pathway: neuronal correlates of phonotactic behavior. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, London, pp 423–458
Schmitz B, Scharstein H, Wendler G (1982) Phonotaxis in Gryllus campestris L. (Orthoptera, Gryllidae). J Comp Physiol A 148:431–444
Schöneich S, Hedwig B (2010) Hyperacute directional hearing and phonotactic steering in the cricket (Gryllus bimaculatus deGeer). PLoS ONE 5(12):e15141
Schöneich S, Hedwig B (2011) Neural basis of singing in crickets: central pattern generation in abdominal ganglia. Naturwissenschaften 98(12):1069–1073
Schöneich S, Hedwig B (2012) Cellular basis for singing motor pattern generation in the field cricket (Gryllus bimaculatus DeGeer). Brain Behav 2(6):707–725
Sobel EC, Tank DW (1994) In vivo Ca2+ dynamics in a cricket auditory neuron: an example of chemical computation. Science 263:823–826
Staudacher E (1998) Distribution and morphology of descending brain neurons in the cricket Gryllus bimaculatus. Cell Tiss Res 294:187–202
Staudacher E, Schildberger K (1998) Gating of sensory responses of descending brain neurones during walking in crickets. J Exp Biol 201:559–572
Staudacher EM (2001) Sensory responses of descending brain neurons in the walking cricket, Gryllus bimaculatus. J Comp Physiol A 187:1–17
Stumpner A, von Helversen D (2001) Evolution and function of auditory systems in insects. Naturwissenschaften 88:159–170
Stout JF, DeHaan CH, McGhee RW (1983) Attractiveness of the male Acheta domesticus calling song to females I. Dependence on each of the calling song features. J Comp Physiol A 153:509–521
Thorson J, Weber T, Huber F (1982) Auditory behavior of the cricket II. Simplicity of calling-song recognition in Gryllus and anomalous phonotaxis at abnormal frequencies. J Comp Physiol A 146:361–378
Tschuch G (1977) Der Einfluss synthetischer Gesänge auf die Weibchen von Gryllus bimaculatus de Geer (Teil 2). Zool Jb Physiol 81:360–372
Ulagaraj S, Walker TJ (1973) Phonotaxis of crickets in flight: attraction of male and female crickets to male calling songs. Science 182:1278–1279
Weber T, Thorson J (1989) Phonotactic behavior of walking crickets. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, London, pp 310–339
Wendler G, Dambach M, Schmitz B, Scharstein H (1980) Analysis of the acoustic orientation behavior in crickets (Gryllus campestris L.). Naturwissenschaften 67:99–101
Wenzel B, Hedwig B (1999) Neurochemical control of cricket stridulation revealed by pharmacological microinjections into the brain. J Exp Biol 202:2203–2216
Wiese K, Eilts-Grimm K (1985) Functional potential of recurrent lateral inhibition in cricket audition. In: Kalmring K, Elsner N (eds) Acoustic and vibrational communication in insects. Parey, Berlin, pp 33–40
Witney AG, Hedwig B (2011) Kinematics of phonotactic steering in the walking cricket Gryllus bimaculatus (de Geer). J Exp Biol 214:69–79
Wohlers DW, Huber F (1982) Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket, Gryllus campestris L. J Comp Physiol A 146:161–173
Wyttenbach RA, May ML, Hoy RR (1996) Categorical perception of sound frequency by crickets. Science 273:1542–1544
Zorovic M, Hedwig B (2011) Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J Neurophysiol 105:2181–2194
Acknowledgments
I am grateful to the members of the lab that contributed to the presented data and fruitful discussions: Stefan Schöneich, Kostas Kostarakos, Hannah ter Hofstede, James Poulet, Tom Baden and Alice Witney and also to the BBSRC, the Isaac Newton Trust and the Royal Society for funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hedwig, B. (2014). Towards an Understanding of the Neural Basis of Acoustic Communication in Crickets. In: Hedwig, B. (eds) Insect Hearing and Acoustic Communication. Animal Signals and Communication, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40462-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-40462-7_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40461-0
Online ISBN: 978-3-642-40462-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)