Abstract
Species-specific acoustic signals of grasshoppers serve to attract mates; they are pivotal in avoiding hybridisation with sympatric species and to evaluate a potential mate’s quality. This necessitates a high precision of neuronal processing, which is constrained by the noisy nature of neuronal activity. Applying a spike train metric to estimate the variability of auditory responses, we quantified the respective impacts that external degradation of acoustic signals and intrinsic neuronal noise exert on signal processing. Unexpectedly, the variability of spike patterns increases from the afferents to the neurons whose axons ascend to the brain and reduces their ability to discriminate between similar communication signals. Between thoracic local and ascending neurons a change of coding principles seems to occur, leading to a population code with labelled-line characteristics. Thoracic auditory processing is conserved between distantly related species, suggesting that during evolution the communication signals have been adapted to match properties of the receiver’s sensory system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
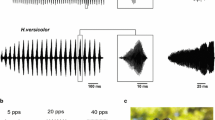
Many species of acridid grasshoppers produce acoustic signals in the context of mate attraction, and this communication is pivotal to avoid hybridisation with sympatric species (von Helversen and von Helversen 1975a, b; Stumpner and von Helversen 1994; Gottsberger and Mayer 2007). Hence, a high premium lies upon a reliable neuronal processing of these signals, allowing for their identification and classification. What cues of acoustic signals do grasshoppers use to infer species identity? The production as well as the recognition of songs is a genetically inherited, species-specific trait (von Helversen and von Helversen 1975a, b). Grasshopper ears exhibit only a poorly developed frequency resolution (Römer 1976; Stumpner and von Helversen 2001; Hennig et al. 2004). In addition, the frequency spectra of songs, generated by rubbing the hind legs against the front wings, overlap considerably between species (Meyer and Elsner 1996). Hence it is unlikely that frequency analysis contributes substantially to species identification. Indeed, the major differences between the songs of different species reside in the species-specific patterns of amplitude modulations produced by the characteristic patterns of hind leg movements underlying sound production (e.g. Elsner 1974; Elsner and Popov 1978; von Helversen 1986; Hedwig 1992, 1994; Stumpner and von Helversen 1994; Mayer et al. 2010). In a pioneering study on Chorthippus biguttulus Dagmar von Helversen showed that it is indeed the pattern of amplitude modulations, i.e. the sound envelope, which provides the major cues for species identification (von Helversen 1972; see also Stumpner and von Helversen 1992; von Helversen and von Helversen 1994, 1997, 1998; Klappert and Reinhold 2003). C. biguttulus females respond in a band-pass like manner to simple song models that are composed of block-like sound syllables separated by pauses (Fig. 11.1b), and they seem to evaluate the ratio between syllable and pause durations (von Helversen 1979; von Helversen and von Helversen 1994).
a Hindleg movements and song pattern of an intact Chorthippus biguttulus male (top) and detail of a male song produced with only one hind leg (bottom). Since sound is not produced at the upper and lower reversal points of the leg movement, small gaps result if only one leg is used for sound production (lower trace in a). In the syllables of intact males there are no silent gaps due to a phase shift between the movements of the two legs. b Responses of ten females to model songs (rectangularly modulated broadband noise, syllable duration 80 ms). c Response of a female to song models containing gaps of different durations (stippled curve) and relative responses of three AN4-neurons to these stimuli. a combined from Ronacher et al. (2004), Krahe and Ronacher (1993); b Sträter and Ronacher unpublished; c combined after von Helversen (1972) and Franz and Ronacher (2002)
There is evidence that specific features of acoustic signals may also be used in the context of sexual selection, i.e. for evaluating a potential mate’s quality (Kriegbaum 1989; Kriegbaum and von Helversen 1992; Klappert and Reinhold 2003, 2005; Einhäupl et al. 2011; Stange and Ronacher 2012). To achieve this, the grasshoppers’ auditory system must be able to detect subtle differences between songs of conspecifics which is obviously more difficult than to discriminate against the dissimilar signals of other species. We may therefore expect that the auditory system of these grasshoppers is particularly well adapted to process species-specific sound patterns. Indeed, behavioural experiments revealed an astonishing temporal resolution of the grasshoppers’ auditory system: C. biguttulus females reject the songs of males that have lost one hind leg. The syllables of these males contain short silent gaps—which in intact males do not occur due to a phase shift between the movements of left and right hind leg (Elsner 1974). The specific rejection of such gappy songs demonstrates that grasshopper females detect gaps as small as 2–3 ms (Fig. 11.1; von Helversen 1972, 1979; von Helversen and von Helversen 1997). Thus, their auditory system resolves very fast amplitude modulations, and with respect to gap detection these insects are not inferior to most vertebrates (for a detailed comparison see Prinz and Ronacher 2002). Hence, grasshoppers became a well-established model system for investigating the processing of acoustic signals in a small nervous system.
The songs’ temporal pattern of amplitude modulations is crucial for signal recognition and mate choice in grasshoppers. Starting from the observation of a remarkable gap detection capacity, I will focus in this review on the following questions:
-
(1)
How are the acoustic signals represented in the auditory system and how do the animals achieve such a high temporal resolution with a small number of auditory neurons and in spite of rather unreliable neuronal signals?
-
(2)
How well can similar signals be discriminated—a question that is particularly relevant in the context of sexual selection? What coding strategies are used at different stages of the auditory pathway?
-
(3)
How are the acoustic signals and the receiver’s auditory pathway mutually adapted to each other, to guarantee an effective communication?
11.2 High Temporal Resolution in Spite of Variable Spike Trains
11.2.1 Basic Features of the Auditory System of Grasshoppers
The ears of acridid grasshoppers are located on both sides of the first abdominal segment. About 60–80 receptor cells assembled in Müller’s organ attach to the inside of each tympanum. The majority of them is tuned to the range between 4 and 7 kHz, only around 10–15 afferent neurons are tuned to high frequencies, above 15 kHz (Michelsen 1971; Römer 1976; Halex et al. 1988; Jacobs et al. 1999). The axons of the sensory afferents enter the metathoracic ganglion complex and form an auditory neuropil with thoracic interneurons (Fig. 11.2a). Several interneuron types have been identified on the basis of their characteristic morphologies and activity patterns (Römer and Marquart 1984; Stumpner and Ronacher 1991). This auditory neuropil harbours an important stage of processing (Ronacher et al. 1986; Stumpner and Ronacher 1991; Stumpner et al. 1991). The basic organisation seems to be a 3-layer feed-forward network with afferent neurons connected to about 15 types of local neurons which in turn forward activity to about 20 different ascending neurons that send their axons to the brain (Marquart 1985; Römer et al. 1988; Stumpner 1988; Stumpner and Ronacher 1991; Boyan 1999; Vogel and Ronacher 2007). At least some afferent axons ascend even to the meso- and prothoracic ganglia (Halex et al. 1988; Römer et al. 1988; Stumpner 1988; Stumpner and Ronacher 1991), however, lesion experiments showed that these anterior projections of the sensory neurons are not necessary for song recognition (Ronacher et al. 1986). The spike patterns of ascending interneurons thus are the only activity which is forwarded to auditory networks in the brain and available to decide whether or not to accept a mating signal (Bauer and von Helversen 1987). A variety of physiological response types exists among thoracic interneurons, ranging from tonic, phasic-tonic and strongly phasic responses with different combinations of inhibition and excitation patterns (Römer and Marquart 1984, Römer et al. 1988; Stumpner and Ronacher 1991, Stumpner et al. 1991; Vogel et al. 2005; Vogel and Ronacher 2007).
a Schematic diagram of the auditory pathway of grasshoppers. Aff primary afferent neurons, LN local neurons, AN neurons with an axon ascending to the brain. b Spike raster plot of the responses of a local neuron and an ascending neuron to eight repeated presentations of an identical stimulus, a section of a C. biguttulus male song. Each small bar represents the timing of an action potential. b from Ronacher et al. (2008), with permission
For any recognition of acoustic signals based on the processing of amplitude modulations, the precision and reliability of a neuron’s spike patterns is fundamental. It is a general observation that auditory neurons do not produce identical responses if one and the same sensory stimulus is presented repeatedly. The responses vary in the timing of spikes, in spike count, or in both (Fig. 11.2b). This trial-to-trial variability results from stochastic events at different stages of the sensory pathway, e.g. stochastic ion channel openings during sensory transduction and spike generation and stochastic transmitter release at synapses (e.g. Zador 1998; White et al. 2000). For the central nervous system that has to decide how the animal should react to objects and events in the outer world this “intrinsic” variability of spike trains poses a potentially difficult problem: if two spike trains differ to some extent, do they still represent the same object or different ones? To pursue this question we must take the view point of the central nervous system that has no other information about the surrounding world than the spike trains provided by sensory neurons. In other words, the question of whether two similar acoustic signals can be discriminated or not, converts to the question: are the sensory spike trains corresponding to the two acoustic signals sufficiently different to be distinguishable for down-stream neurons?
11.2.2 Comparing the Respective Impacts of Intrinsic and External Noise Sources
We first have to consider how relevant the intrinsic neuronal noise really is for the animals. As a rule, acoustic signals arriving at the receiver’s ears will often be severely degraded compared to the emitted signal, due to a variety of external noise sources acting on sound transmission in the biotope (e.g. Michelsen and Larsen 1983; Lang 2000; Römer 2001; Brumm and Slabbekoorn 2005; Kostarakos and Römer 2010; Schmidt and Römer 2011). Conceivably, the intrinsic noise may play only a secondary role when we compare it with a strong degradation of acoustic signals occurring on the way between sender and receiver?
To compare the respective impacts of intrinsic neuronal noise versus external signal degradation we stimulated grasshopper males with a female song whose envelope was degraded by different amounts of random amplitude fluctuations (Fig. 11.3a). We quantified the dissimilarities between spike trains by means of a spike train metric (van Rossum 2001) and compared the spike trains that were elicited by the normal song with the spike trains produced in response to increasingly degraded songs (Neuhofer et al. 2011). The spike train metric describes the dissimilarity between two spike trains by a single number—small values indicating a high similarity (van Rossum 2001; Ronacher et al. 2008; Ronacher and Stange 2012). As the results for an auditory afferent and two thoracic interneurons show (Fig. 11.3b) the spike train distances increased linearly with increasing degradation level but they started already at a rather high distance value even for the normal stimulus (marked with orig at the abscissa). This distance value (filled arrow) reflects the trial-to-trial variation of the spike trains in response to repeated presentations of an identical stimulus. Most remarkably, even at the highest degradation levels the contribution of external signal degradation to the total spike train distance (open arrow in Fig. 11.3b) was not larger than the intrinsic distance. These examples clearly demonstrate that the intrinsic neuronal noise cannot be neglected in relation to the signal degradation imposed by external noise from the environment. Using this experimental approach, our original intention was to establish a kind of ‘noise titration’ by which we could determine that amount of external acoustic noise that would be equivalent to the contribution of intrinsic neuronal noise. However, in many neurons this ‘titration’ was not possible since the intrinsic noise was too dominant (Neuhofer et al. 2011). This was the case in most ascending neurons, for which the contribution of intrinsic noise to spike train distance was particularly strong compared to the additional distance introduced by external signal degradation (see Fig. 11.2 in Neuhofer et al. 2011).
a Song of a C. biguttulus female (top trace) and detail of two subunits. Lower traces: envelope of two original syllables and envelopes at two degradation levels (from Neuhofer et al. 2011). b Metric distances between spike trains obtained with the van Rossum metric at a resolution of τ = 5 ms. For details of the procedure see Neuhofer et al. (2011). Filled arrow indicates the distance due to intrinsic noise, open arrow the additional distance caused by the external stimulus degradation. Data of an auditory afferent and two thoracic neurons (Neuhofer et al. unpublished)
We now can ask how this strong influence of intrinsic noise may affect the representation of acoustic signals in the auditory pathway, and in particular the resolution of fast amplitude modulations which are crucial for signal recognition as well as discriminating signals in the context of mate choice. The precision of spiking will influence the detection of subtle changes between signals—and thus the discrimination of similar songs—as well as the limits of temporal resolution, which will be discussed next.
11.2.3 Modulation Transfer Functions Only Partly Reveal the Temporal Resolution Capacities of Auditory Neurons
A widely applied method to measure the temporal resolution of time varying stimuli by sensory systems or by behaving animals is the modulation transfer function (MTF) paradigm (for reviews see Viemeister and Plack 1993; Joris et al. 2004). Using stimuli with sinusoidal amplitude modulations of different frequencies one can figure out if there are specific modulation frequencies to which the system responds particularly well, and what range of modulation frequencies the system is able to represent (Fig. 11.4a).
Assessing temporal resolution with modulation transfer functions (MTF). a Response of a local neuron (BSN1) to a broadband noise stimulus with sinusoidal amplitude modulations of 20 and 40 Hz. b, c Rate and temporal MTFs of an afferent neuron, a local and an ascending neuron; vertical lines in c indicate the respective corner frequencies (for details see Wohlgemuth and Ronacher 2007). d Corner frequencies of auditory afferents (N = 14), local neurons of the primary-like response type (N = 24), other local neurons (N = 18) and ascending neurons (N = 28). Boxes indicate medians and quartile ranges; the corner frequencies of ANs are different from all others (p < 0.001), whereas all other data do not differ significantly (Kruskal–Wallis). a–c from Ronacher et al. (2008), with permission; d data combined from Weschke and Ronacher (2008) and Wohlgemuth et al. (2011)
Applying the MTF paradigm to neuronal data one can focus on two variables: rate-MTF (rMTF) evaluate spike rates measured over a longer time period, while temporal-MTF (tMTF) describe how well spikes are phase locked to the stimulus envelope (Fig. 11.4b, c). rMTF of auditory neurons cover a wide range between all-pass, band-pass and band-stop characteristics (Weschke and Ronacher 2008; Wohlgemuth et al. 2011). Focusing on the question how well auditory neurons may resolve fast amplitude modulations, the tMTF paradigm reveals how well spikes are locked to the stimulus envelope in period histograms. The vector strength of the response is calculated and the upper limit of temporal resolution is described by the corner frequency, i.e. the upper limit up to which the spike pattern still follows the amplitude modulations (details in Prinz and Ronacher 2002; Weschke and Ronacher 2008; Wohlgemuth et al. 2011). Examples for the vector strength-based tMTF of an afferent, a local, and an ascending interneuron are shown in Fig. 11.4c. The two auditory interneurons show both a band-pass characteristic in their tMTF, but differ strongly in their corner frequencies, indicated by the vertical line. When the corner frequencies of a large sample of neurons belonging to different processing stages are compiled (Fig. 11.4d) an interesting picture is revealed: afferents and local neurons with primary-like responses have a high temporal resolution capacity, median corner frequencies are around 150 Hz. Other local neurons exhibit a shift to a somewhat reduced temporal resolution (median 131 Hz) but operate still up to the same frequency range. However, the ascending neurons differ markedly by occupying a much lower range of modulation frequencies (median 48 Hz). This graph thus reveals a major result: the upper limits of temporal resolution are drastically reduced at the level of ascending neurons, as their median corner frequency of ~50 Hz corresponds to a time scale of 20 ms. This reduction of temporal resolution at the level of ascending neurons—which are the bottleneck for the information transfer to the brain—seems at odds with the capacity of behaving animals to detect gaps of 2–3 ms duration (see Fig. 11.1b).
How can we reconcile this specific behavioural response of the grasshopper females with the low corner frequencies of ascending neurons? The solution is found in the characteristic response type of an ascending neuron AN4 (Ronacher and Stumpner 1988). This neuron responds to a stimulus onset first with a pronounced IPSP preceding the spike response (arrows in Fig. 11.5a). However, if a continuous sound stimulus is interrupted by small gaps, this IPSP is triggered anew by each steep intensity rise, which leads to a quite efficient suppression of the neuron’s spike response (bottom trace in Fig. 11.5a). When tested with different gap durations the spike activity of the AN4 neuron closely parallels the behaviour of females, with a substantial reduction of spike count at 2–3 ms gap duration (Fig. 11.1c)—in spite of its low corner frequency (median: 58 Hz). The answer to this apparent contradiction is found in Fig. 11.5b. This neuron may signal the presence of silent gaps larger than 2–3 ms by the reduction of its spike activity—likely in combination with at least one other neuron that signals whether sound is at all present. However, in suppressing its spike response to gappy stimuli AN4 activity disregards any specific information about the details of a stimulus’ temporal structure: it represents a large range of combinations of pulse and gap durations always in the same way, i.e. by a spike rate close to zero (Fig. 11.5b). This highlights a specific filter property of this neuron, which is due to the characteristic inhibitory input to this neuron. Unfortunately, so far the source of this inhibitory input has not yet been identified.
Responses of the AN4 neuron to noise stimuli with silent gaps. a Morphology of the neuron and responses to a song model with uninterrupted syllables (upper traces) or syllables with 5 ms gaps (lower); arrows point at the inhibitory postsynaptic potentials (IPSPs) elicited by sound onsets. b Responses of an AN4 to different combinations of sound pulses and pause durations. a from Ronacher and Stumpner (1988), b A. Vogel unpublished results
The characterisation of neurons by means of the MTF paradigm also intended to explore if in these insects a kind of filter bank exists for the evaluation of amplitude modulations, similar as it has been proposed for the auditory system of mammals (Langner and Schreiner 1988; Joris et al. 2004). This relates to the question of whether the amplitude modulations of acoustic signals are processed in the time domain or possibly in the frequency domain (von Helversen and von Helversen 1998). However, in a large sample of rMTF obtained from identified neuron types we found no indication for a filter bank (Weschke and Ronacher 2008; Wohlgemuth et al. 2011). Furthermore, also behavioural experiments indicate that acoustic communication signals in grasshoppers are processed in the time domain and not in the frequency domain of amplitude modulations (von Helversen and von Helversen 1998; Schmidt et al. 2008, for a similar result in crickets see also Hennig 2009).
11.3 Discrimination of Signals in the Context of Sexual Selection
The spike train metric method and the MTF paradigm differ in one important aspect: the latter neglects the trial-to-trial variability by assessing spike rates over a longer time period (rMTF) or by using period histograms that sample spike times over many periods as basis for the tMTF evaluation. Therefore, if we want to estimate how well two similar signals can be discriminated on the basis of sensory spike trains, the knowledge of the MTF does not always help and the spike train metric is the better option.
11.3.1 Afferent Responses Allow a Good Discrimination of Similar Signals
Auditory afferents exhibit tonic responses and faithfully represent the sound envelope in their spiking pattern, up to high modulation frequencies (Fig. 11.4). Their response is rather precise and reliable, although some trial-to-trial variability is observed, depending on sound intensity and envelope characteristics (Krahe and Ronacher 1993; Machens et al. 2001; Rokem et al. 2006). Among the population of local neurons we can discern those with primary-like responses that resemble the afferents in their firing pattern and precision. Others, e.g. the BSN1 neuron show phasic-tonic to phasic firing patterns and intermediate levels of reliability and precision (Stumpner 1989; Wohlgemuth and Ronacher 2007). In contrast, the responses of ascending neurons are definitely less precise and less reliable, which seems at odds with their function to provide the brain with essential information about auditory events (Fig. 11.2 and Vogel et al. 2005; Wohlgemuth and Ronacher 2007).
An efficient signal representation should enable the discrimination of relevant signals. Returning to the discrimination of similar communication signals in the context of sexual selection, we address the question posed above: if two spike trains differ to some extent, do they still represent the same object? Whether two similar acoustic signals can be discriminated or not depends on whether the respective sensory spike trains are sufficiently different to be distinguished by down-stream neurons.
Using the songs of 8 C. biguttulus males, we investigated how well natural calling songs of this species can be discriminated on the basis of the spike trains of auditory afferents. To remove spectral and intensity cues, the envelopes of these songs were filled with the carrier frequency spectrum of one male and presented at the same maximum intensity. In addition the length of the subunits (syllable plus pause) was equalised to remove the interindividual differences of the subunit periods as potential cues for discrimination (Machens et al. 2003). In behavioural tests C. biguttulus females still discriminated between these modified songs (Einhäupl et al. 2011). The same songs were used in electrophysiological recordings from auditory receptors (Machens et al. 2003) and interneurons (Wohlgemuth 2008) and it was tested how well the respective spike trains could be assigned to the different songs. For each of the 8 songs one spike train was chosen as a template, and all other spike trains were assigned to that template to which they had the smallest distance according to the spike train metric. The procedure was then repeated for different template spike trains, yielding an average classification success for the song stimuli. The classification success thus indicates the proportion of the spike trains that were correctly assigned to the corresponding acoustic stimulus (for details of the procedure see Wohlgemuth and Ronacher 2007).
The result obtained with spike trains of auditory afferent neurons was remarkable: in spite of the trial-to-trial variability already single spike trains of a single afferent neuron allowed for a very good discrimination of the eight songs (classification success in the range of 90 % correct, Machens et al. 2003). This was confirmed in a study using stimuli comprising sinusoidal amplitude modulations (Wohlgemuth and Ronacher 2007, cf. Fig. 11.4a). Here the maximum classification success was somewhat lower, around 80 %, due to the fact that a third of the stimuli were amplitude modulated at high frequencies, which were beyond the corner frequencies of most neurons. The high average classification success indicates that the remaining modulation frequencies (between 10 and 167 Hz) could be perfectly discriminated on the basis of auditory afferents (Fig. 11.6). Thus, at the input level of the system, even a single auditory afferent conveys surprisingly reliable information in its responses.
Discrimination of amplitude modulated stimuli at different stages of the auditory pathway (AFF auditory afferents, p-l LN local neurons with primary-like responses, other LN other local neurons, AN ascending neurons). The percentage of correct classifications (discrimination success) of nine sinusoidally amplitude-modulated stimuli was determined on the basis of spike train distances evaluated with the van Rossum (2001) metric at different temporal resolutions (for details see Wohlgemuth and Ronacher 2007). Black columns show the contribution of spike count differences to the classification success, and grey columns the contribution of spike timing differences. The stippled horizontal line indicates chance level. From receptors to ascending neurons the overall classification success, and in particular the impact of spike timing information, is reduced while at the same time spike count differences become more important; adapted from Wohlgemuth and Ronacher (2007)
Local neurons with primary-like responses performed with the same discrimination accuracy of ~80 % correct classifications (Fig. 11.6). However, the picture changed particularly at the third processing stage, the ascending neurons: here the classification success—again based on single neurons—dropped markedly to values around 50 % correct (Fig. 11.6). This reduction was obviously due to the increased spike train variability in these neurons: both interspike interval variability as well as spike count variability increase from afferents to local interneurons and ascending interneurons (see Fig. 11.2b and Vogel et al. 2005; Wohlgemuth and Ronacher 2007; Wohlgemuth 2008).
11.3.2 The Coding Principle Changes Between Local and Ascending Neurons
The spike train metric offers the advantage to explore a continuum of different neural coding schemes. By varying the width τ of the filter function by which the spikes are replaced for the spike metric evaluation (van Rossum 2001; Machens et al. 2003) one can adjust the temporal resolution of the spike train metric, to focus, for example, on the two special cases of a rate code, in which only spike numbers are relevant, or on a spike time code, in which the temporal position of spikes as well as differences in spike count are evaluated (van Rossum 2001; Ronacher et al. 2008; Ronacher and Stange 2012). This allowed us to disentangle the respective contributions of spike count differences and spike timing to the total stimulus discrimination success (black and grey parts of columns in Fig. 11.6). For receptor neurons and local neurons with primary-like responses the discrimination depended almost exclusively on the timing of spikes. In contrast, spike count differences became more important among ascending neurons, and their contribution to the—overall lower—classification success became approximately equal to the contribution of spike timing cues (Fig. 11.6, Wohlgemuth and Ronacher 2007; Wohlgemuth 2008).
The increased relevance of spike count and the decreased contribution of spike timing among ascending neurons suggest a change of coding principles at the output stage of the thoracic processing module. Ascending neurons exhibit a higher diversity of feature selectivity and seem to be specialised to encode different features of auditory stimuli with an emphasis on rate coding. They seem to trade the “when” for “what” in their spike response wherefore the reduced spike timing precision (Fig. 11.2) becomes tolerable (Clemens et al. 2012). Prominent examples are AN4 which may signal the presence or absence of gaps (Fig. 11.5) in its spike rate, or AN12 which is thought to encode the duration of the pauses that separate the sound syllables (Fig. 11.1; Creutzig et al. 2009, 2010). The specific filter properties of other ascending neurons are less obvious, but see below. Thus, the information about a sound stimulus’ envelope appears to be distributed among the set of ascending neurons. Indeed, applying a multi-neuron metric (Houghton and Sen 2008) yielded quantitative evidence for such a change of coding principles, i.e. that among ascending neurons a population code based on neurons with distinct filter properties is implemented (Clemens et al. 2011).
Unfortunately, our knowledge of the recognition centres in the brain is fragmentary. So far, no neurons with distinct species-specific filter characteristics have been described in C. biguttulus (in contrast to the cricket’s brain: Schildberger 1994, Zorović and Hedwig 2011). However, in this context a recent modelling study on grasshoppers’ song recognition has to be mentioned. Using a genetic learning algorithm (Mitchell 1998), Jan Clemens trained a set of classifiers on behavioural data obtained with a large set of artificial song stimuli. Starting from a set of 500 random solutions the feature detectors “evolved” to reproduce the behavioural data (for details see Clemens and Ronacher 2013). A characteristic of this classifier model was that the output of each feature detector was integrated over time, to yield a single value. Thus, any information about the exact temporal position of a specific song feature was omitted. The temporal integration was motivated by behavioural data with artificial songs in which syllable and pause durations were scrambled without destroying their attractiveness (von Helversen and von Helversen 1998), and fits also to the reduced spike timing precision observed in ascending neurons (Vogel et al. 2005; Wohlgemuth and Ronacher 2007; Wohlgemuth et al. 2011).
This modelling study yielded two interesting results: (i) Already two feature detectors—one excitatory, the other suppressive—sufficed to reproduce the behavioural data very well (for example the band pass tuning for pause duration, Fig. 11.1b, and additional parameters as the influence of intensity and onset accentuation), explaining 87 % of their variance (Clemens and Ronacher 2013). Inclusion of a third detector only led to a marginally further improved performance. Thus, the model gives a hint that the decision centres in the brain may rely on some cross-fibre patterns of the ascending neurons, with a focus on a spike rate code. In that scenario, the ascending neurons serve as filters that extract certain features from the sound envelopes, which then are evaluated in the brain for species-specific feature combinations. (ii) While the output of one detector showed a significant correlation with the behavioural data, the second detector, taken per se, did not correlate at all with the behavioural data. Only by a linear combination of both detectors the high performance of ~90 % was achieved (Clemens and Ronacher 2012). This result has important consequences when we search for neuronal correlates of behaviour: a neuron’s tuning may be highly relevant for behavioural decisions, but nonetheless show no obvious correlation to the usual test stimuli. By considering each neuron in isolation we may overlook neurons with inconspicuous or variable responses that nevertheless contribute significantly to signal recognition.
11.4 The Auditory Pathway Is Highly Conserved Between Grasshopper Species: Consequences for Signal Evolution
For successful communication it is essential that sender and receiver are matched to each other to at least some degree (von Helversen and von Helversen 1994). As mating success and reproduction are at the stake for grasshoppers, we could expect that their auditory pathway is specialised to process the species-specific signal patterns particularly well. The efficient coding hypothesis (Barlow 1961) predicts that coding properties of sensory neurons are optimised with respect to the relevant natural stimuli they process (Simoncelli and Olshausen 2001; Machens et al. 2005). We compared the coding properties of identified, homologous auditory neurons in two grasshoppers, C. biguttulus and the locust Locusta migratoria; two species which differ strongly in the relevance of acoustic signals for mate finding. For a most stringent, quantitative comparison we applied the van Rossum spike train metric to recordings from identified neurons. Both species were stimulated with the song of C. biguttulus, which is evidently not relevant for the locust. We recorded from several specimens of both species and compared the spike train similarities between homologous neurons in different specimens of the same species as well as between both species. Remarkably, the spike trains of one neuron type were not more different if we determined distances between the two species as compared to the intraspecific distances (Fig. 11.7; Neuhofer et al. 2008). This agreement suggests that the thoracic neurons and network properties were strongly conserved during evolution, although C. biguttulus and L. migratoria are not closely related: locusts and gomphocerine grasshoppers have been separated since about 50 Mio years (Flook and Rowell 1997). This has an interesting evolutionary implication: probably the communication signals have evolved to optimally match the properties of the sensory pathway of the receiver. The same conclusion was also drawn in relation to intensity invariance properties of grasshopper auditory neurons (Clemens et al. 2010). In contrast, according to the ‘efficient coding hypothesis’ (Barlow 1961; Machens et al. 2005) one would rather postulate the reverse sequence, i.e. that the processing capacities of the auditory pathway would have adapted to these highly relevant natural signals.
Comparison of coding properties of homologous neurons of two grasshopper species (Locusta migratoria, Chorthippus biguttulus). Top: spike raster plots of responses of 5 AN12 neurons recorded in both species to repeated presentations of a natural song of C. biguttulus. Each point indicates the occurrence of an action potential. Distances between two spike trains were determined at high temporal resolution (τ = 5 ms) for all possible combinations of spike trains with the van Rossum metric. Bottom: Distribution of distances between spike train combinations comparing different specimens of AN12 neurons of the locust (L.m., green), or between AN12 neurons of C. biguttulus (C. big. orange), or between C. biguttulus and L. migratoria (inter, black). Note that the interspecific comparison yielded no larger values than the intraspecific comparisons. This result was also observed with six additional neuron types. Figure from Neuhofer et al. (2008), with permission
The postulate of the ‘efficient coding hypothesis’—that sensory neurons should adjust their specific coding properties to the statistics of relevant natural stimuli—fits to the typical situation in evolution driven by natural selection, as a sensory system can not influence the properties of environmental signals. In communication systems, however, the situation may be different: the necessity for a match between signals and receiver properties entails a reciprocal coevolutionary adaptation of both parts driven by sexual selection. Thus, it depends on the respective plasticity of sender and receiver properties and on the selective pressures acting on both, whether the signals or the receiver properties may evolve more easily (Clemens et al. 2010). The neuronal hardware of the thoracic auditory pathway is obviously a highly conserved trait in grasshoppers (Neuhofer et al. 2008). In contrast, the specific communication signals of many acridid grasshopper species seem to have evolved rather recently, for example many species of the European C. biguttulus group underwent a rapid radiation only after the last glacial epoch (Bugrov et al. 2006; Mayer et al. 2010; Berger et al. 2010). This recent radiation may have been based on an evolution of the acoustic communication signals to match the properties of the auditory pathway of the receiver in a sensory exploitation scenario (e.g. Ryan et al. 2001; Arnqvist 2006).
The manifold of at least 20 identified types of ascending neurons in grasshoppers (Fig. 11.2a) is remarkable if we compare it with the auditory system of crickets and bush crickets, for which in general only two to three neurons have been found that ascend to the brain (Wohlers and Huber 1982; Römer et al. 1988; Stumpner and von Helversen 2001; Stumpner and Molina 2006; Triblehorn and Schul 2009). This difference between the auditory systems of Caelifera and Ensifera suggests an expansion of the parameter space which is potentially available for communication signals in grasshoppers. The capacity of a more sophisticated analysis of sound patterns may have enabled the evolution of highly complex songs in acridid grasshoppers, while crickets and bush crickets mostly produce rather simple song patterns (von Helversen 1986; Stumpner and von Helversen 1992; Gerhardt and Huber 2002; Vedenina et al. 2007; for bushcricket songs see, e.g. Heller 1988; Schul 1998; Bush et al. 2009).
References
Arnqvist G (2006) Sensory exploitation and sexual conflict. Phil Trans R Soc Lond B 361:375–386
Barlow HB (1961) Possible principles underlying the transformations of sensory messages. In: Rosenblith WA (ed) Sensory communication. MIT Press, Cambridge, pp 217–234
Bauer M, von Helversen O (1987) Separate localisation of sound recognizing and sound producing neural mechanisms in a grasshopper. J Comp Physiol A 165:687–695
Berger D, Chobanov DP, Mayer F (2010) Interglacial refugia and range shifts of the alpine grasshopper Stenobothrus cotticus (Orthoptera: Acrididae: Gomphocerinae). Org Divers Evol 10:123–133
Boyan GS (1999) Presynaptic contributions to response shape in an auditory neuron of the grasshopper. J Comp Physiol A 184:279–294
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Study Behav 35:151–209
Bugrov A, Novikova O, Mayorov V, Adkinson L, Blinov A (2006) Molecular phylogeny of Palaearctic genera of Gomphocerinae grasshoppers (Orthoptera, Acrididae). Syst Entomol 31:362–368
Bush SL, Beckers OM, Schul J (2009) A complex mechanism of call recognition in the katydid Neoconocephalus affinis (Orthoptera: Tettigoniidae). J Exp Biol 212:648–655
Clemens J, Ronacher B (2013) Feature extraction and integration underlying perceptual decision making during courtship in grasshoppers. J Neurosci 33:12136–12145
Clemens J, Weschke G, Vogel A, Ronacher B (2010) Intensity invariance properties of auditory neurons compared to the statistics of relevant natural signals in grasshoppers. J Comp Physiol A 196:285–297
Clemens J, Kutzki O, Ronacher B, Schreiber S, Wohlgemuth S (2011) Efficient transformation of an auditory population code in a small sensory system. Proc Natl Acad Sci USA 108:13812–13817
Clemens J, Wohlgemuth S, Ronacher B (2012) Nonlinear computations underlying temporal and population sparseness in the auditory system of the grasshopper. J Neurosci 32:10053–10062
Creutzig F, Wohlgemuth S, Stumpner A, Benda J, Ronacher B, Herz AVM (2009) Time-scale invariant representation of acoustic communication signals by a bursting neuron. J Neurosci 29:2575–2580
Creutzig F, Benda J, Wohlgemuth S, Stumpner A, Ronacher B, Herz AVM (2010) Timescale-invariant pattern recognition by feed-forward inhibition and parallel signal processing. Neural Comput 22:1493–1510
Einhäupl A, Stange N, Hennig RM, Ronacher B (2011) Attractiveness of grasshopper songs correlates with their robustness against noise. Behav Ecol 22:791–799
Elsner N (1974) Neuroethology of sound production in Gomphocerine grasshoppers (Orthoptera: Acrididae) 1. Song patterns and stridulatory movements. J Comp Physiol 88:67–102
Elsner N, Popov AV (1978) Neuroethology of acoustic communication. Adv Insect Physol 13:229–355
Flook PK, Rowell CHF (1997) The phylogeny of the Caelifera (Insecta, Orthoptera) as deduced from mtrRNA gene sequences. Mol Genet Evol 8:89–103
Franz A, Ronacher B (2002) Temperature dependence of temporal resolution in an insect nervous system. J Comp Physiol A 188:261–271
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. University of Chicago Press, Chicago
Gottsberger B, Mayer F (2007) Behavioral sterility of hybrid males in acoustically communicating grasshoppers (Acrididae, Gomphocerinae). J Comp Physiol A 193:703–714
Halex H, Kaiser W, Kalmring K (1988) Projection areas and branching patterns of the tympanal receptor cells in migratory locusts Locusta migratoria and Schistocerca gregaria. Cell Tissue Res 253:517–528
Hedwig B (1992) On the control of stridulation in the acridid grasshopper Omocestus viridulus L. I Interneurons involved in rhythm generation and bilateral coordination. J Comp Physiol A 171:117–128
Hedwig B (1994) A cephalothoracic command system controls stridulation in the acridid grasshopper Omocestus viridulus L. J Neurophysiol 72:2015–2025
Heller K-G (1988) Bioakustik der europäischen Laubheuschrecken. Josef Margraf Verlag, Weikersheim
Hennig RM (2009) Walking in Fourier’s space: algorithms for the computation of periodicities in song patterns by the cricket Gryllus bimaculatus. J Comp Physiol A 195:971–987
Hennig RM, Franz A, Stumpner A (2004) Processing of auditory information in insects. Microsc Res Tech 63:351–374
Houghton CJ, Sen K (2008) A new multineuron spike train metric. Neural Comput 20:1495–1511
Jacobs K, Otte B, Lakes-Harlan R (1999) Tympanal receptor cells of Schistocerca gregaria: correlation of soma positions and dendrite attachment sites, central projections, and physiologies. J Exp Zool 283:270–285
Joris PX, Schreiner CE, Rees A (2004) Neural processing of amplitude-modulated sounds. Physiol Rev 84:541–577
Klappert K, Reinhold K (2003) Acoustic preference functions and sexual selection on the male calling song in the grasshopper Chorthippus biguttulus. Anim Behav 65:225–233
Klappert K, Reinhold K (2005) Local adaptation and sexual selection: a reciprocal transfer experiment with the grasshopper Chorthippus biguttulus. Behav Ecol Sociobiol 58:36–43
Kostarakos K, Römer H (2010) Sound transmission and directional hearing in field crickets: neurophysiological studies outdoors. J Comp Physiol A 196:669–681
Krahe R, Ronacher B (1993) Long rise times of sound pulses in grasshopper songs improve the directionality cues received by the CNS from the auditory receptors. J Comp Physiol A 173:425–434
Kriegbaum H (1989) Female choice in the grasshopper Chorthippus biguttulus: mating success is related to song characteristics of the male. Naturwissenschaften 76:81–82
Kriegbaum H, von Helversen O (1992) Influence of male songs on female mating behavior in the grasshopper Chorthippus biguttulus (Orthoptera, Acrididae). Ethology 91:248–254
Lang F (2000) Acoustic communication distances of a gomphocerine grasshopper. Bioacoustics 10:233–258
Langner G, Schreiner CE (1988) Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. J Neurophysiol 60:1799–1821
Machens CK, Stemmler MB, Prinz P, Krahe R, Ronacher B, Herz AVM (2001) Representation of acoustic communication signals by insect auditory receptor neurons. J Neurosci 21:3215–3227
Machens CK, Schütze H, Franz A, Stemmler MB, Ronacher B, Herz AVM (2003) Auditory receptor neurons preserve characteristic differences between conspecific communication signals. Nature Neurosci 6:341–342
Machens CK, Gollisch T, Kolesnikova O, Herz AVM (2005) Testing the efficiency of sensory coding with optimal stimulus ensembles. Neuron 47:447–456
Marquart V (1985) Local interneurons mediating excitation and inhibition onto ascending neurons in the auditory pathway of grasshoppers. Naturwissensch 72:42–43
Mayer F, Berger D, Gottsberger B, Schulze W (2010) Non-Ecological radiations in acoustically communicating grasshoppers? In: Glaubrecht M (ed) Evolution in action. Springer, Berlin Heidelberg, pp 451–464
Meyer J, Elsner N (1996) How well are frequency sensitivities of grasshopper ears tuned to species-specific song spectra? J Exp Biol 199:1631–1642
Michelsen A (1971) The physiology of the locust ear. I. Frequency selectivity of single cells in the isolated ear. Z vergl Physiol 71:49–62
Michelsen A, Larsen ON (1983) Strategies for acoustic communication in complex environments. In: Huber F, Markl H (eds) Neuroethology and behavioural physiology. Springer, Berlin, pp 321–331
Mitchell M (1998) An introduction to genetic algorithms. MIT press, Boston
Neuhofer D, Wohlgemuth S, Stumpner A, Ronacher B (2008) Evolutionarily conserved coding properties of auditory neurons across grasshopper species. Proc R Soc Lond B 208:1965–1974
Neuhofer D, Stemmler M, Ronacher B (2011) Neuronal precision and the limits for acoustic signal recognition in a small neuronal network. J Comp Physiol A 197:251–265
Prinz P, Ronacher B (2002) Temporal modulation transfer functions in auditory receptor fibres of the locust (Locusta migratoria L.). J Comp Physiol A 188:577–587
Rokem A, Watzl S, Gollisch T, Stemmler MB, Herz AVM, Samengo I (2006) Spike-timing precision underlies the coding efficiency of auditory receptor neurons. J Neurophysiol 95:2541–2552
Römer H (1976) Die Informationsverarbeitung tympanaler Rezeptorelemente von Locusta migratoria. J Comp Physiol A 109:101–122
Römer H (2001) Ecological constraints for sound communication: from grasshoppers to elephants. In: Barth FG, Schmid A (eds) Ecology of sensing. Springer, Berlin, pp 59–77
Römer H, Marquart V (1984) Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J Comp Physiol A 155:249–262
Römer H, Marquart V, Hardt M (1988) Organization of a sensory neuropile in the auditory pathway of two groups of Orthoptera. J Comp Neurol 275:201–215
Ronacher B, Stange N (2012) Processing of acoustic signals in grasshoppers – a neuroethological approach towards female choice. J Physiol Paris 107:41–50
Ronacher B, Stumpner A (1988) Filtering of behaviourally relevant temporal parameters of a grasshopper′s song by an auditory interneuron. J Comp Physiol A 163:517–523
Ronacher B, von Helversen D, von Helversen O (1986) Routes and stations in the processing of auditory directional information in the CNS of a grasshopper, as revealed by surgical experiments. J Comp Physiol A 158:363–374
Ronacher B, Franz A, Wohlgemuth S, Hennig RM (2004) Variability of spike trains and the processing of temporal patterns of acoustic signals – problems, constraints, and solutions. J Comp Physiol A 190:257–277
Ronacher B, Wohlgemuth S, Vogel A, Krahe R (2008) Discrimination of acoustic communication signals by grasshoppers: temporal resolution, temporal integration, and the impact of intrinsic noise. J Comp Psychol 22:252–263
Ryan MJ, Phelps SM, Rand AS (2001) How evolutionary history shapes recognition mechanisms. Trends Cogn Sci 5:143–148
Schildberger K (1994) The auditory pathway of crickets: adaptations for intraspecific acoustic communication. In: Schildberger K, Elsner N (eds) Neural basis of behavioural adaptations. G. Fischer, Stuttgart, pp 209–225
Schmidt AKD, Römer H (2011) Solutions to the cocktail party problem in insects: selective filters, spatial release from masking and gain control in tropical crickets. PLoS ONE 6:e 28593
Schmidt A, Ronacher B, Hennig RM (2008) The role of frequency, phase and time for processing amplitude modulated signals by grasshoppers. J Comp Physiol A 194:221–233
Schul J (1998) Song recognition by temporal cues in a group of closely related bushcricket species (genus Tettigonia). J Comp Physiol A 183:401–410
Simoncelli EP, Olshausen BA (2001) Natural image statistics and neural representation. Annu Rev Neurosci 24:1193–1216
Stange N, Ronacher B (2012) Grasshopper calling songs convey information about condition and health of males. J Comp Physiol A 198:309–318
Stumpner A (1988) Auditorische thorakale Interneurone von Chorthippus biguttulus L.: morphologische und physiologische Charakterisierung und Darstellung ihrer Filtereigenschaften für verhaltensrelevante Lautattrappen. Ph.D. thesis, Friedrich-Alexander Universität Erlangen-Nürnberg
Stumpner A (1989) Physiological variability of auditory neurons in a grasshopper. Naturwissenschaften 76:427–429
Stumpner A, Molina J (2006) Diversity of intersegmental auditory neurons in a bush cricket. J Comp Physiol A 192:1359–1376
Stumpner A, Ronacher B (1991) Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus biguttulus. 1. Morphological and physiological characterization. J Exp Biol 158:391–410
Stumpner A, von Helversen O (1992) Recognition of a two-element song in the grasshopper Chorthippus dorsatus (Orthoptera: Gomphocerinae). J Comp Physiol A 171:405–412
Stumpner A, von Helversen O (1994) Song production and song recognition in a group of sibling grasshopper species (Chorthippus dorsatus, C. dichrous, and C. loratus: Orthoptera, Acrididae). Bioacoustics 6:1–23
Stumpner A, von Helversen D (2001) Evolution and function of auditory systems in insects. Naturwissenschaften 88:159–170
Stumpner A, Ronacher B, von Helversen O (1991) Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus biguttulus. 2. Processing of temporal patterns of the song of the male. J Exp Biol 158:411–430
Triblehorn JD, Schul J (2009) Sensory-encoding differences contribute to species-specific call recognition mechanisms. J Neurophysiol 102:1348–1357
van Rossum MCW (2001) A novel spike distance. Neural Comput 13:751–763
Vedenina VY, Panyutin AK, von Helversen O (2007) The unusual inheritance pattern of the courtship songs in closely related grasshopper species of the Chorthippus albomarginatus-group (Orthoptera: Gomphocerinae). J Evol Biol 20:260–277
Viemeister NF, Plack CJ (1993) Time analysis. In: Yost WA, Popper AN, Fay RR (eds) Human psychophysics. Springer, Berlin, pp 116–154
Vogel A, Ronacher B (2007) Neural correlations increase between consecutive processing levels in the auditory system of locusts. J Neurophysiol 97:3376–3385
Vogel A, Hennig RM, Ronacher B (2005) Increase of neuronal response variability at higher processing levels as revealed by simultaneous recordings. J Neurophysiol 93:3548–3559
von Helversen D (1972) Gesang des Männchens und Lautschema des Weibchens bei der Feldheuschrecke Chorthippus biguttulus (Orthoptera, Acrididae). J Comp Physiol A 81:381–422
von Helversen O (1979) Angeborenes Erkennen akustischer Schlüsselreize. Verh Deutsch Zool Ges 1979:42–59
von Helversen O (1986) Gesang und Balz bei Feldheuschrecken der Chorthippus albomarginatus-Gruppe. Zool Jahrb Systematik 113:319–342
von Helversen D, von Helversen O (1975a) Verhaltensgenetische Untersuchungen am akustischen Kommunikationssystem der Feldheuschrecken (Orthoptera, Acrididae). I Der Gesang von Artbastarden zwischen Chorthippus biguttulus und C. mollis. J Comp Physiol 104:273–299
von Helversen D, von Helversen O (1975b) Verhaltensgenetische Untersuchungen am akustischen Kommunikationssystem der Feldheuschrecken (Orthoptera, Acrididae). II Das Lautschema von Artbastarden zwischen Chorthippus biguttulus und C. mollis. J Comp Physiol 104:301–323
von Helversen O, von Helversen D (1994) Forces driving coevolution of song and song recognition in grasshoppers. In: Schildberger K, Elsner N (eds) Neural basis of behavioural adaptations. G. Fischer Verlag Stuttgart, pp 253–284
von Helversen D, von Helversen O (1997) Recognition of sex in the acoustic communication of the grasshopper Chorthippus biguttulus (Orthoptera, Acrididae). J Comp Physiol A 180:373–386
von Helversen D, von Helversen O (1998) Acoustic pattern recognition in a grasshopper: processing in the frequency or time domain? Biol Cybern 79:467–476
Weschke G, Ronacher B (2008) Influence of sound pressure level on the processing of amplitude modulations by auditory neurons of the locust. J Comp Physiol A 194:255–265
White JA, Rubinstein JT, Kay AR (2000) Channel noise in neurons. Trends Neurosci 23:131–137
Wohlers D, Huber F (1982) Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket, Gryllus campestris L. J Comp Physiol A 146:161–173
Wohlgemuth S (2008) Repräsentation und Unterscheidbarkeit amplitudenmodulierter akustischer Signale im Nervensystem von Feldheuschrecken. PhD thesis Humboldt- Universität zu Berlin
Wohlgemuth S, Ronacher B (2007) Auditory discrimination of amplitude modulations based on metric distances of spike trains. J Neurophysiol 97:3082–3092
Wohlgemuth S, Vogel A, Ronacher B (2011) Encoding of amplitude modulations by auditory neurons of the locust: influence of modulation frequency, rise time, and modulation depth. J Comp Physiol A 197:61–74
Zador A (1998) Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol 79:1219–1229
Zorović M, Hedwig B (2011) Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J Neurophysiol 105:2181–2194
Acknowledgements
I thank all members of my lab who contributed to the work reported here, in particular Matthias Hennig for years of fruitful collaboration and many stimulating discussions. I also thank Daniela Neuhofer, Jana Sträter, Astrid Vogel and Sandra Wohlgemuth for providing partially unpublished data. Special thanks to Stefanie Krämer for making the figures. Berthold Hedwig and Carl Gerhardt gave valuable suggestions that helped to improve the manuscript. The American Psychological Association, the Royal Society, and Springer Science and Business Media very kindly allowed the reproduction of figures. Financial support from the German Research Council (SFB 618, GRK 837, GRK 1589/1) and the Bernstein Centre for Computational Neuroscience (Federal Ministry of Education and Research, Germany, grant 01GQ1001A) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ronacher, B. (2014). Processing of Species-Specific Signals in the Auditory Pathway of Grasshoppers. In: Hedwig, B. (eds) Insect Hearing and Acoustic Communication. Animal Signals and Communication, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40462-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-40462-7_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40461-0
Online ISBN: 978-3-642-40462-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)