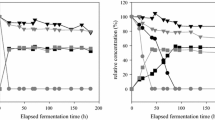
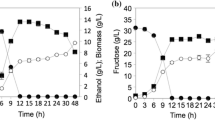
Abstract
Ethanol fermentation using the hydrolysate obtained after the saccharification of biomass is the last step in lignocellulosic bioethanol production process. The hydrolysate contains large amount of fermentable sugars that can be directly used by the ethanologenic microorganisms. Yeast is the most commonly and widely used microorganism for commercial ethanol production due to its some special characteristics such as fast growth rates, efficient glucose repression, efficient ethanol production, and a tolerance for environmental stresses, like high ethanol concentration and low oxygen levels. In addition to yeast, there are several other fungi and bacteria that can produce ethanol under various fermentation conditions. This chapter describes the most common wild-type microorganisms used for the fermentative production of ethanol.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
With the ever increasing demand for energy and the fast depleting petroleum resources, there is an increased interest in alternative fuels, especially liquid transportation fuels. The use of lignocellulosic biomass for the production of biofuels, especially bioethanol, will be unavoidable if the fossil fuels are to be replaced by renewable and sustainable alternatives. Ethanol accounts for majority of biofuels worldwide and its production from lignocellulosic biomass through biological route seems very attractive and sustainable due to several reasons, among which the renewable and ubiquitous nature of biomass and its non-competitiveness with food crops, and the higher reduction in greenhouse gas emission.
There are a limited number of microorganisms which ferment carbohydrates, mainly pentose sugars or hexose sugars, into alcohols. The major bacterial strains producing ethanol include Clostridium acetobutylicum, Klebsiella pneumoniae, Leuconostoc mesenteroides, Sarcina ventriculi, Zymomonas mobilis. Several fungal species are also reported to be the producer of ethanol. These include Aspergillus oryzae, Endomyces lactis, Kloeckera sp., Kluyreromyees fragilis, Mucor sp., Neurospora crassa, Rhizopus sp., Saccharomyces beticus, S. cerevisiae, S. elltpsoideus, S. oviformis, S. saki,Torula sp., Trichosporium cutaneum.
The major characteristics of an organism to be used in ethanol production are the ability to give a high yield of ethanol, to produce it with a high productivity and to withstand high ethanol concentration. In addition, the organism should possess the ability to utilize multiple sugars as well as that to tolerate inhibitors that are usually present in the hydrolysate obtained after pretreatment and enzymatic saccharification. It should also possess the ability to tolerate temperature and low pH, in order to minimize the risk of contamination. From an industrial point of view, high temperature tolerant strains are preferred so as to eliminate the other contaminating mesophilic microbes by increasing the fermentation temperature which in turn reduces the step of sterilization and thus the process become more cost-effective. Simultaneous hydrolysis and fermentation (SSF) is the main route to produce lignocellulosic ethanol. It consists on the use of a unique reactor in which enzymatic hydrolysis and fermentation of the obtained sugars by the ethanologenic microorganisms, mainly yeasts, are carried out. For a successful SSF process, temperature and pH values should be modulated with the aim to optimize the operative conditions of both enzymatic hydrolysis and fermentation, without negatively affecting both the yield of sugars release and ethanol production.
7.2 Fermentative Production of Ethanol
Fermentation is the term used to describe any process for the production of a product by means of the mass culture of a microorganism. In simple way, it is a chemical change brought on by the action of microorganisms. The two key components in the fermentation process are the microorganism and substrate. Control of the process, absence of contaminations, high fermentation rate and yield are the major factors which determine the total fermentation efficiency. The major steps in ethanol production process are shown in Fig. 7.1. The fermentation technique in lignocellulosic biomass to ethanol process is the same as that of conventional fermentation except the source of carbon is from the biomass.
Most of industrial ethanol fermentations are carried out by submerged fermentation (SmF), where a supply of oxygen is essential. SmF can be operated in batch culture, fed-batch culture, perfusion batch culture, and continuous culture.
As far as lignocellulosic bioethanol production is concerned, two main routes can be followed for ethanol production, namely separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) (Fig. 7.2). In SHF the bioconversion of lignocellulose takes place in two separate reactors, thus separating the saccharification and the fermentation processes. In this process each step can be conducted at optimal conditions of pH and temperature. The major steps involved in SHF are pretreatment, hydrolysis, and fermentation. Both pretreatment and hydrolysis are very crucial for obtaining fermentable sugars. The major aim of pretreatment is to separate cellulose and hemicelluloses from lignin. Pretreatment can be performed by physical, chemical, and biological means and each method has its own advantageous and disadvantageous. Chemical method is the most preferred way of pretreatment as it is very easy to perform. The major drawback in chemical method is the formation of inhibitors and generation of waste chemical effluents. The formation of inhibitors adversely affect during fermentation. Microbe can tolerate inhibitors up to a certain concentrations beyond that it dies. To avoid that it needs to detoxify the pretreated as well as hydrolyzed liquor before fermentation. The inhibitors that affect fermentation include acetic acid, formic acid, levulinic acid, furfural, hydroxymethyl furfural, phenol, and vanillin. The effect of furfural on cultivation of yeast has been studied well. Among known effects for batch cultivations are decreased ethanol production rate and specific growth rate. The mode in which furfural inhibit yeast metabolism is not completely known, but it has been suggested that it inhibit central enzymes in glycolysis. In addition, enzymes coupled to the citric acid cycle and ethanol formation (e.g., alcohol dehydrogenase and aldehyde dehydrogenase) have also been suggested (Taherzadeh 2000).
Studies on SSF process has shown a potential for high rates and high ethanol yields from lignocellulosic materials. SSF allows performing the enzymatic hydrolysis of polysaccharides components of lignocelluloses, together with the fermentation, using a unique reactor. This results in the decrease of inhibition effects of the end-product on the enzymatic hydrolysis and an immediate availability of fermentable sugars. On the other side, the main drawback is the need to find favorable conditions (e.g., temperature and pH) for both the enzymatic hydrolysis and the fermentation and the difficulty to recycle the fermenting organism and the enzymes. Techno-economical analyses have shown that SSF is a much more competitive process in comparison to SHF. In fact, the use of a unique bioreactor results in a strong reduction of investment and operational costs.
A number of yeast and several bacterial strains have been studied for ethanol production under SSF.
7.2.1 Up-Stream Operations in Ethanol Fermentation
All the operations before starting the fermentation are generally called up-stream operations such as sterilization of reactor, preparation and sterilization of culture media, preparation and growth of suitable inoculums of microbial strains. All the process of up-stream operations is important for a successful fermentation among them media preparation and fermentation parameters play important roles. In the case of lignocellulosic ethanol production, the pretreatment of biomass is a key step to assure availability of polysaccharides to be converted into fermentable sugars.
The reaction environment should contain source for energy, water, carbon sources, nitrogen sources, vitamins, minerals, buffers, chelating factors, air, and antifoaming agents. Pretreated lignocellulose not always guarantees all these supplies thus pushing to the need of adding other components which can assure ethanologenic microorganism’s growth and fermentation.
The culture medium should produce the desired product at a faster rate, and low yield of undesired products. Thus, the type and the amount of nutrient components of a medium are critical.
In the case of SSF, where enzymes are directly added for the hydrolysis of the polysaccharides, fermentable sugars, both pentoses and hexoses, are prompt available to be fermented, differently from SHF where an accumulation of sugars results in the inhibition of the enzymes involved in the hydrolysis and a decrease of the sugars production rate as a consequence. Carbon serves as a major energy source for the organisms.
The product formation depends on the rate at which the carbon source is metabolized and main product of fermentation depends on the type of carbon source used. Carbon enters the pathways of energy yielding respiratory mechanism. The carbon sources for fermentation can be simple or complex carbohydrates, organic acids, proteins, peptides, amino acids, oils, fats, and hydrocarbons. Many microorganisms can use a single organic compound to supply both carbon and energy needs.
Followed by carbon, nitrogen is the next most plentiful substance used in the fermentation media. Few microbes can utilize nitrogen as the energy source. It occurs in the organic compounds of the cell and also as reduced form in amino acids. The commonly used nitrogen sources in the fermentation media are yeast extract, ammonium salts, and urea. Other nitrogen sources include amino acids, proteins, sulfite waste liquor, corn steep liquor, and molasses. Nitrogen sources are added in the SSF reactor to assure the growth of the ethanologenic microorganisms.
For instance, yeast extract is generally added to SSF process, thus assuring a proper amount of nitrogen. Minerals supply the essential elements required for the cells during their cultivation. The essential minerals for all media include calcium, chlorine, magnesium, phosphorous, potassium, and sulfur. Other minerals like copper, cobalt, iron, manganese, molybdenum, and zinc are required in trace amounts. The trace elements may contribute to both primary and secondary metabolite production. The specific concentration of the different minerals depends upon the type of microorganism being used. The functions of trace elements include coenzyme functions to catalyze many reactions, vitamin synthesis, and cell wall support (Vogel and Todaro 1996). Primary metabolite function is not very sensitive to trace element composition while secondary metabolite production is sensitive to trace element concentration.
Oxygen is normally present at very low levels in commercial-scale ethanol fermentations. In practice, the process cannot be completely anaerobic because oxygen is required for production of unsaturated fatty acids that are essential for yeast growth and ethanol production. It is generally recommended to avoid yeast stress factors such as high temperatures, high osmotic pressure, high sodium and other ionic concentrations, and high concentration of organic acids. Prevention of bacterial contaminants is critical in successful ethanol fermentation.
Besides nutrition, the yeast dose rate also affects on the total performance and it must be optimized for cost-effective performance. For instance, a higher dose rate results in a faster start of fermentation, which helps the control of contamination.
Saccharomyces cerevisiae is the most favored organism for ethanol production from hexoses while Pichia stipitis and Candida shehatae are yeasts capable of fermenting both hexose and pentose sugars to ethanol (Parekh and Wayman 1986). Bacteria belonging to the species Clostridia and Zymomonas, and fungi such as Fusarium spp. have been investigated for ethanol production. The success of fermentation depends on the nature of the lignocellulosic biomass, thus the effect of the pretreatment on its structure. The parameters like temperature, pH, degree of agitation, oxygen concentration must be monitored throughout the fermentation process, so that any deviation from the optimum conditions can be corrected by a control system.
In the case of SSF, the effects of enzymes and biomass loading should be even studied to optimize the process, particularly the saccharification from which fermentable sugars arise.
It is worth noting that several yeast growth inhibitors produced during the pretreatment can negatively affect the fermentation process.
7.2.2 Down-Stream Operations
The major down-stream operations in ethanol fermentation involve distillation. After distillation yield 95 % ethanol known as rectified spirit. It is not possible to remove the remaining water from rectified spirit by straight distillation, as ethanol forms a constant boiling mixture with water at this concentration known as azeotrope. In order to extract water from ethanol, it is necessary to use some dehydrants which are capable of separating water from ethanol. A simple dehydrant is the unslaked lime which is added to rectified spirit and left overnight for complete reaction. The mix is then distilled in a fractionating column to get absolute alcohol. This process is mainly used in small-scale processes.
Dehydration by molecular sieve is another approach used in industry. In this technique the rectified spirit is superheated with steam in feed super-heater. It is then passed to one of the pair of molecular sieve beds for several minutes. On a time basis, the flow of the rectified spirit vapor is switched to the alternate bed of the pair. A portion of the anhydrous ethanol vapor leaving the fresh adsorption bed is used to regenerate the loaded bed.
The advantages of molecular sieve technology are the simplicity of the process and the fact that it is very easy to automate the process, reducing the labor. The process is inert and there is no use of chemicals. The desiccant material has very long life span. A properly designed molecular sieve can dehydrate 160− proof ethanol to more than 190+ proof ethanol and near theoretical recovery of ethanol is possible.
7.3 Ethanologenic Microbes
The ethanologenic microorganisms should satisfy a specific criterion for isolation which includes utilization of a cheap media for growth. It should convert the substrate into the product rapidly and the product should be easily recovered from the culture medium.
Efficiency or yield, throughput and consistency are the major objectives for selecting the organism for any fermentation processes. Several techniques were employed for isolating and screening of ethanologenic microorganisms from various sources. This includes the liquid culture method and solid culture method. The liquid culture is carried out in shake flasks containing liquid culture medium while the solid culture is carried out in solid culture medium containing a substrate. A number of high-throughput screening methods have been proposed by Qi et al. 2011. After isolating microbes from various sources, they can be cultured either in liquid or solid medium, such as nutrient agar, which contains the desired carbon containing feedstock as well as the other nutrients required for microbial growth, such as ammonia, salts, and trace metals. Ethanol produced by microbes is excreted into the extracellular culture medium. The secreted ethanol can then be detected and quantified by any suitable means such as GC or HPLC. It is highly desirable to employ a detection method that can at least partially quantify the ethanol produced by each individual microbe or the microbes in an individual microbial colony. As such, a preferred screening method is one which is applied in a solid phase screen, in which a very large number of individual microbial colonies can be easily separated. This offers a much higher throughput compared to a solely liquid phase screening in which samples must be manually separated and measured in liquid assay. A colorimetric assay method was developed by Fotheringham et al. (2009) where the assay solution contains alcohol oxidase, peroxide, and a peroxide co-substrate. The oxidase reaction upon alcohol produces hydrogen peroxide which reacts with a second enzyme such as Horseradish Peroxidase, in the presence of a peroxidase co-substrate which is responsible for generating the color.
Yeast is the most commonly used microorganism for ethanol production by fermentation. There are certain unique properties of yeasts that make them outstanding for ethanol production. Some of these properties are: fast growth rates, efficient glucose repression, efficient ethanol production, and a tolerance for environmental stresses, such as high ethanol concentration and low oxygen levels. Of the different types of yeast, S. cerevisiae is the industrially important yeast for alcohol fermentation, even if it is able to ferment only hexose sugars.
One of the best opportunities to further reduce the cost of cellulosic bioethanol is to enhance the sugar recovery from lignocellulose. This includes the exploitation of the hemicelluloses portion of biomass, mainly made of pentose sugars. Therefore, microorganisms which could ferment other sugars such as xylose, mannose, arabinose, or galactose are required for an economically viable conversion from lignocellulose to ethanol.
Besides S. cerevisiae, other examples of yeasts used for ethanol production are Schizosaccharomyces pombe, Kluyveromyces lactis, Candida spp., Pichia spp that are able to ferment even pentose sugars.
Yeast can grow aerobically as well as anaerobically. Aerobic conditions favors yeast cell production, which is not of interest to ethanol producers. However, growth during anaerobic condition is very marginal and major reaction is conversion of sugar to ethanol for energy production. For growth and multiplication, yeast requires utilizable organic carbon (sugars), nitrogen source, and various organic and inorganic trace growth factors. During the conversion of sugar to ethanol, energy is produced, which is utilized by cells for different functions. In addition to yeast, a large number of bacteria are capable of ethanol production, but most of them produce other end products like butanol, isopropyl alcohol, acetic acid, formic acid, arabitol, glycerol, acetone, methane, etc., as well as ethanol. Bacteria that produce ethanol as the major product (i.e., a minimum of 1 mol ethanol produced per mol of glucose utilized) are shown in Table 7.1.
The major wild microbes used in fermentation processes are described below.
7.3.1 Yeast
7.3.1.1 Saccharomyces sp
Worldwide, nearly all ethanol production is accomplished using a single genus and species of yeast, namely Saccharomyces cerevisiae. Specific strains producing ethanol from sugarcane juice and molasses and also from beet juice and molasses have been reported and marketed also for commercial production. Yeast has been so far shown able to produce ethanol from hexose sugars obtained from lignocelluloses saccharification. Recently, high performance yeast strains have been selected and commercialized for dry grind corn ethanol production utilizing batch fermentation processes. Some yeast strains ferment faster or are able to convert substrate to ethanol with increased yields. Several inducers and stress factors also affect the yeast growth and ethanol production. Genetically enhanced microorganisms for ethanol production are in various stages of development (described in Chap. 8).
An optimal process for fermentation uses a broth containing S. cerevisiae supplemented with 22 % (w/v) sugar, 1 % (w/v) of each of ammonium sulfate and potassium dihydrogen phosphate, and fermented at pH 5.0 and 30 °C (Junior et al. 2009). Under such conditions a typical strain of S. cerevisiae is capable of producing 46.1 g ethanol/l broth (Maziar 2010). Cane molasses conditioned with EDTA, ferrocyanide or zeolites, and fermented under similar conditions have been shown to enhance ethanol production (Ergun et al., 1997). Further, addition of minimal concentrations of hops acids to the fermentation broth has been shown to prevent bacterial growth and thus enhances ethanol yields (Maye 2006). Fermentation using immobilized yeast and broth supplemented with Mg, Zn, Cu or Capantothenate has also been shown to increase fermentation efficiency by almost 20 % (Nikolic et al. 2009). Ethanol production using steam pretreated barley straw with low enzyme loadings and low yeast concentration was evaluated by Linde et al. (2007). The highest ethanol yield and ethanol concentration of 82 % and 15.5 g/l, respectively, were obtained with 5 % solid loading, enzyme loading of 20 FPU/g and with 5 g/l of yeast. It was observed that with increase in solid loading and decrease of enzyme loading, there is a reduction in ethanol yield. Ethanol production using hydrothermal pretreated wheat straw by thermo-tolerant flocculating S. cerevisiae was recently evaluated by Ruiz et al. 2012. The study revealed that ethanol concentration was affected by enzyme loading and biomass loading. Maximum ethanol concentration of 14.84 g/l was obtained at 45 °C, with 3 % biomass loading and 30 FPU of enzyme loading.
Rodrigues et al. (2011) evaluated cashew apple bagasse as a potential substrate for bioethanol production using yeast. The fermentation of the hydrolyzate by S. cerevisiae resulted in ethanol concentration and productivity of 5.6 g/l and 1.41 g/l/h, respectively. Simultaneous saccharification and fermentation (SSF) of steam exploded citrus peel waste to ethanol by S. cerevisiae was reported by Wilkins et al. (2007). Steam explosion removed D-limonene, an inhibitor present in citrus peel waste. The highest ethanol concentrations were obtained when the initial pH of citrus peel was adjusted to 6.0.
Choi et al. (2012) reported bioethanol production from coffee residue by S. cerevisiae, achieving ethanol concentration and yield (based on sugar content) of 15.3 g/l and 87.2 %, respectively.
The previous studies show that sodium ion concentration has significant effects on ethanol production by S. cerevisiae and there is interactive effect between calcium and magnesium. The optimum sodium concentration was found to be 930 mg/l (Soyuduru et al. 2009) and increase in sodium concentration decreased ethanol production due to its negative effect on glycolysis as well as due to competitive inhibition of potassium uptake leading to depletion of potassium in the cell and increased level of sodium.
7.3.1.2 Schizosaccharomyces sp
Schizosaccharomyces is a genus of fission yeasts, able to ferment xylose to ethanol under microaerophilic or oxygen limited conditions. The studies carried out by Lastick et al. (1990) revealed that simultaneous fermentation and isomerization of xylose (SFIX) allows the total fermentation of xylose in a single step. SFIX provides a significant improvement for fermentation of xylose to ethanol since it is faster and more tolerant to higher concentrations of xylose and ethanol.
7.3.1.3 Kluveromyces sp
Direct fermentation of D-xylose to ethanol using Kluveromyces marxianus SUB-80-S was reported by Margaritis and Bajpai (1982). The strain produced ethanol under aerobic conditions in a medium containing 20 g/l xylose. The ethanol concentration and yield were 5.6 g/l and 0.28 g of ethanol/g of xylose after 48 h of incubation. Ethanol production from poplar and eucalyptus biomass by simultaneous saccharification and fermentation using thermo-tolerant yeast strain K. marxianus CECT 10875 was evaluated by Ballesteros et al. (2004). The results indicated that it is possible to reach SSF yields in the range of 50–72 % of the maximum theoretical SSF yield, in 72–82 h. Maximum ethanol contents from 16 to 19 g/l were obtained in fermentation media, depending on the material tested. The use of thermo-tolerant strains at high process temperatures (42 °C) will minimize the risk of contamination comparable with other fermenting yeasts. This allows for working under non-sterile conditions which is very favorable for process scale up. Tomás-Pejo et al. (2009) developed a simultaneous saccharification and fermentation fed–batch process for bioethanol production by the thermo-tolerant strain Kluveromyces marxianus CECT 10875. The ethanol yield was 36.2 g/l which is 20 % more ethanol yield when compared with batch SSF. Garcia-Aparicio (2011) reported an economic process for high ethanol yield from steam exploded barley straw by K. marxianus CECT 10875. The ethanol concentration was 4 % (w/v) with a substrate loading of 15 %, after 72 h of fermentation.
Toyoda and Ohtaguchi (2008) reported ethanol production by K. lactis NBRC 1903 using cheese whey as lactose source. The study revealed that dissolved oxygen level has a key role for ethanol production in K. lactis NBRC 1903. The ethanol yield in batch culture was 63.7 g/l after 24 h of incubation. Ethanol production using switch grass in SSF with thermo-tolerant yeast strain, K. marxianus IMB3 was reported by Pessani et al. (2011), achieving ethanol concentration and yield of 22.5 g/l and 86 %, respectively, after 168 h of incubation. The coproduction of ethanol and polygalacturonase by K. marxianus in a pilot scale batch fermenter, using yeast extract-glucose-sugar beet molasses medium (SBM), was reported by Serrat et al. (2004). The ethanol productivity was 1.94 g/l/h and the fermentation efficiency was 95.1 %. Ethanol production using steam exploded and liquid hot water pretreated poplar (Populus nigra) by SSF was evaluated using K. marxianus CECT 10875 by Negro et al. (2003). The results indicate that fermentation using steam exploded pretreated poplar gave better SSF yield of 60 % of theoretical when compared to liquid hot water pretreated poplar.
7.3.1.4 Candida sp
The conversion of wood sugars to ethanol has been limited to the hexoses because xylose was not fermentable; however, xylose is a major component of lignocellulosic residues. Most xylose-metabolizing yeasts do not produce ethanol. Most of the yeasts can grow on xylose under aerobic conditions, but very few of them will ferment xylose.
One of the first examples regards Candida tropicalis, which is capable of fermenting xylose under oxygen limited conditions in the presence of increasing concentrations of polyethylene glycol (Hagerdal et al. 1985).
Jeffries and Alexander (2012) produced ethanol from xylose using C. shehatae grown under continuous and fed-batch conditions. The concentration of ethanol produced is proportional to the vigor, viability, and growth rate of the starting culture. This group has developed a two-phase process for ethanol production. In the first phase, a continuous culture was used to generate a vigorous cell suspension and in the second phase, fed-batch fermentation was carried out by pumping in a concentrated sugar feed under semi-aerobic conditions. The cells adapt to oxygen limitation by synthesizing alcohol dehydrogenase (ADH) and ferment the xylose rapidly to ethanol. For the cost-effective production of bioethanol, the yeast strain should be able to convert both glucose and xylose at elevated temperature. Tanimura et al. (2012) isolated a novel yeast strain C. shehatae which is capable of ethanolic fermentation at elevated temperature. The ethanol production yield was 71.6 % in SX medium (3 % xylose and 0.67 % YNB (Yease Nitrogen Base) without amino acid) after 24 h of incubation at 37 °C. This strain produced ethanol even from rice straw and it was found to be superior to S. cerevisiae for producing ethanol from lignocellulosic biomass.
In a study carried out by Watanabe et al. (2010) using respiratory deficient C. glabrata, higher ethanol production ability was observed in SSF. High temperature (45 °C) and agitation (150 rpm) are advantageous for ethanol production from insoluble feed stock using SSF. Nakayama et al. (2008) reported C. krusei IA-1 producing 55 g/l of ethanol from 150 g/l of glucose. The study revealed that C. krusei can be used as a potential alternative to S. cerevisiae for cost-effective production of ethanol.
Dahiya and Vij (2012) reported ethanol production from whey using different strains of immobilized Candida species, C. inconspicua W16, and C. xylopsoci W 23. C. inconspicua W16 was shown to be more efficient in ethanol (3.03 % v/v) production from whey when it is immobilized. Candida tropicalis can convert xylose to ethanol under aerobic conditions and the ethanol production is accelerated by aeration. In order to convert xylose to ethanol under aerobic conditions, it is necessary to have active Embden Meyerhoff and pentose phosphate pathways which are not repressed by air under the conditions employed.
Alexander et al. (1988) evaluated continuous xylose fermentation by C. shehatae in a two-stage reactor. This can overcome the major factor preventing continuous production of ethanol in batch culture. The steady influx of fresh cells and continuous removal of spent cells helps minimize loss of fermentative activity due to anaerobiosis and exposure to high levels of ethanol concentration. The final ethanol yield was 37 g/l in two-stage while in batch it was 0.38 g/l.
7.3.1.5 Pachysolen sp
Saharan and Sharma (2010) investigated the role of trehalose in ethanol induced oxidative condition in Pachysolen tannophilus. It was observed that there was a marked increase in trehalose content after ethanol stress. In addition there was an increase in protein carbonyl content, Reactive Oxygen Species (ROS) generation and lipid peroxidation and there was a decrease in reduced and total glutathione. This study revealed the protective role of trehalose in oxidative stress conditions generated by ethanol. In a study conducted by Kruse and Schuger (1996) by employing batch, fed-batch, and continuous cultivation of Pachysolen tannophilus on various substrates under aerobic, anaerobic, and microaerobic conditions in stirred tank reactor it was observed that under anaerobic conditions low cell biomass and low amount of ethanol were formed. Highest ethanol was produced under microaerobic conditions.
7.3.1.6 Pichia sp
Among the pentose fermenting organisms, P. stipitis has been shown to have most promise for industrial applications (Agbogbo et al. 2006). For example, the hemicellulosic hydrolysates of Prosopis juliflora (18.24 g sugar/l broth) when fermented with P. stipitis produced 7.13 g/l ethanol (Gupta et al. 2009). Detoxified xylose rich hydrolysate of L. camara when fermented with P. stipitis 3498 at pH 5.0 and 30 C for 36 h resulted 0.33 g alcohol/g lignocellulose used (Kuhad et al. 2010). In yet another example, the detoxified water hyacinth hemicellulose acid hydrolysate (rich in pentose sugars) fermented with P. stipitis NCIM-3497 at pH 6.0 and 30 C resulted in 0.425 g ethanol/g lignocellulose.
Canilha et al. (2010) evaluated hemicellulosic hydrolyzate from sugarcane bagasse for ethanol production by Pichia stipitis DSM 3651. Fermentation was carried out by supplementing yeast extract and malt extract at 3 g/l level and peptone 5 g/l level, respectively. It was observed that detoxification of hemicellulosic hydrolyzate by changing the pH and using active charcoal improved bioconversion of hemicelluloses into ethanol. The fermentation yields with detoxified and non- detoxified hydrolyzate were 0.30 g/g and 0.20 g/g, respectively. The effect of various process parameters affecting ethanol production from rice straw hemicellulosic hydrolyzate by P. stipitis NRRL Y-7124 was evaluated by Silva et al. 2010. Parameters like initial xylose concentration, agitation, and aeration were evaluated. Initial xylose concentration of 50 g/l was found to be optimum while increase in aeration and agitation caused a deviation in yeast metabolism from ethanol to biomass production. Under optimized conditions a process efficiency of 72.5 % was achieved. Shupe and Liu 2012 studied the effect of agitation rate on ethanol production from sugar maple hydrolyzate by P. stipitis. It was reported that the highest ethanol yield (29.7 g/l) was observed when the air flow rate was set at 100 cm3 and agitation rate at 150 rpm. Increasing or decreasing the agitation rate in the range 300–350 rpm resulted in a decline in ethanol production. An improved method for ethanol production from undetoxified hemicellulosic hydrolyzate from steam exploded corn stover was evaluated using P. stipitis CBS 5776 by Yong et al. 2012. It was observed that domestication of P. stipitis improved sugar consumption and ethanol yield by increasing the ratio of hydrolyzate in the medium. The ethanol yield was 80 % and the sugar consumption was 90 %.
7.3.2 Bacteria
7.3.2.1 Clostridium sp
The ability of Clostridium beijerinckii in acetone butanol ethanol (ABE) fermentation using degermed corn was reported by Campos et al. (2002). Batch fermentation resulted in 8.93 g/l of total ABE production as compared with 24.80 g/l of total ABE when supplemented with P2 medium nutrients. Several studies report the cost-effective production of ethanol using filter paper, corn steep liquor, cysteine HCl, magnesium chloride and ferrous sulfate and these nutrients play an important role in growth as well as ethanol production by Clostridium sp.
7.3.2.2 Zymomonas sp
Lawford and Rousseau (1997) reported ethanol production by Zymomonas using corn steep liquor as a cost-effective medium. 1 % (v/v) corn steep liquor was found to be optimum and sugar to ethanol conversion efficiency as well as product recovery were 98 % and 100 %, respectively. Immobilized Z. mobilis showed high productivity and conversion compared to free cells (Davison and Scott 1988). The theoretical ethanol yield was reported as 97 % under incubation temperature 30 °C and pH 5.0. Ethanol production from starch hydrolyzates using Z. mobilis and glucoamylase entrapped in polyvinyl alcohol hydrogel was carried out by Rebros et al. (2009). Ethanol productivity increased 2.1 times with immobilized glucoamylase compared to free enzyme—free microorganism system.
7.3.2.3 Thermanaerobacter sp
Lacis and Lawford (1991) studied the potential of Thermanerobacter ethanolicus for ethanol yield form glucose or xylose. It was observed that the ethanol yield depend on the cultivation time and growth rate. The highest ethanol yield (0.42 g/g) was attained at low growth rates. Thermophilic ethanol production by thermophilic bacterium Thermanerobacter BG1L1 in a continuous reactor was investigated by Georgieva et al. (2008) using wet exploded wheat straw. Fermentation was carried out in a fluidized bed reactor at 70 °C. The ethanol yield using non-detoxified hydrolysate was 0.39–0.42 g/g. This study revealed the potential of Thermanaerobacter using fluidized bed reactor for anaerobic ethanol fermentation.
7.3.3 Filamentous fungi
Several fungal species are also reported as a producer of ethanol. The studies carried out on various fungal species for ethanol production are described in the following section.
7.3.3.1 Fusarium sp
Joshi and Verma (1990) evaluated ethanol production from wood hydrolysate by Fusarium oxysporum. Ethanol production at pH 5.5 and 30 C after 96 h of fermentation was of 12.3 g/l and 11.7 g/l by F. oxysporum strain D-140 and NCIM-1072, respectively. The ethanol production in presence of yeast extract and minerals was 13.2 g/l after 108 h of incubation.
Brewer's spent grain is an attractive low cost feed stock for bioethanol production. Xiros and Christakopoulos (2009) evaluated bioethanol production by Fusarium oxysporum by submerged fermentation adopting a consolidated bioprocess strategy. Effects of various process parameters affecting ethanol production were evaluated. Hydrolysis seems to be the bottleneck while the bioethanol yield of 109 g kg−1 of dry material by F. oxysporum was achieved which constitute 60 % of theoretical yield making the process economically feasible for commercial application. F. oxysporum has the ability to ferment xylose which is present in Brewer’s spent grain. The effect of initial sugar concentration and aeration rate affects the fermentation performance of F. oxysporum. The SSF of cellulose by F. oxysporum was investigated by Panagiotou et al. (2005). It was found that F. oxysporum grows with a maximum specific growth rate of 0.023 h−1 on cellulose at aerobic conditions and that it can produce ethanol with a volumetric productivity of 0.044 g/l/h under anaerobic conditions. Ruiz et al. (2007) evaluated ethanol production from lignocellulosic residues by F. oxysporum, achieving an ethanol yield of 0.28 g/g from a 50 % xylose/50 % glucose mixture. The fermentation efficiency was lower but its ability for SSF is a potential advantage.
7.3.3.2 Aspergillus sp
Pushalkar and Rao (1998) reported a cellulolytic fungus Aspergillus terreus which showed an additional property of fermenting glucose, other hexoses, pentoses, and disaccharides to ethanol. Of the various carbon sources tested, glucose yielded maximum ethanol (2.46 % w/v). The ethanol values and the theoretical yields produced by A. terreus with glucose and cellobiose were comparable to or higher than that reported by other fungal species.
7.3.3.3 Mucor sp
Sues et al. (2005) identified Mucor indicus as a potential ethanol producing strain capable of growing aerobically as well as anaerobically on different pentoses and hexoses with yield and productivity same as that of S. cerevisiae. Asachi et al. (2011) developed a cost-effective medium for ethanol production using the fungal extract of M. indicus biomass which is a by-product of fermentation showed improved ethanol production. Yeast extract in the fermentation medium was replaced with fungal extract of M. indicus. The ethanol yield and productivity were 0.46 g/g and 0.69 g/l h, respectively. Ethanol production was higher during aerobic growth on glucose under non-oxygen limiting conditions.
7.3.3.4 Neurospora sp
Dogaris et al. (2012) reported bioethanol production from dilute acid pretreated sweet sorghum bagasse using Neurospora crassa. The study revealed that the bioconversion ability of N. crassa was superior to S. cerevisiae, while their mixed cultures have negative impact on ethanol production.
7.4 Conclusions
Lignocellulosic biomass offers as excellent raw material for ethanol production. There occurs several technological challenges in lignocellulosic biomass to ethanol conversion process and the major challenge in fermentation process is the selection of suitable microorganism. The formation of inhibitors during the pretreatment and hydrolysis stages limits its application and hence ethanologenic organisms capable of tolerating these inhibitors are necessary. Exploration and exploitation of wild and extreme environmental niches may provide novel ethanologenic microorganisms with higher inhibitor tolerance. The search for new ethanologenic microorganisms as well as the improvement in the techniques of fermentation may help in the advancement of cost-effective production of lignocellulosic ethanol.
References
Agbogbo FK, Coward-Kelly G, Torry-Smith M, Wenger KS (2006) Fermentation of glucose/xylose mixtures using Pichia stipitis. Process Biochem 41:2333–2336
Alexander MA, Chapman TW, Jeffries TW (1988) Continuous xylose fermentation by Candida shehatae in a two-stage reactor. Appl Biochem Biotechnol 17:221–229
Asachi R, Karimi K, Taherzadeh MJ (2011) Ethanol production from Mucor indicus using the fungal autolysate as a nutrient supplement. World Renewable Energy Congress, Likoping, Sweden, 8–13 May 2011
Ballesteros M, Oliva JM, Negro MJ, Manzanares P, Ballesteros I (2004) Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem 39:1843–1848
Campos EJ, Qureshi N, Blaschek HP (2002) Production of acetone butanol ethanol from degermed corn using Clostridium beijerinckii BA101. Appl Biochem Biotechnol 98–100:553–561
Canilha L, Carvalho W, Felipe MdG, Silva JB, Giuli M (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Appl Biochem Biotechnol 161:84–92
Choi S, Wi SG, Kim S-B, Bae H-J (2012) Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour Technol 125:132–137
Dahiya M, Vij S (2012) Comparative analysis of bioethanol production from whey by different strains of immobilized thermotolerant yeast. Int J Sci Res Publ 2(3). ISSN 2250-3153
Davison BH, Scott CD (1988) Operability and feasibility of ethanol production by immobilized Zymomonas mobilis in a fluidized-bed bioreactor. Appl Biochem Biotechnol 18:19–34
Dogaris I, Gkounta O, Mamma D, Kekos D (2012) Bioconversion of dilute acid pretreated sorghum bagasse to ethanol by Neurospora crassa. Appl Biochem Biotechnol 95:541–550
Ergun M, Mutlu SF, Gurel O (1997) Improved ethanol production by Saccharomyces cerevisiae with EDTA, ferrocyanide and zeolite X addition to sugar beet molasses. J Chem Technol Biotechnol 68:147–150
Fotheringham I, Kaftzik N, Oswald N (2009) Method for detecting biofuel producing microbes, Patent WO/2009/014722, WIPO
García-Aparicio MP, Oliva JM, Manzanares P, Ballesteros M, Ballesteros I, González A, Negro MJ (2011) Second-generation ethanol production from steam exploded barley straw by Kluyveromyces marxianus CECT 10875. Fuel 90:1624–1630
Georgieva TI, Mikkelsen MJ, Ahring BK (2008) Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Appl Biochem Biotechnol 145:99–110
Gupta R, Sharma KK, Kuhad RC (2009) Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, woody substrate for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis NCIM 3498. Bioresour Technol 100:1214–1220
Hagerdal BH, Jonsson B, Vogel EL (1985) Shifting product formation from xylitol to ethanol in pentose fermentations using Candida tropicalis by adding polyethylene glycol (PEG). Appl Microbiol Biotechnol 21:73–175
Jeffries TW, Alexander MA (2012) Production of ethanol from xylose by Candida shehatae grown under continuous or fed-batch conditions. In: Kirk TK, Chang H-M (eds) Biotechnology in pulp and paper manufacture. Proceedings of the fourth international conference on biotechnology in the pulp and paper industry, Butterworth-Heinermann, USA, pp 311–321
Joshi SK, Verma J (1990) Production of ethanol from sugars in wood hydrolysate by Fusarium oxysporum. World J Microbiol Biotechnol 6:10–14
Junior MM, Batistote M, Cilli EM, Ernandes JR (2009) Sucrose fermentation by Brazilian ethanol production yeast in media containing structurally complex nitrogen sources. J Inst Brewing 115:191–197
Kruse B, Schuger K (1996) Investigation of ethanol formation by Pachysolen tannophilus from xylose and glucose/xylose co-substrate. Process Biochem 31:389–407
Kuhad RC, Gupta R, Khasa YP, Singh A (2010) Bioethanol production from Lantana camara (red sage): pretreatment, saccharification and fermentation. Bioresour Technol 101:8348–8354
Lacis LS, Lawford HG (1991) Thermoanaerobacter ethanolicus growth and product yield from elevated levels of xylose or glucose in continuous cultures. Appl Environ Microbiol 57(2):579–585
Lastick SM, Mohagheghi A, Tucker MP, Grohmann K (1990) Simultaneous fermentation and isomerization of xylose to ethanol at high xylose concentrations. Appl Biochem Biotechnol 24(25):431
Lawford HG, Rousseau JD (1997) Corn steep liquor as a cost-effective nutrition adjunct in high-performance Zymomonas ethanol fermentations. Appl Biochem Biotechnol 63–65:287–304
Linde M, Glabe G, Zacchi G (2007) Simultaneous saccharification and fermentation of steam-pretreated barley straw at low enzyme loadings and low yeast concentration. Enzym Microb Technol 40:1100–1107
Margaritis A, Bajpai P (1982) Direct fermentation of D-xylose to ethanol by Kluyveromyces marxianus strains. Appl Environ Microbiol 44:1039–1041
Maye JP (2006) Use of hop acids in fuel ethanol production. US patent. Patent US2006263484
Maziar SA (2010) A study on some efficient parameters in batch fermentation of ethanol using S. cerevisiae SC1 extracted from fermented Siahe Sardasht Pomace. Afr J Biotechnol 9:2906–2912
Nakayama S, Morita T, Negishi H, Ikegami T, Sakaki K, Kitamoto D (2008) Candida krusei produces ethanol without production of succinic acid: a potential advantage for ethanol recovery bypervaporation membrane separation. FEMS Yeast Res 8:706–714
Negro MJ, Manzanares P, Ballesteros I, Oliva JM, Cabañas A, Ballesteros M (2003) Hydrothermal pretreatment conditions to enhance ethanol production from poplar biomass. Appl Biochem Biotechnol 105–108:87–100
Nikolic S, Mojovic L, Pejin D, Rakin M, Vucurovic V (2009) Improvement of ethanol fermentation of corn Semolina hydrolyzates with immobilized yeast by medium supplementation. Food Technol Biotechnol 47:83–89
Panagiotou G, Villas-Boas SG, Christakopoulos P, Nielsen J, Olsson L (2005) Intracellular metabolite profiling of Fusarium oxysporum converting glucose to ethanol. J Biotechnol 115:425–434
Parekh S, Wayman M (1986) Fermentation of cellobiose and wood sugars to ethanol by Candida shehatae and Pichia stipitis. Biotechnol Lett 8:597–600
Pessani NK, Atiyeh HK, Wilkins MR, Bellmer DD, Banat IM (2011) Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresour Technol 102:10618–10624
Pushalkar S, Rao KK (1998) Ethanol fermentation by a cellulolytic fungus Aspergillus terreus. World J Microbiol Biotechnol 14:289–291
Qi X, Zhang Y, Tu R, Lin Y, Li X, Wang Q (2011) High-throughput screening and characterization of xylose-utilizing, ethanol-tolerant thermophilic bacteria for bioethanol production. J Appl Microbiol 110:1584–1591
Rebros M, Rosenberg M, Grosová Z et al (2009) Ethanol production from starch hydrolyzates using Zymomonas mobilis and glucoamylase entrapped in polyvinylalcohol hydrogel. Appl Biochem Biotechnol 158:561–570
Rodrigues TH, Rocha MV, de Macedo GR, Goncalves LR (2011) Ethanol production from cashew apple bagasse: improvement of enzymatic hydrolysis by microwave-assisted alkali pretreatment. Appl Biochem Biotechnol 164:929–943
Ruiz E, Romero I, Moya M, Sanchez S, Bravo V, Castro E (2007) Sugar fermentation by Fusarium oxysporum to produce ethanol. World J Microbiol Biotechnol 23:259–267
Ruiz HA, Silva DP, Ruzene DS, Lima LF, Vicente AA, Teixeira JA (2012) Bioethanol production from hydrothermal pretreated wheat straw by a flocculating Saccharomyces cerevisiae strain—effect of process conditions. Fuels 95:528–536
Saharan RK, Sharma SC (2010) Correlation studies of trehalose with oxidative stress in ethanol stressed yeast Pachysolen tannophilus. Curr Res J Biol Sci 2:300–305
Serrat M, Bermúdez IRC, Villa TG (2004) Polygalacturonase and ethanol production in Kluyveromyces marxianus, potential use of polygalacturonase in foodstuffs. Appl Biochem Biotechnol 117:49–64
Shupe AM, Liu Shijie (2012) Effect of agitation rate on ethanol production from sugar maple hemicellulosic hydrolysate by Pichia stipitis. Appl Biochem Biotechnol 168:29–36
Silva JPA, Mussatto SI, Roberto IC (2010) The influence of initial xylose concentration, agitation, and aeration on ethanol production by Pichia stipitis from rice straw hemicellulosic hydrolysate. Appl Biochem Biotechnol 162:1306–1315
Soyuduru D, Ergun M, Tosun A (2009) Application of a statistical technique to investigate calcium, sodium, and magnesium ion effect in yeast fermentation. Appl Biochem Biotechnol 152:326–333
Sues A, Millati R, Edebo L, Taherzadeh MJ (2005) Ethanol production from hexoses, pentoses and dilute acid hydrolyzate by Mucor indicus. FEMS Yeast Res 5:669–676
Taherzadeh MJ, Gustafsson L, Niklasson C, Liden G (2000) Inhibition effects of furfural on aerobic batch cultivation of Saccharomyces cerevisiae growing on ethanol and/or acetic acid. J Biosci Bioeng 90:374–380
Tanimura A, Nakamura T, Watanabe I, Ogawa J, Shima J (2012) Isolation of a novel strain of Candida shehatae for ethanol production at elevated temperature SpringerPlus 2012. 1:27. doi:10.1186/2193-1801-1-27
Tomás-Pejó E, Oliva JM, González A, Ballesteros I, Ballesteros M (2009) Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 88:2142–2147
Toyoda T, Ohtaguchi K (2008) Production of Ethanol from Lactose by Kluyveromyces lactis NBRC 1903. Thammasat Int J Sci Technol 13:30–35
Vogel HC, Todaro CL (1996) Fermentation and biochemical engineering hand book, 2nd edn. Noyes publications, New Jersy, pp 122–160
Watanabe I, Nakamura T, Shima J (2010) Strategy for simultaneous saccharification and fermentation using a respiratory-deficient mutant of Candida glabrata for bioethanol production. J Biosci Bioeng 110:176–179
Wilkins MR, Widmer WW, Grohmann K (2007) Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem 42:1614–1619
Xiros C, Christakopoulos P (2009) Enhanced ethanol production from brewer’s spent grain by a Fusarium oxysporum consolidated system. Biotechnol Biofuels 2:4
Yong Q, Li X, Yuan Y, Lai C, Zhang N, Chu Q, Xu Y, Yu S (2012) An improved process of ethanol production from hemicellulose: bioconversion of undetoxified hemicellulosic hydrolyzate from steam-exploded corn stover with a domesticated Pichia stipitis. Appl Biochem Biotechnol 167:2330–2340
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Binod, P., Sindhu, R., Pandey, A. (2013). The Alcohol Fermentation Step: The Most Common Ethanologenic Microorganisms Among Yeasts, Bacteria and Filamentous Fungi. In: Faraco, V. (eds) Lignocellulose Conversion. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37861-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-37861-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37860-7
Online ISBN: 978-3-642-37861-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)