Abstract
The trend for material and energy recovery from biomass-waste along with the need to reduce green house gases has led to an increased interest in the thermal processes applied to biomass. The thermal process applied to biomass produces either liquid fuel (bio-oil) or gaseous fuel. Liquid fuel is more preferred because it is easier to transport from one point to another and also it can be used for production of chemicals. One of the biomass obtained in Tanzania is sugarcane bagasse. The sugarcane bagasse is the fibrous materials that remain after sugarcane is crushed to extract juice. Currently, it is burnt directly in the boilers for production of steam, but it can be used for production of bio-oil. The bio-oil can be optimally obtained by fast pyrolysis, which is a fast thermal decomposition of biomass material at temperature range 523–800 K in the absence of an oxidizing agent. In order to undertake a parametric study on the fast pyrolysis of sugarcane bagasse, it is imperative to establish its thermal characteristics. The paper reports the proximate and ultimate analysis, and thermal degradation of sugarcane bagasse in nitrogen as heating agent. The thermal degradation was conducted in a thermo-gravimetric analyzer from room temperature to 1,000 K at different heating rates of 5, 10, 20 and 40 K min−1. The thermo-gravimetric analyzer was used to study the effect of heating rate on the thermal degradation characteristics and to determine mass loss kinetics. The sugarcane bagasse was observed to be suitable for use in pyrolysis since it contains high volatile level of 80.5 % and fixed carbon of 8.2 %. The peak temperature was observed at 573 K at 10 K min−1 and corresponding activation energy was 387.457 kJ/mol.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Short Introduction
In order to reduce the emissions of green house gases, an increased interest in the thermal processes applied to biomass-waste became a common trend in the world. Sugarcane bagasse (the fibrous materials that remain after sugarcane is crushed to extract juice) is a one of the important biomass-wastes of Tanzania. This paper reports the proximate and ultimate analysis, and thermal degradation of sugarcane bagasse in nitrogen as heating agent. The sugarcane bagasse was observed to be suitable for use in pyrolysis since it contains high volatile level of 80.5 % and fixed carbon of 8.2 %.
Introduction
Biomass is in fourth position in the ranks of energy resources on a global basis, providing 14 % of the world energy need (Garcia-Perez et al. 2001). In developing countries like Tanzania 90 % of the energy consumed is from biomass, in the form of wood and charcoal, while petroleum and electricity account for 8 and 1.2 % respectively, the remaining source of energy is 0.8 %, such as coal (Mshandete 2011).
Sugarcane is one of the largest products that are obtained in Tanzania; there are about 17,000 hectares of sugarcane. All the sugarcane farms are owned by sugarcane companies; these are Kilombero Sugar Company, Mtibwa Sugar Estate, Tanganyika Planting Company (TPC) and Kagera Sugar. Each company produces about 450,000 tones of bagasse per year (Gwang’ombe and Mwihava 2005).
The produced sugarcane bagasse from Sugar Company has a moisture content of about 50 %, with a calorific value of 10 MJ/kg. The mill bagasse has a low bulk density of 130 kg/m3, which cause a storage problem, because it requires a large space and also equipment for handling (Deepchand 2001). In most of the sugar company the bagasse produced is directly burnt in the boiler for production of steam which can be used for production of electricity. This process generates residues that could lead to high disposal costs or pose environmental problem when disposed by means of open air burning (Szogs and Wilson 2008).
An advanced thermal technology is required to harness energy with the intimacy of minimizing disposal cost and environmental pollution. The thermal technology requires knowledge of thermal behavior of sugarcane bagasse before application. Thermo-gravimetric analysis (TGA) is one of the techniques that are used to study the thermal behavior of solid fuels. The TGA assists to calculate mass loss rate per unit time, reaction constant (k) and the value of activation energy (Ea) of bagasse. The results are useful during application in process such as pyrolysis.
Pyrolysis is one of the thermal process which favoring the upgrading of energy content of biomass. Pyrolysis can produce both charcoal and bio-oil. The bio-oil produced from sugarcane bagasse has the gross calorific value of about 22.4 MJ/kg, which is twice the original bagasse. Also charcoal has heating value of 36 MJ/kg (Garcia-Perez et al. 2002). The transportation and storing is easier for bio-oil than charcoal, also synthesis gas and valuable chemicals can be produced from bio-oil.
The theme of this paper is to characterize the sugarcane bagasse in relation to the frequency factor and activation energy and also to determine the quality of bio-oil that can be obtained from sugarcane bagasse.
The Sugarcane Bagasse Reaction Kinetics
Sugarcane bagasse mainly consists of cellulose, hemicellulose and lignin, the cellulose is in the range of 32–44 wt %, hemicellulose is between 27 and 32 wt %, and lignin is around 19–24 wt % (Sanchez 2009). Also bagasse has small amount of cane wax, organic acids and other materials, the composition of bagasse depends on the sugar beat content, age, nature of soil which the sugarcane plant was grown. The properties of each component are different, the cellulose fibers provide material strength and its degradation occurs at 240–350 °C to produce an hydrocellulose and levoglucosan. The hemicelluloses decomposition occurs at a temperature of 200–260 °C giving rise to more volatiles, but less tar and less char than cellulose. The third major component is lignin, which is more thermally stable and accounts for the production of residual char (Mohan et al. 2006).
The variation of the components in biomass materials result to complexity of thermal decomposition, due to that reason several researches have been done to determine the behavior of each component. Broido-Shafizadeh studied the pyrolysis kinetics of cellulose, observed that the cellulose reacts at elevated temperature to form active cellulose and then decomposes into volatiles and, gas and solid materials as shown in Eq. 31.1 (Bradbury et al. 1979).

In the Broido-Shafizadeh mechanism there is no change of mass in the transformation of cellulose to active cellulose, the transformation of the active cellulose to product is accompanied by a mass loss. In addition to that most of the pyrolysis mechanisms are based on cellulose since it is considered as a primary component of a lignocellulosic biomass. Finally, a mechanism which suggests the degradation of the biomass is the sum of the contribution of the individual degradation of the three components was applied, this is known as three-step mechanism, Eq. 31.2, in this paper the degradation of the sugarcane bagasse is assumed to follow three step mechanism.
There are different methods for determination of pyrolysis kinetics from Thermo-gravimetric analysis. These are Coats and Redfern (Cai and Bi 2008), Agrawal sivasubramanian (Safi et al. 2004), Freeman and Carroll (Criado et al. 1982), Kissinger’s method (Criado and Ortega 1986) among others. This study will consider Kissinger’s method, since the process that used during thermo-gravimetric analysis of bagasse was non-isothermal.
The Kissinger’s method does not depend on reaction mechanism for determination of activation energy, although the determination of the frequency factor assumes first order reaction mechanism (Tsamba 2008). The peak temperature (Tmax) is used to determine the activation energy (Ea). Thermal decomposition rate are measured at different heating rate, through sequence of experiments. The pyrolysis rate is expressed by using Arrhenius Eqs. (31.3) and (31.4), k is the rate constant, which depends on temperature.
where x is the reacted fraction as shown in Eq. (31.5), T is the absolute temperature, Ea is the activation energy, A is the pre exponential factor, R is the gas constant and f(x) is the algebraic function depending on the reaction mechanism.
If temperature rises at a constant heating rate (β), which is expressed as Eq. 31.6, the differentiation of Eq. 31.4 will result Eq. 31.7.
The maximum rate occurs at a temperature Tmax, defined by equating Eq. 31.7 to zero. Approximations from the calculations give the Eq. 31.8.
A straight line graph is obtained by plotting of \( ln\left({\frac{\beta}{{T_{max}^{2}}}} \right)\,{\text{v/s}}\,{1 \mathord{\left/{\vphantom {1 {T_{max}}}} \right. \kern-0pt} {T_{max}}}, \) the slope is \( {\raise0.7ex\hbox{${E_a}$} \!\mathord{\left/{\vphantom {{Ea} R}}\right.\kern-0pt} \!\lower0.7ex\hbox{$R$}} \) and the intercept on the vertical axis is an \( ln\left(\frac{AR}{E_a} \right) \).
Methodology
Materials
The bagasse used in this study was collected from Tanganyika Planting Company, which is located in Moshi region, Tanzania. Pure nitrogen gas was used as heating agent. The Thermo-gravimetric analyser was used to study the thermo-degradation of the bagasse, fixed bed pyrolyzer was used for production of bio-oil, bomb calorimeter was used to determine heating value, furnace was used for determination of proximate analysis of the bagasse and atomic absorption spectrometer (AAS) was used for ultimate analysis.
Method
The experiments were divided into three parts; the first part was to study the characteristics of rice husk through proximate, ultimate analysis and high heating value (HHV). Secondly, the determination of pyrolysis kinetics of the bagasse and finally, the production and analysis of bio-oil obtained from bagasse.
The proximate analysis of the bagasse was carried out according to ASTMD 3172 method, ultimate analysis was done according to ASTMD 3176 and the HHV was obtained by ASTM D2015.
The experiments under non isothermal conditions were carried out in the TGA. The sample of a ground bagasse was at an average particle size of 100 μm and 30 mg of the sample was used for each experiment. The TGA experiments were conducted at heating rates 5, 10, 20 and 40, in nitrogen atmosphere. The weight change of sample was recoded by thermo-balance.
The fast pyrolysis process was applied to the 100 gm of sugarcane bagasse by using fixed bed pyrolyzer. The operation of the fixed bed pyrolyzer is divided into, heating and experimental phase. The heating phase is done by heating the ceramic honeycomb to 600 °C by using methane burner. The flue gases that remain in the system are thereafter purged by nitrogen gas with the assistance of exhaust fan until the system is free of flue gases. The flue gases are monitored by using gas chromatography, at this stage the system temperature reduced to 500 °C.
The sample is stored in the chamber just above the reactor and it is continually blown with nitrogen gas so as to remove air in the sample. After the temperature of the system stabilizes, the nitrogen gas which is blown to sample is stopped and the sample is introduced to the reactor. The temperature of the reactor and the sample temperature will be measured by using thermocouples.
The sampling line has bottles which are immersed in the ice bath, which act like a condenser, volatile matters that passes through the bottle will condense, gases will go to Gas chromatography for analysis, while char will remain in the basket.
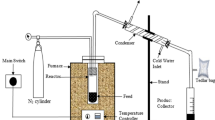
The pyrolyzer is shown in Fig. 31.1 and it consist of A is primary chamber, B is secondary chamber, C is ceramic honeycomb, D and E are Oxygen and methane line for the burner, F is sample, G is weighing machine, H is ice bath, I is sampling bottle and GC is Gas chromatography.
Result and Discussion
The sample characteristics are shown in Table 31.1.
The carbon and hydrogen contents are the indicative of hydrocarbons that can be released during pyrolysis process. On the other hand the high oxygen content reduces the energy density of the fuel. The presence of chlorine and sulphur are not preferred since they contribute to the formation of corrosive compounds.
Thermo-gravimetric analysis of the sugar cane bagasse is shown in Fig. 31.2; it is the thermo-gravimetric (TG) against temperature. It is divided into three sections, the moisture content section which shows the mass change of the bagasse due to moisture released, secondly, the abrupt mass change due volatile matter and the last section is the constant section where there is no mass change or the mass change is significantly small.
There are differences between proximate analysis and thermo-gravimetric analysis, in thermo-gravimetric analysis the moisture content was 9.52 %, volatile matters were 83.5 %, while in proximate analysis the moisture and volatile matter are 9.00 and 80.5 % respectively.
The constant section in the thermo-gravimetric analysis is know as char, which consists of ash and fixed carbon, in Fig. 31.2 the char was 7.40 %, while in proximate analysis the fixed carbon and ash sum up to 10.5 %.
The thermo-gravimetric analysis shows that the heating rate affects the thermo-degradation of sugarcane bagasse. Figure 31.3 describes that when 5 °C/min was applied to sugarcane bagasse the char collected was higher than when 10, 20 and 40 °C/min were applied. It also shows that the suitable heating rate that provides minimum char at low temperature (i.e., 405 °C), this was 10 °C/min, while in 20 and 40 °C/min, the small amount of char obtained were at 420 and 500 °C respectively.
Figure 31.4 is the derivative of the Fig. 31.3. It shows clearly that after 500 °C there was no any weight change of the sample for all used heating rate, this described that the thermo-degradation of the sugarcane bagasse stopped. The remaining material was only char, which contains fixed carbon and ash.
The final temperature to degrade sugar bagasse increases as heating rate increases, The final temperature for weight change of sugar bagasse of 5, 10, 20 and 40 °C/min were 306, 340, 400 and 500 °C respectively. This observation gives evidence that the degradation of sugarcane bagasse a slow reaction process, it requires a low heating rate.
Figure 31.5 was obtained by plotting the natural logarithm of the ratio of heating rate and maximum temperature against the reciprocal of maximum temperature. The heating rate and the maximum temperature were obtained from Fig. 31.4. The determination of kinetic parameters was done by using Fig. 31.5. The activation energy obtained was 387.457 kJ/mol and the pre-exponential factor is 0.74 s−1.
The bio-oil obtained was dark brown liquid, with a density of 1200 kg/m3 and viscosity of 23 cP at 40 °C, the pH was 3, High Heating Value (HHV) was 23.2 MJ/kg and ash content was 0.02 wt %.
Conclusion
The overall calculations of the thermochemical kinetics of sugarcane bagasse was done by using Kissinger’s method, which assume that the reaction order was one, the activation energy and frequency factor obtained were 387.457 kJ/mol and 0.74 s−1.
On the other hand the liquid fuel obtained during the pyrolysis process is more suitable since it has higher calorific value than the original sugarcane bagasse; this is an advantage because a little amount of bio-oil is able to produce enough energy.
Abbreviations
- Symbol:
-
Description
- A:
-
Pre-exponential factor
- AAS:
-
Atomic Absorption Spectrometer
- C:
-
Carbon
- Cl:
-
Chlorine
- Ea :
-
Activation energy
- H:
-
Hydrogen
- k:
-
Reaction constant
- k1:
-
Rate constant for gas
- k2:
-
Rate constant for tar
- k3:
-
Rate constant for char
- N:
-
Nitrogen
- O:
-
Oxygen
- R:
-
Gas constant
- S:
-
Sulphur
- T:
-
Absolute temperature
- TG:
-
Thermo-gravimetric
- TGA:
-
Thermo-gravimetric analysis
- Tmax :
-
Maximum temperature
- w:
-
Weight of a sample at time t
- wo :
-
Initial weight
- w∞ :
-
Final weight
- x:
-
Reacted fraction
- β:
-
Heating rate
References
Bradbury A, Sakai Y, Shafizadeh F (1979) A kinetic model for pyrolysis of cellulose. J Appl Polym Sci 23:3271–3280
Cai J, Bi L (2008) Precission of the coats and Redfern method for the determination of activation energy without neglecting the low temperature end of the temperature integral. Energy Fuels 22:2172–2174
Criado J, Ortega A (1986) Non isothermal transformation kinetics: remarks on Kissinge method. J Non Cryst Solid 87:302–311
Criado J, Dollimore D, Heal G (1982) Critical study of the suitability of the Freeman and Carroll method for the kinetic analysis of the reactions of thermal decomposition of solids. Thermochim Acta 54:159–165
Deepchand K (2001) Bagasse-based cogeneration in Mauritius-A model for Eastern and Southern Africa, AFREPREN
Garcia-Perez M, Chaala A, Yung J, Roy C (2001) Co-pyrolysis of sugarcane bagasse with petroleum residue part 1: Thermo gravimetric analysis. Fuel 80:1245–1258
Garcia-Perez M, Chaala A, Roy C (2002) Vacuum pyrolysis of sugarcane bagasse. J Anal Appl Pyrol 65:111–136
Gwang’ombe F, Mwihava N (2005) Renewable in Tanzania status and prospects of biomass-based cogeneration and geothermal technology, AFREPREN
Mohan D, Pittman C, Steele P (2006) Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 20:848–889
Mshandete A (2011) Biofuel in Tanzania: status, opportunities and challenges. J Appl Biosci 40:2677–2705
Safi M, Mishra I, Prasad B (2004) Global degradation kinetics of pine needles in air. Thermochimica Acta 412:155–162
Sanchez C (2009) Lignocellulosic residues: biodegradable and bioconversion by fungi. Biotechnol Adv 27:185–194
Szogs A, Wilson L (2008) A system of innovation? Biomass digestion technology in Tanzania. J Technol Soc 30:94–103
Tsamba A (2008) Fundamental study of two selected tropical biomass for energy: coconut and cashew nut shells, Doctoral Thesis, Energy and Furnace Technology, Stockholm, Sweden
Acknowledgments
The Authors would like to thank the SIDA SAREC program and the University of Dar es Salaam for financing the project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Said, M., John, G., Mhilu, C., Manyele, S. (2013). Fast Pyrolysis and Kinetics of Sugarcane Bagasse in Energy Recovery. In: Leal Filho, W., Mannke, F., Mohee, R., Schulte, V., Surroop, D. (eds) Climate-Smart Technologies. Climate Change Management. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37753-2_31
Download citation
DOI: https://doi.org/10.1007/978-3-642-37753-2_31
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37752-5
Online ISBN: 978-3-642-37753-2
eBook Packages: Business and EconomicsEconomics and Finance (R0)