Abstract
Pristine and partially iodized noble metal thin films are of interest in plasmonic, ‘plexitonic’ (involving Plasmon-exciton interaction and transition) and nanophotonic applications as well as potential substrates for SERS. It is crucial to basically characterize such films for optimization and implementation of structures for specific practical applications. Iodization offers an attractive, simple and effective experimental procedure to modify the surface of noble metal films and also to grow semiconductor nanostructures. Interestingly one can observe the development of excitonic and fundamental electronic band structure of the semiconductor which is AgI in the present case. In this paper we focus on structural and optical characterization of (a) <30 nm thick Ag films obtained by thermal evaporation and (b) the same Ag films surface-modified by partial iodization. The Structural properties, the crystal structure, Surface morphology and chemical composition of the deposited Ag films were studied by X-Ray diffraction (XRD), Field Emission Scanning electron microscopy (FESEM). XRD Pattern showed that the films had preferred orientation along (111) of the face centered cubic (fcc) structure. The mechanical properties (The intrinsic stress, Dislocation density, grain size) are also calculated. The optical properties pristine and partially iodized Ag films of were investigated by measuring the absorbance from 300 to 800 nm.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Metal nanostructures have attracted considerable attention both fundamentally and technologically because of their unique physical and chemical properties and functionalities compared to their bulk counterparts. One of the most fascinating aspects is their optical properties. Pristine and partially iodized noble metal thin films are of interest in plasmonic, ‘plexitonic’ (involving Plasmon-exciton interaction and transition) and nanophotonic applications as well as potential substrates for SERS [1–3]. On the nano scale many metals, e.g., silver and gold exhibit strong absorption in the visible region of the spectrum. The origin of this absorption is attributed to collective conduction band electron oscillation in response to the electrical field of the electromagnetic radiation of light. This optical absorption is termed surface plasmons, partly because net charges are displaced transiently on the particle surface during electron oscillation. Surface plasmon absorption band are somewhat dependent on the size of spherical nanostructures, they strongly depend on the shape of the nanostructures. For a metal such as silver or gold, almost any color or absorption in any part of the visible spectrum can be produced by controlling the shape or structure of the nano material. Examples of various non spherical metal nanostructures reported in the literature include aggregates, nano rods, nano wires, nano cages, nano spheres, nano prisms, and nano plates or sheets [4–12]. The controllable and tunable optical properties of metal nanostructures are highly desired for many applications that rely on light absorption of metal, including surface Plasmon resonance (SPR), surface-enhanced Raman scattering (SERS), sensing, imaging, therapy, and photo catalysis. Therefore, the optical properties of metal nano materials can be altered at will by manipulating the details of the structure in a controllable manner. The modification of the surfaces for an enhancement of optical absorption, hence, provides a good method to improve efficiency of an optoelectronic device involving photon absorption [13].
In this paper, the control of the aggregation of spherical silver nano particles were examined by reaction of iodine vapors with Ag film at normal conditions. It has been proved that the partial iodization modifies the Ag film surface and produces reshaped silver nano particles on the surface of the Ag film.
2 Experimental Part
Ag films were deposited on borosilicate glass substrates by thermal evaporation using a molybdenum filament. The material was highly pure Ag wire (Aldrich, 99.999) placed in the filament which was thermally evaporated on to the cleaned borosilicate glass substrates kept at an ambient temperature. The borosilicate substrates were cleaned as follows: glass substrates were first immersed 10 % soap solution with 90 % water, rubbed with cotton 10–15 min with single distilled water to remove weathering, kept in chromic acid up to boiling point for removing organic contaminates, washed in cold water to remove surface contaminants followed by ultra-sonification in iso-propyl alcohol for 10 min duration, and, finally these substrates were dried in air before loading into the system for deposition. The glass substrates were kept at a distance of 20 cm from the source for deposition [14]. Ag film thickness measured as 20 nm with 0.1 nm/s rate of deposition by using thermal evaporator attached thickness monitor (Model CTM-200) during the experiment, and also the thickness was measured using Stylus profiler XP-1, Ambios Technology, USA. The deposition rate was controlled by adjusting the current through the filament. Vacuum of the order of 4.5 × 10−6 mbar was maintained in the chamber thought the deposition. The thermal evaporated films were iodized for selected durations up to 8 min using a set up described earlier [2].
Crystallographic study was carried out using a PHILIPS X-Ray diffractometer with Co-Kα (λ = 1.78897 Å) in the 2θ range from 200 to 1000. The microstructure of the Ag thin films on the glass substrate was studied by using a Field emission scanning electron microscope (FESEM) (Model ULTRA-55) attached with an Energy Dispersive Scattering (EDS) analyzer to measure quantitatively the Ag film chemical stoichiometry. In order to study optical properties, optical absorption spectra were recorded in the wavelength range of 300–800 nm using JASCO V-570 UV–VIS-NIR double beam spectrometer in visible range from 300 nm to 800 nm with scanning rate 4 nm/s.
3 Results and Discussion
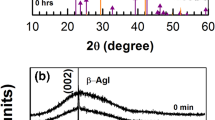
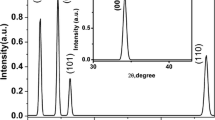
X-ray diffraction (XRD) is a popular and powerful technique for determining crystal structure of crystalline materials. By examining the diffraction pattern, one can identify the crystalline phase of the material. Small angle scattering is useful for evaluating the average inter particle distance while wide angle diffraction is useful for refining the atomic structure of nano particle [15]. The width of the diffraction lines are closely related to strain and defect size and distribution in nano crystals. As the size of the nano crystals decrease, the line width is broadened due to loss of long-range order relative to the bulk. The presence of peaks at 2θ values 44.60, 520, 76.60, 93.20, 98.70 correspond to (111), (200), (220), (311) Planes of silver respectively. Thus the XRD pattern confirmed the completely crystalline nature of silver thin film. These data agree with the standard JCPDS data card [16]. The lattice constant of silver deduced from the pattern is a = 4.085Å compares well to the standard data of silver. This XRD line width can be used to estimate the size of the particle by using the Debye–Scherrer formula (Fig. 1).
where D is the nano crystal diameter, λ is the wavelength of light, W is the full width half-max (FWHM) of the peak in radians, and θ is the Bragg angle. The crystallite size of silver Nanoparticles has been found to be 14 nm. In order to distinguish the effect of crystallite size induced broadening and strain induced broadening at FWHM of XRD profile,
Where W is FWHM in radians, t is the grain size in nm, ε is the strain in nm, l is X-ray wavelength in nanometers, and C is a correction factor taken as 0.9. The grain size and strain of the sample have been found to be 14 nm and 88.35 × 10−2, respectively. The dislocation density (δ) in the nanoparticles has been determined using expression [17]
The X-ray line profile analysis has been was used to determine the dislocation density of silver nano particles and found to be as 4.024 × 10−4 m-2 respectively.
3.1 Optical Properties of Ag Films
When the Ag film was exposed to iodine vapors at room temperature, the film surface will modify according to the time of iodine expose, before iodization a broad absorption band was observed at 522 nm. The gradual changes of the peak position and FWHM observed in 0–8 min of iodization seem to be responsible for reshaped silver nano particles. If increases the iodization time the absorption band full width half maxima (FWHM) also increased, at 2 min iodization time the broad absorption band was absorbed at 517 nm. Within this duration iodine vapors will try to modify the film surface and at the 4, 6, 8 min iodization time silver film the surface particles will stabilized and form a reshaped spherical nano particles in Fig. 2b, c. The variation of absorption spectrum with iodization time is given in Fig. 2. In the absorption spectrum a small shoulder at 380 nm this peak is assigned to the silver clusters less than 1 nm in size (Loginov et al. 2002). When iodization is continued, the small peak at 380 nm gets reduced in intensity, and the absorbance of the long wavelength tail is increased. Equally significantly, the minimum in absorbance preceding the absorption maximum which is related to the reflectivity rises upon progressive iodization. These observations indicate the growth and aggregation of silver.
Absorption co-efficient (Δα) versus iodization time for the SPR minimum, maximum is shown in the Fig. 2b. It shows two roughly linear segments with a break at 4 min. But Δα versus iodization time plot for the SPR maximum is nonlinear saturating up to 8 min, while SPR peak shows a linear increase (Fig. 2c). These features are quantitatively similar to the Δα versus iodization time for UV minimum. The linear increases in SPR absorbance with increasing iodization time offers an energy calibration which could be used for applications.
3.2 FESEM
Field emission scanning electron micrographs (FE-SEM) of the 20 nm Ag films before and after 2, 8 min iodization and corresponding electron dispersive scattering (EDS) spectrum are shown in Fig. 3. Before iodization, the film surface looked rough and silver cracks were observed on the whole surface in Fig. 3a. Within two minutes iodization, the silver particles (size 10–20 nm) are growing because iodine vapors are modifying the Ag film surface (Fig. 3b). When the iodization was continued up to 8 min, the surface was covered with silver nano particles of relatively uniform size ~40 nm and density of silver nano particles was increased and the particles grow in size. Most of the particles were in spherical shape and the distribution of silver nano particles at the film surface was almost homogeneous particles [18, 19]
Field emission scanning electron microscopy (FE-SEM) graphs of the 20nm Ag film before and after iodization time: (a) 0 min, (b) 2 min, (c) 8 min iodization shows that silver nano particles are spherical in shape having smooth surface and are well dispersed (d) EDS spectrum of reshaped Ag nano particles
.
The elemental analysis of Ag film has been performed using EDS spectroscopy (Fig. 3d). The peaks observed at 2.6–3.2 keV correspond to the binding energies of Ag. The remaining peaks came due to carbon coated tape; glass substrate and gold paint, no peaks of any other impurity have been detected. Therefore, the EDX profile of Ag film indicates silver nanoparticles only in which case we are using iodine for surface modification purpose only. Within the 8 min iodization time AgI has not yet formed [20].
4 Conclusion
Formation and aggregation of reshaped silver nanoparticles at the surface of silver film of have been investigated by partial iodization. The Structural properties, the crystal structure, Surface morphology and chemical composition of the deposited Ag films were studied by X-Ray diffraction (XRD), FE-SEM observations have been carried out to characterize silver nanoparticles at the film surface. Silver nano particles of ~40 nm size were formed on the surface. According to the FE-SEM observation, the growth of the particle diameter and aggregation of Nanoparticles took place after partial iodization.
References
C.S. Sunandana, M. Gnanavel, in Photonics 2010: X International conference on fiber optics and photonics, ed. by S Khijwania, A K Sharma. (Viva Books, New Delhi, 2010) p. 127
M. Gnanavel, C.S Sunandana, in SPIE Proceedings, Plasmonic: Metllic Nanostrutures and their Optical Properties VII, ed. by M.I. Stockman. 7394, 73942D (2009)
C. Yang, Y.T. Xie, M.M.F. Yuen, X. Xiong, C.P. Wong, A facile chemical approach for preparing a SERS active silver substrates. Phys. Chem. Chem. Phys. 12, 4459 (2010)
J.Z. Zhang, C. Noguez, Plasmonic optical properties and applications of metal nanostructures. Plasmonics 3, 127 (2008)
C.J. Murphy, T.K. Sau, A.M. Gole, C.J. Orendorff, J. Gao, L. Gou, S.E. Hunyadi, T. Li, Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 109, 13857 (2005)
L. Gou, C.J. Murphy, Fine-tuning the shape of gold nanorods. Chem. Mater. 17, 3668 (2005)
C.J. Murphy, A.M. Gole, S.E. Hunyadi, C.J. Orendorff, One-dimensional colloidal gold and silver nanostructures. Inorg. Chem. 45, 7544 (2006)
B. Nikoobakht, M.A. El-Sayed, Preparation and growth mechanism of gold nanorods (nrs) using seed-mediated growth method. Chem. Matter 15, 1957 (2003)
Y. Sun, B.T. Mayers, Y. Xia, Template-engaged replacement reaction: a one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2, 481 (2002)
Y. Sun, B. Mayers, Y. Xia, Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process. Nano Lett. 3, 675 (2003)
J. Chen, J.M. McLellan, A. Siekkinen, Y. Xiong, Z.Y. Li, Y. Xia, Facile synthesis of gold-silver nanocages with controllable pores on the surface. J. Am. Chem. Soc. 128(46), 14776 (2006)
A.M. Schwartzberg, J.Z. Zhang, Novel optical properties and emerging applications of metal nanostructures. J. Phys. Chem C 128(28), 10323 (2008)
M. Ihara, K. Tanaka, K. Sakaki, I. Honma, K. Yamada, Enhancement of the absorption coefficient of cis-(NCS)2 Bis(2,2‘-bipyridyl-4,4‘-dicarboxylate)ruthenium(II) Dye in Dye-Sensitized solar cells by a silver island film. J. Phys. Chem. B. 101, 5153 (1997)
E.N. Economou, surface plasmons in thin films. Phys. Rev. 182, 539 (1969)
A.P. Alivisatos, Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933 (1996)
J. Spreadborough, J.W. Christian, J. Sci. Instru. 36, 116 (1959)
Y.P. Venkata Subbaiah, P. Prathap, K.T. Ramakrishna Reddy, Structural, electrical and optical properties of ZnS films deposited by close-spaced evaporation. Appl. Surf. Sci. 253, 2409 (2006)
D. Rajesh, C.S. Sunandana, Briefly brominated Ag thin films: XRD, FESEM, and optical studies of surface modification. Appl. Sur. Sci. 259, 276 (2012)
D. Rajesh, C.S. Sunandana, XRD, optical and AFM studies on pristine and partially iodized Ag thin film. Results Phys. 2, 22, (2012)
D. Rajesh, C.S. Sunandana, Controlled structural and optical properties of nano structured Ag films reshaped by brief iodization. Thin Solid Films 524, 316 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Rajesh, D., Sunandana, C.S. (2013). Structural and Optical Evaluation of Pristine and Partially Iodized Ag Thin Films. In: Giri, P.K., Goswami, D.K., Perumal, A. (eds) Advanced Nanomaterials and Nanotechnology. Springer Proceedings in Physics, vol 143. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-34216-5_28
Download citation
DOI: https://doi.org/10.1007/978-3-642-34216-5_28
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-34215-8
Online ISBN: 978-3-642-34216-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)