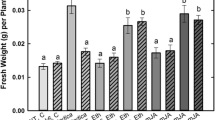
Abstract
In nature, plants are exposed to a large number of quite diverse microorganisms, which can be beneficial (mutualistic interaction), harmful (pathogenic interaction), or neutral (commensalistic interaction) for plant performance. Root-colonizing rhizobacteria, mycorrhizal fungi, or beneficial endophytes often promote plant growth and biomass production and establish tolerance against biotic and abiotic stresses.
We study the beneficial interaction between the growth- and biomass-promoting endophyte Piriformospora indica and plant roots. Using a genetic approach, we identified genes and proteins which are required for the beneficial interaction between the two symbionts. We isolated two classes of mutants: those which do not respond to P. indica and grow like uncolonized plants in the presence of the fungus and those for which P. indica was pathogenic. For some of these mutants, the genes were identified by map-based cloning strategies. Interestingly, several of these mutants were defective in defense compounds.
Here, we discuss four defense-related processes which are required for the beneficial interaction between P. indica and Arabidopsis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Defense in Beneficial Plant/Microbe Interactions: An Introduction
In nature, plants are exposed to a large number of quite diverse microorganisms, which can be beneficial (mutualistic interaction), harmful (pathogenic interaction), or neutral (commensalistic interaction) for plant performance. Root-colonizing rhizobacteria, mycorrhizal fungi, or beneficial endophytes often promote plant growth and biomass production and establish tolerance against biotic and abiotic stresses. They compete with soil-borne microbes which are harmful for the plant (Johnson and Oelmüller 2009). These two types of symbiotic interactions are extremes. Therefore, it is not surprising that the mode of interaction between two symbionts is never stable, and all types of transitions have been observed in nature, depending on environmental conditions and genetic programs. Genetic studies have uncovered that single gene loci in both plants and microbes determine the mode of interaction and manipulation of crucial genes may cause severe alterations in the symbiosis (Johnson and Oelmüller 2009 and references therein).
We study the beneficial interaction between the growth- and biomass-promoting endophyte Piriformospora indica and plant roots. The endophytic fungus, a basidiomycete of the Sebacinaceae family, interacts with many plant species, including Arabidopsis. Like other members of the Sebacinales, P. indica colonizes the roots, grows inter- and intracellularly, and forms pear-shaped spores in the roots as well as on the root surface. The endophyte promotes nutrient uptake, allows plants to survive under abiotic (water and salt) stress, confers resistance to toxins, heavy metal ions, and pathogenic organisms, and stimulates growth and seed production (cf. Oelmüller et al. 2004, 2005, 2009; Peškan-Berghöfer et al. 2004; Pham et al. 2004; Sahay and Varma 1999; Shahollari et al. 2005, 2007; Sherameti et al. 2005; Varma et al. 1999, 2001). P. indica is a cultivable fungus and can grow on synthetic media without a host (Peškan-Berghöfer et al. 2004; Varma et al. 2001; Verma et al. 1998). The host range includes trees, agricultural, horticultural, and medicinal plants, monocots, dicots, and mosses (Barazani et al. 2005; Glen et al. 2002; Peškan-Berghöfer et al. 2004; Shahollari et al. 2005, 2007; Sherameti et al. 2005; Varma et al. 2001; Waller et al. 2005; Weiss et al. 2004), suggesting that the interaction is based on general recognition and signaling processes.
Using a genetic approach, we identified genes and proteins which are required for the beneficial interaction between the two symbionts (Oelmüller et al. 2004; Shahollari et al. 2005, 2007; Sherameti et al. 2008; Camehl et al. 2010). For this screen, we used independent plant responses which are induced by P. indica in Arabidopsis roots and leaves (e.g. growth promotion, seed yield, resistance against drought or leaf pathogens, marker gene expression, protein phosphorylation pattern, spore germination) and identified those mutants in which these responses are not induced by the fungus. We isolated two classes of mutants: those which do not respond to P. indica and grow like uncolonized plants in the presence of the fungus and those for which P. indica was pathogenic (Camehl et al. 2010). For some of these mutants, the genes were identified by map-based cloning strategies. Interestingly, several of these mutants were defective in defense compounds. Their analysis uncovered that mutualism depends on a balanced activation of defense mechanisms. For some of the defense compounds, we also discovered novel functions in this beneficial symbiosis.
Defense responses have been intensively studied in beneficial microbial and mycorrhizal communications. As long as the microbial partner is not recognized as a friend (e.g. during early phases of mycorrhizal interaction, when the plants have not yet benefited from the fungus and nutrient exchange has not yet started), the plant often initiates defense responses against the symbiont. Güimil et al. (2005) have shown that over 40% of the genes in the roots of rice seedlings respond to beneficial and non-beneficial fungi and many of them are involved in plant defense. When the establishment of a beneficial symbiosis proceeds and the plant recognizes the microbial partner as a friend, the expression of defense genes becomes downregulated. The molecular mechanism which causes the shutdown of defense responses is unclear at present. It can be an active process initiated by either the plant or the fungus or simply a passive process since defense activating compounds are no longer present in the beneficial symbiosis (Harrison 2005).
Plant hormones such as salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) play major roles in regulating plant defense responses. Simplified, SA is involved in the reaction against biotrophic and hemi-biotrophic pathogens while JA and ET are associated with defense against necrotrophic pathogens and herbivorous insects. The two main defense mechanisms in plants are the systemic acquired resistance (SAR) where SA is essential and the induced systemic resistance (ISR) which is mainly based on ET and JA signaling. ISR results from colonization of roots by certain nonpathogenic bacteria (van Loon et al. 1998) but also friendly fungi such as Trichoderma spp. (Shoresh et al. 2010) or P. indica (Stein et al. 2008).
Furthermore, reactive oxygen species (ROS), in particular H2O2, are important signaling compounds in plants dealing with pathogenic microorganisms (Apel and Hirt 2004) but also with rhizobia (Puppo et al. 2005) or arbuscular mycorrhizal fungi (Fester and Hause 2005). Tanaka et al. (2006) have shown the important role of ROS in regulating the mutualistic interaction between a clavicipitaceous fungal endophyte, Epichloë festucae, and its grass host, Lolium perenne. E. festucae grows systemically in intercellular spaces of leaves as infrequently branched hyphae parallel to the leaf axis. A fungal mutant defective in a NADPH oxidase gene (noxA) altered the interaction from mutualistic to antagonistic. Plants infected with the noxA mutant lose apical dominance, become severely stunted, show precocious senescence, and eventually die. The fungal biomass in these associations is increased dramatically. ROS accumulation was detected cytochemically in the endophyte extracellular matrix and at the interface between the extracellular matrix and host cell walls of meristematic tissue in wild-type but not in noxA mutant associations. These results demonstrate that not only plant-synthesized ROS but also fungal ROS production is critical in mutualistic fungus–plant interactions, presumably by restricting root colonization.
These examples demonstrate that defense gene activation is an important aspect in beneficial symbioses, which is further highlighted by the identification of genes involved in defense processes in the P. indica/Arabidopsis screen outlined above. Here, we discuss four defense-related processes which are required for the beneficial interaction between P. indica and Arabidopsis.
2 PYK10
Glucosinolate biosynthesis plays an important role in plant/pathogen interactions (Halkier and Gershenzon 2006). Many genes for glucosinolate biosynthesis and/or degradation are upregulated in P. indica-colonized Arabidopsis roots. Several of them appear to be involved in establishing a mild defense response against the fungus. One of the genes codes for the putative myrosinase PYK10 (Nitz et al. 1999), an abundant protein in the roots of Brassicaceae. The putative β-glucosidase of 65 kDa is located in so-called endoplasmic reticulum (ER) bodies and contains the ER-retention signal KDEL (Matsushima et al. 2003b). ER bodies are spindle-shaped structures of ∼10 μm in length and ∼1 μm in width (cf. Matsushima et al. 2003a; Haseloff et al. 1997; Hawes et al. 2001; Hayashi et al. 1999; Ridge et al. 1999) which have been found in more than 50 plant species (Behnke and Eschlbeck 1978; Bones et al. 1989; Bonnett and Newcomb 1965; Gunning 1998; Iversen 1970). ER bodies are surrounded by ribosomes (Hayashi et al. 1999) and are highly enriched in the roots of young seedlings (Matsushima et al. 2002). Interestingly, ER bodies can also be induced in rosette leaves by JA (McConn et al. 1997), and the JA-insensitive coronatine insensitive1 (coi1; Xie et al. 1998) mutant does not form ER bodies (Matsushima et al. 2002). This suggests that PYK10 might be involved in JA-induced defense responses.
PYK10 has been identified as a target of P. indica in Arabidopsis roots (Peškan-Berghöfer et al. 2004). Within minutes after the contact of the roots with the fungus, a shift in the electrophoretic mobility of PYK10 can be observed on two-dimensional gels, suggesting that the protein becomes modified in response to signals from the fungus. We identified an EMS and a T-DNA insertion line, which are defective in PYK10 expression. These mutants do not respond to P. indica, indicating that PYK10 is required for the establishment of the beneficial interaction between the two symbionts (Sherameti et al. 2008). This observation is further supported by an independent mutant with a lesion in the transcription factor NAI1. The basic helix-loop-helix domain-containing transcription factor NAI1 is responsible for PYK10 expression. Closer inspection of plants with altered PYK10 levels uncovered that the putative myrosinase controls the degree of root colonization: lower PYK10 mRNA levels result in higher root colonization, while plants overexpressing PYK10 under the control of the 35S promoter are less colonized. Although the physiological role and natural substrate(s) of PYK10 are unknown at present, these observations suggest that enzymatic activities associated with PYK10 may restrict root colonization. Apparently, the beneficial interaction between Arabidopsis and P. indica is based on a highly sophisticated balance between the two symbiotic partners. It is conceivable that increasing quantities of fungal hyphae lead to a degree of root colonization that provokes plant defense responses and represses beneficial responses, whereas decreasing quantities of hyphae in the root environment result in suboptimal exchanges of information and nutrients between the two partners. This resembles mycorrhizal symbioses, in which initially activated defense responses against the symbiont are reduced during later phases of the interaction or are even actively repressed (cf. Pozo and Azcón-Aguilar 2007). Although not studied in detail, Zeng et al. (2003) have also shown that myrosinase activity controls the growth of ectomycorrhiza fungi.
β-glucosidases and myrosinases hydrolyze β-glucosidic bonds of aryl and alkyl β-d-glucosides, as well as glucosides with carbohydrate moieties such as cellobiose and other β-linked oligosaccharides (Esen 1993). In particular, myrosinases hydrolyze nontoxic glucosinolates to biologically active isothiocyanates, thiocyanates, nitriles, or epithionitriles (cf. Bones and Rossiter 1996; Poulton 1990; Rask et al. 2000; Wittstock and Halkier 2002), and the biological function of a myrosinase depends upon the nature of the aglycon moieties released from the substrates. A well-studied role of these agylcons is their involvement in plant defense against herbivores and microbes (Rask et al. 2000; Stotz et al. 1999, 2000; Tierens et al. 2001; Sanchez-Vallet et al. 2010). PYK10 is released from the endosomal system and reacts with PBP1, forming a multimeric complex. Thus, the substrate(s) of PYK10 is likely to be separated from the enzyme through membranes (cf. references in Nagano et al. 2005), and destruction of the cell and cellular compartments is required to bring these components together. One might speculate that this occurs during root colonization after the two organisms come into contact with each other. Overcolonization might result in more damage to the root cells and thus more activation of glucosinolate-based defense responses.
Although the role of PYK10 in the interaction between Arabidopsis and P. indica is unclear at present, the observation that Arabidopsis lines with reduced PYK10 protein levels are more susceptible to fungal colonization/association supports the idea that the enzyme is involved in defending the root cells against an excess of invading hyphae, which could result in a disturbance of the balanced mutualistic interaction. PYK10 exhibits striking sequence similarities to PEN2, a glycosyl hydrolase, which restricts pathogen entry of two ascomycete powdery mildew fungi into Arabidopsis leaf cells (Lipka et al. 2005). Like PEN2, PYK10 belongs to the class of glycosyl hydrolase family 1. Both proteins are located in intracellular organellar structures (PYK10 in ER bodies and PEN2 in peroxisomes), and both proteins share a high degree of sequence similarity. The catalytic domains of both proteins contain two conserved nucleophilic glutamates. Lipka et al. (2005) have shown that glutamate183 is required for PEN2 function in vivo, which suggests that PEN2 catalytic activity is required for restricting pathogen entry. Thus, PYK10 might have a similar biological function in the P. indica/Arabidopsis system.
An important task for the future will be to understand the function of PYK10, one of the most abundant protein in Brassica roots. It is not known whether the enzyme has myrosinase activity. Furthermore, the appropriate substrate(s) and product(s) need to be identified.
3 ET Signaling Components
ET and JA often function synergistically in plant defense response. Defense genes such as PLANT DEFENSIN 1.2 (PDF1.2) and PATHOGENESIS-RELATED PROTEIN (PR)-3 encoding the basic chitinase are activated against necrotrophic fungi primarily by the ET/JA pathway. Both hormones are also required for the ISR which is triggered by beneficial rhizobacteria and fungi (Pieterse et al. 1998; van Wees et al. 2008). In contrast, biotrophic pathogens are more efficiently countered by SA-controlled defense mechanisms (Thomma et al. 1998, 1999) and the activation of PR-1, PR-2, and PR-5.
We identified Arabidopsis mutants which are smaller in the presence of the fungus compared to the uncolonized control. This suggests that these mutants consider P. indica as a foe and that the interaction is shifted from mutualism to parasitism. Several mutated genes were identified as components of the ET signaling pathway (Camehl et al. 2010).
ET is perceived by a family of membrane-associated two-component systems at the ER, including ETR1/ETR2, ET response sensor (ERS) 1 and 2, and EIN4 in Arabidopsis (Chang et al. 1993; Hua et al. 1995; Hua and Meyerowitz 1998; Sakai et al. 1998). EIN2, EIN3, EIN5, and EIN6 are positive regulators of ET responses, acting downstream of CTR1. CTR1 derepresses EIN2, and this leads to the activation of EIN3 and EIN3-like (EIL) transcription factors. EIN2 is an integral membrane protein of unknown function with similarities to NRAMP metal transporters (Alonso et al. 1999). Growth of etr1, ein2, and ein3/eil1 plants is not promoted or even inhibited by the fungus. The plants produce less seeds, and the roots are more colonized compared to the wild type roots. This results in a mild activation of defense responses. These results clearly demonstrate that restriction of fungal growth by ET signaling components is required for the beneficial interaction between the two symbionts. Furthermore, overexpression of the ETHYLENE RESPONSE FACTOR1 (ERF1) constitutively activates defense responses, which also abolishes the benefits for the plants. Therefore, ET signaling components and ET-targeted transcription factors are required for balancing beneficial and non-beneficial traits in the symbiosis. Manipulation of signaling components of this pathway, including crucial target transcription factors, results in an unstable symbiosis which is no longer beneficial for the plant (Camehl et al. 2010).
Several questions remain unanswered. The exact target genes of the ET signaling pathway, which are required for the establishment of the beneficial interaction, are still unknown. Furthermore, it is interesting to note that growth promotion is abolished at the seedlings level. Thus, ET signaling is already required during early phases of the interaction. Again, a link between ET signaling and the control of the growth response is unknown. Finally, we and others have demonstrated that leaves of P. indica-colonized plants are more resistant against leaf pathogens. It is likely that the information flow from the roots to the leaves is mediated by a mechanism that resembles an ISR response (Pieterse and Van Loon 2004). Stein et al. (2008) have shown that P. indica SAR in Arabidopsis requires JA signaling and the cytoplasmic function of NPR1. Since the ISR in Arabidopsis depends on ET and JA signaling, a putative role of ET in the P. indica-induced ISR needs to be defined.
4 OXI1/PDK1
One of the mutants which do not respond to P. indica has a lesion in OXI1. OXI1 is a serine/threonine kinase which is necessary for oxidative burst-mediated signaling in Arabidopsis roots (Anthony et al. 2004; Rentel et al. 2004). The enzyme is a member of the AGC protein kinase family and was originally identified because its expression was induced by H2O2 in vivo (Rentel et al. 2004). OXI1 is required for full activation of the two MITOGEN-ACTIVATING PROTEIN KINASES 3 and 6 after treatment with ROS or elicitors and for different ROS-mediated processes including basal resistance to Peronospora parasitica infection and root hair growth (Rentel et al. 2004). Besides ROS, OXI1 is also activated by the PHOSPHOLIPID-BINDING KINASE (PDK)1 (Anthony et al. 2004). The main phospholipid in plants is phosphatidic acid (PA) which functions as a second messenger in many stress response pathways. The active OXI1 phosphorylates and thus activates the downstream serine/threonine kinase PTI1-2 in response to ROS and PA signals (Anthony et al. 2006), and many of these signals derive from microbial pathogens or elicitors, such as cell wall fragments or specific protein factors released by pathogens (van der Luit et al. 2000; Yamaguchi et al. 2005).
P. indica does not induce ROS production in Arabidopsis roots (Vadassery et al. 2009a), while the PA level is stimulated. Furthermore, under beneficial, growth-promoting cocultivation conditions, defense genes are downregulated in Arabidopsis. Genetic studies established the PA-activated PDK1-OXI1 pathway as a novel signaling event which is crucial for a beneficial interaction between the two symbionts. Thus, like in mammalian systems, this pathway is required for P. indica-induced growth promotion and proliferation rather than activation of defense processes. Even under non-beneficial cocultivation conditions of the two symbionts, activation of defense genes is independent of the PA-PDK1-OXI1 pathway. This novel function of the originally identified defense pathway and the role of AGC kinase in beneficial plant/microbe interactions may be of general importance (cf. Pislariu and Dickstein 2007).
5 AtHSPRO2
In our screen for Arabidopsis mutants which recognizes P. indica as a pathogen rather than a beneficial fungus, we identified hspro2. HSPRO2 is required for basal resistance against the bacterial pathogen Pseudomonas syringae pv. tomato. The Arabidopsis protein exhibits striking sequence similarities to a nematode resistance protein from Beta procumbens (Cai et al. 1997). HSPRO2 appears to function downstream of SA and is negatively regulated by signaling through JA and ET (Murray et al. 2007). We are only at the beginning to understand the role of this protein in pathogenic and beneficial plant–microbe interactions.
6 Conclusions
The initially activated defense response of a plant against beneficial microbes resembles that against pathogenic microbes. The same genes and signaling pathways are activated by microbe-associated molecular patterns from pathogens and beneficial fungi. We show that defense gene activation plays a crucial role in the beneficial symbiosis between Arabidopsis and P. indica. These defense responses appear to function at different levels (Fig. 14.1). Glucosinolates and enzymes involved in their breakdown appear to be activated only after cell damage. This may occur, at least to some extent, if the fungus enters the plant cell. It is conceivable that this defense strategy becomes important if uncontrolled hyphal growth occurs in the roots. Reduced levels of PYK10, for instance, allows overcolonization of the root cells, presumably because the roots cannot restrict hyphal growth. Quite similar, ET signaling controls the interaction between the two symbionts at early stages. Inactivation of components of the ET pathway has a severe impact on root colonization, whereas higher ET signaling represses and lower ET signaling promotes hyphal growth. Overcolonization of the roots is associated with a mild defense response for the restriction of fungal growth. On the other hand, overexpression of ERF1 induces a mild defense response, which also restricts root colonization. As a consequence, the roots are less colonized as wild-type roots, which—in turn—is less beneficial for the plant. These two examples support classical concepts developed for mycorrhizal symbiosis that some defense strategies are necessary for long-term harmony between symbionts.
In contrast, the PDK1/OXI1 pathway, previously identified to activate defense responses against pathogens, appears to have a different function in beneficial interactions. The kinases are required for long-term harmony between the two symbionts, and they are not involved in defense gene activation against or restriction of hyphal growth of P. indica. Finally, the role of HSPRO2 in the P. indica/Arabidopsis interaction is unclear. All available information suggests that HSPRO2 has another/additional function than activating defense processes against P. indica.
Taken together, a sophisticated network of defense responses which need to be active in a time- and space-dependent manner is required to maintain a beneficial P. indica/Arabidopsis symbiosis. The relative large number of mutants which have been identified in our screen demonstrates that defense components are crucial for this beneficial symbiosis. While some of them activate mild defense responses, the function of other is not yet understood.
References
Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284:2148–2152
Anthony RG, Henriques R, Helfer A, Mészáros T, Rios G, Testerink C, Munnik T, Deák M, Koncz C, Bögre L (2004) A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J 23:572–581
Anthony RG, Khan S, Costa J, Pais MS, Bögre L (2006) The Arabidopsis protein kinase PTI1-2 is activated by convergent phosphatidic acid and oxidative stress signaling pathways downstream of PDK1 and OXI1. J Biol Chem 281:37536–3746
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Barazani O, Benderoth M, Groten K, Kuhlemeier C, Baldwin IT (2005) Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146:234–243
Behnke H-D, Eschlbeck G (1978) Dilated cisternae in Capparales—an attempt towards the characterization of a specific endoplasmic reticulum. Protoplasma 97:351–363
Bones AM, Rossiter JT (1996) The myrosinase-glucosinolate system, its organization and biochemistry. Physiol Plant 97:194–208
Bones AM, Evjen K, Iversen T-H (1989) Characterization and distribution of dilated cisternae of the endoplasmic reticulum in intact plants, protoplasts, and calli of Brassicaceae. Isr J Bot 38:177–192
Bonnett HT Jr, Newcomb EH (1965) Polyribosomes and cisternal accumulations in root cells of radish. J Cell Biol 27:423–432
Cai D, Kleine M, Kifle S, Harloff HJ, Sandal NN, Marcker KA, Klein-Lankhorst RM, Salentijn EM, Lange W, Stiekema WJ, Wyss U, Grundler FM, Jung C (1997) Positional cloning of a gene for nematode resistance in sugar beet. Science 275:832–834
Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J, Oelmüller R (2010) Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and non-beneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol 185:1062–1073
Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262:539–544
Esen A (1993) β-glucosidases: overview. In: Esen A (ed) B-Glucosidases. Biochemistry and molecular biology. American Chemical Society, Washington, DC, pp 1–14
Fester T, Hause G (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15:373–379
Glen M, Tommerup IC, Bougher NL, O'Brien PA (2002) Are Sebacinaceae common and widespread ectomycorrhizal associates of Eucalyptus species in Australian forests? Mycorrhiza 12:243–247
Güimil S, Chang HS, Zhu T, Sesma A, Osbourn A, Roux C, Ioannidis V, Oakeley EJ, Docquier M, Descombes P, Briggs SP, Paszkowski U (2005) Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc Natl Acad Sci USA 102:8066–8070
Gunning BES (1998) The mystery organelles in Arabidopsis expressing GFP. Trends Plant Sci 3:417
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Harrison MJ (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Hawes C, Saint-Jore C, Martin B, Zheng H-Q (2001) ER confirmed as the location of mystery organelles in Arabidopsis plants expressing GFP. Trends Plant Sci 6:245–246
Hayashi M, Toriyama K, Kondo M, Hara-Nishimura I, Nishimura M (1999) Accumulation of a fusion protein containing 2S albumin induces novel vesicles in vegetative cells of Arabidopsis. Plant Cell Physiol 40:263–272
Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271
Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269:1712–1714
Iversen T-H (1970) Cytochemical localization of myrosinase (β-thioglucosidase) in root tips of Sinapis alba. Protoplasma 71:451–466
Johnson JM, Oelmüller R (2009) Mutualism and parasitism: life in an unstable continuum. What can we learn from the mutualistic interaction between Piriformospora indica and Arabidopsis thaliana? Endocytobiol Cell Res 19:81–111
Lipka V, Dittgen J, Bednarek P et al (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Matsushima R, Hayashi Y, Kondo M, Shimada T, Nishimura M, Hara-Nishimura I (2002) An endoplasmic reticulum-derived structure that is induced under stress conditions in Arabidopsis. Plant Physiol 130:1807–1814
Matsushima R, Hayashi Y, Yamada K, Shimada T, Nishimura M, Hara-Nishimura I (2003a) The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis. Plant Cell Physiol 44:661–666
Matsushima R, Kondo M, Nishimura M, Hara-Nishimura I (2003b) A novel ER-derived compartment, the ER body, selectively accumulates a β-glucosidase with an ER retention signal in Arabidopsis. Plant J 33:493–502
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94:5473–5477
Murray SL, Ingle RA, Petersen LN, Denby KJ (2007) Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol Plant Microbe Interact 20:1431–1438
Nagano AJ, Matsushima R, Hara-Nishimura I (2005) Activation of an ER-body-localized β-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol 46:1140–1148
Nitz I, Berkefeld H, Puzio PS, Grundler FMW (1999) Pyk10, a seedling and root specific gene and promoter from Arabidopsis thaliana. Plant Sci 161:337–346
Oelmüller R, Shahollari B, Peškan-Berghöfer T, Trebicka A, Giong PH, Sherameti I, Oudhoff M, Venus Y, Altschmied L, Varma A (2004) Molecular analyses of the interaction between Arabidopsis roots and the growth-promoting fungus Piriformospora indica. Endocytobiosis Cell Res 15:504–517
Oelmüller R, Peškan-Berghöfer T, Shahollari B, Sherameti I, Varma A (2005) MATH-domain containing proteins represent a novel gene family in Arabidopsis thaliana and are involved in plant/microbe interactions. Physiol Plant 124:152–166
Oelmüller R, Sherameti I, Tripathi S, Varma A (2009) Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 49:1–17
Peškan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Varma A, Oelmüller R (2004) Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant–microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 122:465–477
Pham GH, Kumari R, An S et al (2004) Axenic cultures of Piriformospora indica. In: Varma A, Abbott L, Werner D, Hampp R (eds) Plant surface microbiology. Springer, Berlin, Heidelberg, pp 593–616
Pieterse CM, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7:456–464
Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10:1571–1580
Pislariu CI, Dickstein R (2007) An IRE-like AGC kinase gene, MtIRE, has unique expression in the invasion zone of developing root nodules in Medicago truncatula. Plant Physiol 144:682–694
Poulton JE (1990) Cyanogenesis in plants. Plant Physiol 94:401–405
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165:683–701
Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol 42:93–113
Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, Knight MR (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427:858–861
Ridge RW, Uozumi Y, Plazinski J, Hurley UA, Williamson RE (1999) Developmental transitions and dynamics of the cortical ER of Arabidopsis cells seen with green fluorescent protein. Plant Cell Physiol 40:1253–1261
Sahay NS, Varma A (1999) Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett 181:297–302
Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95:5812–5817
Sanchez-Vallet A, Ramos B, Bednarek P, López G, Piślewska-Bednarek M, Schulze-Lefert P, Molina A (2010) Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant J 63:115–127
Shahollari B, Varma A, Oelmüller R (2005) Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J Plant Physiol 162:945–958
Shahollari B, Vadassery J, Varma A, Oelmüller R (2007) A leucine-rich repeat protein is required for growth promotion and enhanced seed production mediated by the endophytic fungus Piriformospora indica in Arabidopsis thaliana. Plant J 50:1–13
Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R (2005) The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor which binds to a conserved motif in their promoters. J Biol Chem 280:2641–2647
Sherameti I, Venus Y, Drzewiecki C, Tripathi S, Dan VM, Nitz I, Varma A, Grundler FM, Oelmüller R (2008) PYK10, a β-glucosidase located in the endoplasmic reticulum, is crucial for the beneficial interaction between Arabidopsis thaliana and the endophytic fungus Piriformospora indica. Plant J 50:1–17
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48:21–43
Stein E, Molitor A, Kogel KH, Waller F (2008) Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol 49:1747–1751
Stotz HU, Kroymann J, Mitchell-Olds T (1999) Plant–insect interactions. Curr Opin Plant Biol 2:268–272
Stotz HU, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Bauke A, Mitchell-Olds T (2000) Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against cotton worm but not diamondback moth. Plant Physiol 124:1007–1017
Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B (2006) Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 18:1052–1066
Thomma B, Eggermont K, Penninckx I, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95:15107–15111
Thomma BP, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19:163–171
Tierens KF, Thomma BP, Brouwer M, Schmidt J, Kistner K, Porzel A, Mauch-Mani B, Cammue BP, Broekaert WF (2001) Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol 125:1688–1699
Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R (2009a) A cell wall extract from Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J 59:193–206
van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T (2000) Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol 123:1507–1516
van Loon LC, Bakker PA, Pieterse CM (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
van Wees SC, Van der Ent S, Pieterse CM (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11:443–448
Varma A, Verma S, Sudha S, Sahay NS, Butehorn B, Franken P (1999) Piriformospora indica, a cultivable plant growth promoting root endophyte. Appl Environ Microbiol 65:2741–2744
Varma A, Singh A, Sudha S et al (2001) Piriformospora indica: a cultivable mycorrhiza-like endosymbiotic fungus. In: Hock B (ed) Mycota IX. Springer, Berlin, pp 123–150
Verma SA, Varma A, Rexer K-H, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütehorn B, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90:898–905
Waller F, Achatz B, Baltruschat H et al (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102:13386–13391
Weiss M, Selosse MA, Rexer KH, Urban A, Oberwinkler F (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol Res 108:1003–1010
Wittstock U, Halkier BA (2002) Glucosinolate research in the Arabidopsis era. Trends Plant Sci 7:263–270
Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Yamaguchi T, Minami E, Ueki J, Shibuya N (2005) Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol 46:579–587
Zeng RS, Mallik AU, Setliff E (2003) Growth stimulation of ectomycorrhizal fungi by root exudates of Brassicaceae plants: role of degraded compounds of indole glucosinolates. J Chem Ecol 29:1337–1355
Acknowledgements
We thank IMPRS and DFG for funding and Corinna Drzewiecki for support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Camehl, I., Sherameti, I., Seebald, E., Johnson, J.M., Oelmüller, R. (2013). Role of Defense Compounds in the Beneficial Interaction Between Arabidopsis thaliana and Piriformospora indica . In: Varma, A., Kost, G., Oelmüller, R. (eds) Piriformospora indica. Soil Biology, vol 33. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33802-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-33802-1_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33801-4
Online ISBN: 978-3-642-33802-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)