Abstract
The present study deals with the retrieval and analysis of O3 total columns over the Évora Observatory (South of Portugal) for the period 2007–2010. The data-set presented in this paper is derived from spectral measurements carried out with the UV–Vis. Spectrometer for Atmospheric Tracers Measurements—SPATRAM, installed at the Observatory of the Geophysics Centre of Évora (CGE) –Portugal (38.5ºN; 7.9 ºW, 300 m asl). The results obtained applying Differential Optical Absorption Spectroscopy (DOAS) methodology to the SPATRAM measurements of zenith sky scattered radiation are presented in terms of seasonal variations of O3. The O3 retrieved with SPATRAM instrument confirms the typical seasonal cycle for middle latitudes reaching the maximum during the spring and the minimum during the autumn. The ground-based results obtained for O3 column are also compared with data from Ozone Monitoring Instrument (OMI) instrument onboard Aura Satellite.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stratospheric Ozone
- Total Ozone Column
- Ozone Monitoring Instrument
- Differential Optical Absorption Spectroscopy
- Aura Satellite
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Ozone (O3) is a key compound in the chemistry of the atmosphere. This trace gas represents only about 0.0012 % of the total atmospheric composition but it is involved in several atmospheric phenomena (Antón et al. 2011; Bortoli et al. 2009). For example, ozone plays a relevant role in controlling the chemical composition of the atmosphere. The photolysis of ozone near 300 nm followed by reaction with water leads to production of OH. OH radicals are sometimes called the “cleansing agent” of the atmosphere and therefore a variety of atmospheric species like CO, CH4, NO2 and halocarbons are removed from the atmosphere by reaction with it. Because ozone absorbs thermal radiation it also plays an important role in the energy budget of the troposphere (Platt and Stutz 2008).
While the tropospheric ozone is known as the “bad ozone” for it is a secondary pollutant and its capacity to alter the global climate system, the stratospheric ozone is known for being the “good ozone”. The motives are well known. In the troposphere O3 is harmful to humans and plants and it is a component of smog and an important greenhouse gas. The stratospheric ozone layer, containing 90 % of the atmospheric ozone, is responsible for the absorption of ultraviolet (UV) radiation, particularly UV-B (320–280 nm) and UV-C above 220 nm (Staehelin et al. 2001), harmful for animals and plants.
Ozone is formed by two different mechanisms in the stratosphere and troposphere. In the stratosphere O2 molecules are split by short-wave UV radiation into O atoms which combine with O2 to form O3. This process is the kernel of the Chapman Cycle (Platt and Stutz 2008). On the other hand the tropospheric ozone is formed by reactions involving NOx (NO + NO2) and Volatile Organic Compounds (VOC).
In the troposphere, NO2 is decomposed by solar radiation as shown in Eq. 1
where hν is the energy of solar radiation. Then other reaction (Eq. 2) follows Eq. 1. The O atom combines with O2 in presence of other atmospheric molecule M.
In the end O3 is oxidised by NO leading to NO2 formation (Eq. 3)
The chemical conversion of NO to NO2 is the fundamental factor in the tropospheric O3 formation. If this conversion occurs without the ozone but with other reaction cycles of hydroxyl (HOx), NOx and peroxy radicals there isn’t a net formation of ozone. It is important to note the contribution of anthropogenic sources of NOx in the atmosphere such as combustion of fossil fuel (power stations, industry, and automobiles), forest fires and artificial fertilization. All these sources enhance the presence of NOx in the atmosphere that contributes to the increase in the O3 concentration (Platt and Stutz 2008; Logan 1985).
Increases in ozone are a concern to the community in part because of the adverse effects of the gas on vegetation and human health, but also because the changes in ozone could affect the concentration of OH which in turn could influence the concentration of the other trace gases removed from the atmosphere by reactions with OH.
Concerning the stratosphere S. Chapman proposed in the 1920 s the following mechanism to the stratospheric ozone formation which starts with the photolysis of oxygen molecules (Eq. 4) for radiation with wavelengths below 242 nm.
After that reaction the oxygen atoms (O (3P)) can: (a) combine with an oxygen molecule to form ozone with the collision with a third body that can be N2 or O2 (Eq. 5); (b) can react with an existing ozone molecule (Eq. 6) or (c) combine with other oxygen atoms to form O2 (Eq. 7).
The photolysis of O3, as described in Eq. 8, also provides the O atoms at a much higher rate than Eq. 1.
The above set of reactions explains the steady state of O3 concentration in the atmosphere in which the production of O atoms is in balance with their destruction.
During the 1960s, studies revealed that some other chemical reactions were taking place in the atmosphere beyond the “Chapman Reactions”. In fact there are many other trace gas cycles that affect the O3 concentrations in atmosphere, like ClO, BrO, NO2, HO2 and halocarbon species as CH3Cl, CFCl3. The monitoring of ozone levels gained more importance since the discovering of the depletion in ozone layer—the so called “Ozone Hole” over Antarctica. Nowadays, the interest in studying ozone and other gases directly or indirectly involved in the ozone chemical cycles is one of the main topics of the scientific community. Atmospheric compounds, such as NO2 and BrO, are monitored with ground based instruments as well as with satellite and airborne equipments. Among the different data analysis techniques, the Differential Optical Absorption Spectroscopy (DOAS) is recognized as a powerful tool for the retrieval of the columnar abundances of ozone and other compounds playing a key role in the ozone chemical cycles. DOAS uses the interaction of radiation with matter to determine the presence and abundance of molecules or atoms in a sample and it is based on the Lambert–Beer extinction law. The description of this spectroscopic technique can be found in literature (Platt and Stutz 2008).
The scope of this work is the retrieval of O3 total columns during 2007–2010 from spectral measurements carried out with the UV–Vis. Spectrometer for Atmospheric Tracers Measurements (SPATRAM) installed at the Observatory of the Geophysics Centre of Évora (CGE)-Portugal. In the next sections the description of the SPATRAM instrument and specifics of the data processing methodology used in the retrieval and analysis of O3 total columns over the Évora Observatory are presented. The results are also discussed and compared with the Ozone Monitoring Instrument (OMI) data.
2 Instrumental Setup and Method
2.1 SPectrometer for Atmospheric TRAcers Measurements
The multipurpose UV–Vis. SPATRAM (Bortoli et al. 2010) (Fig. 1c) is a scanning spectrometer and it measures the zenith scattered radiation in the 250–900 nm spectral range. The main products are the daily total column and the vertical profiles of NO2 and O3. The monochromator equipping SPATRAM allows for the decomposition of light in its wavelengths using a grating by Jobin–Yvon of 1200 grooves/mm with a typical dispersion of 2.4 nm/mm at 300 nm and spectral resolution ranging from of 0.05–0.2 nm depending on the spectral interval analyzed.
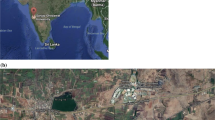
The location of the Observatory (b) of the Geophysics Centre of Évora- CGE in Évora (38.5ºN; 7.9 ºW, 300 m asl), Portugal (a) (adapted from http://maps.google.com/). The SPATRAM (c) instrument installed at the Observatory of the CGE inside a container (red circle) and the VErtical LOoking Device- VELOD (d), installed on the top of the container, which connects to the Optic Fiber Input device of SPATRAM
The equipment (Fig. 1c) installed at Geophysics Centre Observatory in Évora (Fig. 1b) allows for multiple input of the radiation: (1) from the Optic Tower Input (Fig. 2) which is the primary input that is composed by a pair of flat and spherical mirrors that focus the light beam on the entrance slit; (2) from the two lateral inputs called Optic Fiber Inputs (Fig. 2), where the signal is carried to the entrance slit with an optical fiber connected to a very simple optical system, and (3) an additional input for spectral and radiometric calibration. In the optic fiber input the radiation is collected by the VELOD (VErtical LOoking Device) (Fig. 1c). The light beam reaches the monochromator thanks to the title mirror in the Rotating Mirror Module (Fig. 2). This module allows for choosing between the primary input (pointing to the vertical), the optical fiber ones and the additional input for spectral or radiometric calibration. Once the light beam reaches the monochromator is decomposed in their wavelengths thanks to the holographic grating. The latter rotates by means of a stepper motor attached to the grating that allows for the analysis of the whole spectral interval of about 60 nm windows. The radiation is finally collected by a mirror that sends it to the CCD sensor. The CCD sensor is composed of 1024 × 254 pixels over the length of 1 inch and each pixel has the dimension of 24 m, giving a full dimension of 25.4 × 6.6 mm.
Representation of the main modules of the SPATRAM a monochromator, b ventilated box containing the CCD sensor, c Electronic Control Unit, d optic tower input, e optic fiber inputs, f rotating mirrors (Adapted from Bortoli 2009)
The SPATRAM is handled by a software tool (DAS—Data Acquisition System) that was developed in order to manage all the spectrometer devices and for automatic schedule measurements in automatic mode.
The Electronic Control Unit (ECU) of the instrument composed by all the power sources, CCD camera drivers, stepper motors and an industrial mono-board CPU provides also the storage of the measured spectral data as well as the pre-processing of the data and of their first analysis. DOAS methodology is applied to the spectral data measured with SPATRAM.
The output of DOAS algorithms is the Slant Column Density (SCD) which is defined as the integral of the number density along the path of measurement in the atmosphere. The unit of SCD used in this work is molecules per cm2. An important step in the interpretation of DOAS observations is the conversion of SCD in Vertical Column Densities (VCD) using calculated Air Mass Factors (AMF). The AMF is obtained using a Radiative Transfer Model (RTM) (Platt and Stutz 2008; Rozanov et Rozanov 2010). In this particular case the AMF calculation is performed with the Atmospheric Model for Enhancement Factor Computation (AMEFCO) (Petritoli et al. 2002). This RTM is based on the Intensity Weighted Optical Path (IWOP) approach considering a single scattering diffusion process and the a priori assumption of the vertical ozone profile.
2.2 Ozone Monitoring Instrument
OMI onboard the National Aeronautics and Space Administration’s Earth (NASA) Observing System (EOS) Aura satellite, on flight from 15 July 2004, is a nadir viewing imaging spectrograph that measures the solar radiation backscattered by the Earth’s atmosphere and surface over the entire wavelength range from 270 to 500 nm with a spectral resolution of about 0.5 nm and with a very high spatial resolution (13 × 24 km) and daily global coverage. Trace gases derived from OMI instrument include O3, NO2, SO2, HCHO, BrO and OClO (Levelt et al. 2006). The OMI total ozone column data used in this work were obtained with OMI-TOMS algorithm based on the TOMS V8 algorithm. This algorithm uses measurements at 4 discrete 1 nm wide wavelength bands centered at 313, 318, 331 and 360 nm (Kroon et al. 2008).
3 Data Analysis and Results
In this section the diurnal and seasonal evolution of O3 VCD values, for the period comprised between 1st January of 2007 and 6th July of 2010 obtained at Évora-Portugal with the SPATRAM instrument, are presented and discussed. Comparisons of these ground-based measurements with satellite retrievals of O3 VCD values derived from OMI instrument are also shown and examined.
3.1 Daily Variation of Ozone
To obtain the O3 SCD the DOAS algorithms are applied in the 320–340 nm spectral range. The data obtained with SPATRAM instrument comprises all the solar zenith angles (SZAs) between the highest daily solar elevation (minimum SZA) and approximately 93º of SZA. Due to the wavelength dependency of the AMF and since the lowest error associated to the measurements is obtained for the higher values of SZA, the O3 VCD are interpolated for the SZA of 87º. In order to investigate the typical diurnal variation of O3, the SCD of this gas is plotted versus the SZA during one day, as shown in Fig. 3.
The O3 SCD values are very similar during the morning and afternoon. It is evident, by the analysis of Fig. 3, that for ozone the differences between AM and PM values are almost absent. In fact, the diurnal photochemical activity of the stratospheric ozone is not as strong as, for example, the NO2 diurnal photochemical activity, so the PM data series present approximately the same values as the AM ones. The same diurnal behavior is registered on the other days of the study period.
3.2 Seasonal Variation of Ozone
In Fig. 4 the O3 VCD time series are plotted versus the time of the year in order to examine the seasonal behavior of this gas. In addition the OMI O3 values are compared with the O3 VCD values derived from SPATRAM instrument for the same period. The results obtained using the SPATRAM instrument corroborate the well-known fact that the maximum of O3 VCD values in mid-latitudes are found in spring and the minimum values in fall. This O3 cycle is seen in all years with the maximum of monthly mean values (± one standard deviation) registered in May 2007 (373 ± 26) DU, April 2008 (336 ± 6) DU, May 2009 (353 ± 9) DU and April 2010 (399 ± 11) DU and the minimum registered in October 2007 (293 ± 12) DU, January 2008 (242 ± 18) DU, September 2009 (304 ± 11) DU and January 2010 (302 ± 38) DU. There were no data available during the time period between 16th October and 15th December of 2007 due to maintenance of the spectrometer. In summer periods the O3VCD values have tendency to decrease as autumn approaches, but in 2008 this reduction is not so pronounced as in 2007, as it can be seen in Fig. 4.
The seasonal behavior of O3 registered at Évora′s Observatory is consistent with the literature for the northern mid-latitudes (Antón et al. 2011; Schmalwieser et al. 2003; Chen and Nunez 1998). Although the explanation of total ozone tendency has still not been quantified, the likely contribution processes have been identified. In the middle-latitudes and specifically over the Iberian Peninsula the total ozone column presents a strong seasonal variability mainly caused by dynamical factors such as Dobson-Brewer circulation (Antón et al. 2008, 2009, Staehelin et al. 2001, Weber et al. 2011).
The comparison between the SPATRAM and the OMI data describes the same seasonal behavior. However, the values of O3 VCD are normally lower than the ones derived from the OMI satellite instrument. The mean deviation, of the comparison, calculated using the relative error expression, is of about 7 % with higher values during the spring seasons. The main reason for the differences found between the SPATRAM and OMI instruments could be due to the fact that the OMI data presented in Fig. 1, are the O3 total column values corresponding to the daily overpass of the AURA satellite over a pixel of about 300 Km2 containing the Évora station while the SPATRAM Field Of View (FOV), determined by the monochromator f number (f# = 5), is of about 1 × 10−5 sr.
In Fig. 5 the scatter plot of the SPATRAM and OMI dataset is shown. In this plot only the data from the OMI time series obtained for a distance lower than 50 km from the Évora station are taken into account and are obtained from the daily overpass file analyzing the geo-references of each satellite ground pixel. The SPATRAM data and the “filtered” OMI dataset are in good agreement as evidenced from the correlation coefficient obtained (R = 0.83). Considering the full OMI dataset the R decrease to a value of 0.7.
4 Conclusions
The main purpose of this study is the use of the SPATRAM and DOAS methodology for the retrieval of ozone column content at Évora Station from 1st January of 2007 to 6th July of 2010. The comparison of the ground- based SPATRAM derived O3 VCD data with the correspondent satellite (OMI) O3 measurements was also envisaged. The daily variation of the O3 data derived from the SPATRAM reveals that a) the O3 concentration during the day is approximately uniform and b) the O3 seasonal trend shows a maximum in spring months and a minimum in autumn, in the North Hemisphere as expected. The comparison of the SPATRAM data—O3 VCD- with the satellite data (OMI instruments) highlights that the SPATRAM data presents similar patterns as the satellite measurements. The O3 VCD deviation is about 7 %.
References
Antón M, Bortoli D, Costa MJ, Kulkarni PS, Domingues AF, Barriopedro D, Serrano A, Silva AM (2011) Temporal and spatial variabilities of total ozone column over Portugal. Remote Sens Environ 115:855–863
Antón M, López M, Vilaplana JM, Kronn M, Bañon M, Serrano A (2009) Validation of OMI-TOMS and OMI-DOAS total ozone column using five brewer spectroradiometers at the Iberian Peninsula. J Geophys Res 114:D14302
Anton M, Serrano A, Cancillo ML, Garcia JA (2008) Total ozone and solar erythemal irradiance in the Southwestern Spain: Day-to-day variability and extreme episodes. Geophys Res Lett 35:L20804
Bortoli D, Silva AM, Costa MJ, Domingues AF, Giovanelli G (2009) Monitoring of atmospheric ozone and nitrogen dioxide over the south of Portugal by ground-based and satellite observations. Opt Express 17(15):12944–12959
Bortoli D, Silva AM, Giovanelli G (2010) A new multipurpose UV-Vis spectrometer for air quality monitoring and climatic studies. Int J Remote Sens 31(3):705–725
Chen D, Nunez M (1998) Temporal and spatial variability of total ozone in southwest Sweden revealed by two ground-based instruments. Int J Climatol 18:1237–1246
Kroon M, Veefkind JP, Sneep M, Mcpeters RD, Bhartia PK, Levelt PF (2008) Comparing OMI-TOMS and OMI-DOAS total ozone column data. J Geophys Res 113:D16S28
Levelt PF, Van den oord G, Dobber MR, Mälkki A, Visser H, Vries J, Stammes P, Lundell JOV, Saari H (2006) The Ozone Monitoring Instrument. IEEE Trans Geosci Remote Sens 44(5):1093–1101
Logan J (1985) Tropospheric ozone: seasonal behaviour, trends and anthropogenic influence. J Geophys Res 90(D6):10463–10482
Petritoli A, Giovanelli G, Ravegnani F, Bortoli D, Kostadinov I, Oulanovsky A (2002) Off-axis measurements of atmospheric trace gases from an airborne UV-Vis spectroradiometer. Appl Opt: Lasers, Photonics Environ Opt 41(27):5593–5599
Platt U, Stutz J (2008) Differential optical absorption spectroscopy principles and applications. Springer, Berlin, pp 65–75 (135–158)
Rozanov VV, Rozanov AV (2010) Differential optical absorption spectroscopy (DOAS) and air mass factor concept for a multiply scattering vertically inhomogeneous medium: theoretical consideration. Atmospheric Measurements Techniques Discussions 3:697–784
Schmalwieser AW, Schauberger G, Janouch M (2003) Temporal and spatial variability of total ozone content over Central Europe: analysis in respect to the biological effect on plants. Agric For Meteorol 120:9–26
Staehelin J, Harris NRP, Appenzeller C, Eberhard J (2001) Ozone trends: a review. Rev Geophys 39(2):231–290
Weber M, Dikty S, Burrows JP, Garny H, Dameris M, Kubin A, Abalichin J, Langematz U (2011) The Brewer-Dobson circulation and total ozone from seasonal to decadal time scales. Atmos Chem Phys 11:11221–11235
Acknowledgments
The first author is financially supported by the Portuguese FCT grant SFRH/BD/44920/2008. The author acknowledges NASA team for the accomplishments of all satellite missions and to all scientists that participate in the acquirement and provision of the environmental data. This research was partially funded by the FCT project PTDC/AAC-CLI/114031/2009.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Domingues, A.F., Bortoli, D., Silva, A.M., Antón, M., Costa, M.J., Kulkarni, P. (2013). Ozone Seasonal Variation with Ground-Based and Satellite Equipments at Évora Observatory: Portugal During 2007–2010. In: Krisp, J., Meng, L., Pail, R., Stilla, U. (eds) Earth Observation of Global Changes (EOGC). Lecture Notes in Geoinformation and Cartography. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32714-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-32714-8_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32713-1
Online ISBN: 978-3-642-32714-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)