Abstract
Metabolic imaging and early response assessment by positron emission tomography (PET) may guide treatment of localized esophageal cancers. The most consistent and validated results have been obtained during neoadjuvant treatment of adenocarcinoma of the esophago-gastric junction (AEG). It was demonstrated that 18F-Fluorodeoxyglucoe (FDG)-PET is highly accurate for identifying non-responding tumors within 2 weeks after the initiation of neoadjuvant chemotherapy when a quantitative threshold for metabolic response is used. In consecutive phase II studies the metabolic activity, defined by the standardized uptake (SUV) of 18-FDG before and during chemotherapy, was measured. Significant decreases of the SUV after only two weeks of induction chemotherapy were observed. A drop of >35 % 2 weeks after the start of chemotherapy revealed as an accurate cut-off value to predict response after a 12-week course of preoperative chemotherapy. This cut-off was recently confirmed in a US study, where investigators did follow-up PET not 14 days but 6 weeks after initiation of chemotherapy. It was further noticed that the metabolic response to induction chemotherapy revealed as an independent prognostic factor in locally advanced AEG. Therefore, PET could be used to tailor treatment according to the sensitivity of an individual tumor. This concept was realized in the MUNICON-1 and -2 trials. These trials prospectively confirmed that responders to induction chemotherapy can be identified by early metabolic imaging using FDG-PET. Continuing neoadjuvant chemotherapy in the responding population resulted in a favorable outcome. Moreover, MUNICON-1 showed that chemotherapy can be discontinued at an early stage in metabolic non-responders without compromising the patients’ prognosis, but saving time and reducing side effects and costs. MUNICON-2 showed that the addition of neoadjuvant radiation therapy in metabolic nonresponders did not lead to an improvement of their poor prognosis, thus showing that early metabolic nonresponse indicates dismal tumor biology. Future studies need to validate the prognostic and predictive value of PET in multicenter settings and in conjunction with different neoadjuvant chemotherapy and chemo-immunotherapy regimens.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Esophageal Cancer
- Standardize Uptake Value
- Induction Chemotherapy
- Esophageal Squamous Cell Cancer
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Progress has been made in the treatment of locally advanced esophageal cancer. With the introduction of more sophisticated surgical techniques, standardized perioperative care and the introduction of active preoperative chemotherapy, with or without radiation, we have moved toward a more effective and stage-specific approach for every patient.
Novel imaging techniques may enhance the accuracy of clinical staging and thereby improve the estimation of the patients’ prognosis. Molecular imaging may also be of value to predict and assess the response to neoadjuvant therapy.
Positron emission tomography (PET) in combination with computed tomography (CT) in a hybrid imaging modality (PET/CT) offers the unique chance of combining anatomic and functional information of the tumor. PET/CT has been widely investigated in oncology. Some centers routinely use PET imaging when assessing esophageal cancers. However, in some countries, PET is not refunded for this indication as prospective studies are scarce and a positive impact on prognosis by applying this technique has not yet been proven.
This chapter reviews the current literature and attempts to define the role of PET scanning in the management of esophageal cancer. Future clinical research directions in this field are delineated.
2 PET Tracers
The most widely used tracer for PET in oncology is 18F-Fluordeoxyglucose (FDG), which is a glucose analog. It is avidly taken up and retained by most esophageal cancers. About 83–95 % esophageal cancers are FDG avid and therefore can be accurately detected (Flamen et al. 2000; Räsänen et al. 2003).
Other tracers have also been investigated: 3′-deoxy-3′-(18)F-fluorothymidine (FLT) has been reported as stable tracer which accumulates in proliferating tissues and malignant disease (Shields et al. 1998; Shields 2012). A disadvantage of FLT is its high accumulation in the liver which limits its ability to detect liver metastases (Hermann et al. 2007). In a study undertaken in esophageal cancer, uptake of 18F-FDG was shown to be significantly higher compared to 18F-FLT uptake. 18F-FLT scans showed more false-negative findings on the one hand but fewer false-positive findings than 18F-FDG scans on the other hand. Disappointingly, neither uptake of 18F-FDG nor 18F-FLT did correlate with proliferation measured by Ki-67 expression on histopathology (van Westreenen et al. 2005).
3 PET for Staging
Several studies have looked at how PET imaging can improve tumor staging. Due to its physically determined limitations in spatial resolution, PET is per se not a good tool for defining the T category in esophageal cancer where the definition of the T stage is based on the depth of penetration into the esophageal wall. In contrast, PET may add information with regard to N- and M-stage. In a systematic review it was shown that the sensitivity and specificity for CT and PET in lymph node staging (N category) is 51 and 84 %, respectively. For the detection of distant metastases (M category) the corresponding numbers are 67 and 91 %, respectively (van Westreenen et al. 2004). In a more recent meta-analysis the authors come to the conclusion that EUS, CT, and FDG-PET each play a distinctive role in the detection of metastases in esophageal cancer. For the detection of regional lymph node metastases, EUS is the most sensitive investigation, while CT and FDG-PET are more specific. For the assessment of distant metastases, FDG-PET has probably a higher sensitivity than CT. Its combined use could however be of clinical value, with FDG-PET detecting possible metastases and CT confirming or excluding their presence and precisely determining their location (van Vliet et al. 2008). An expert panel recently recommended the use of FDG-PET for the detection of distant metastases in esophageal cancer (Fletcher et al. 2008).
In view of its limited accuracy one may conclude that PET-based treatment decisions have to be taken with some caution. The chance of a false-negative result on FGD-PET is not negligible; therefore, it is recommended that radiation volumes and resection fields should not be downsized based on a negative FDG-PET finding. However, due to the relatively high specificity of FDG-PET enlarging irradiated volumes or extending resections based on positive FDG-PET findings, e.g., in a region without suspected lymph node involvement on CT and/or EUS should be considered (Vrieze et al. 2004).
Of note, the specificity of PET is still limited and false-positive findings are reported in up to 20 % of cases. Therefore, treatment decisions should not be based on PET results alone. Positive findings in PET which would lead to relevant treatment limitations need to be confirmed by other methods, especially by histopathology. Figure 1 gives the example of a positive FDG-PET in the right neck region of a patient who had localized distal esophageal cancer. In case of a lymph node metastasis this finding would define a distant metastasis (cM1). In this particular case histology revealed a lymph node metastasis of a thyroid follicular micro-carcinoma and the patient underwent curative resection for both diseases.
It shows a positive FDG-PET in the right neck region of a patient presenting with a distal esophageal cancer. In case of a lymph node metastasis this would constitute a distant metastasis (cM1) and esophagectomy would not be indicated. In this particular case histology revealed a lymph node metastasis of a follicular thyroid micro-carcinoma and the patient underwent curative resection of two separate malignant diseases
4 PET and Prognosis
Prognosis is linked with the tumor stage on the hand. But an additional question is if the quantification of FDG-uptake gives independent prognostic information.
The standardized uptake value (SUV) is often used for (semi-)quantitative analysis of dynamic data (Schomburg et al. 1996). The SUV is calculated either pixel-wise or over a region of interest (ROI) for each image of a dynamic series at time points (t) as the ratio of tissue radioactivity concentration (e.g. in MBq/kg = kBq/g) at time t, c(t), and injected dose (e.g. in MBq) at the time of injection (t = 0) divided by body weight (e.g. in kg). Some authors prefer to use the lean body weight or the body surface area instead of the body weight. Also, for c(t) either the maximum or the mean value of a ROI is taken (Boellard et al. 2004). In the newer literature, a change from region of Interest-based SUV calculation to volume of Interest-based SUV calculation can be observed (Boellard et al. 2008).
Investigators from New York analyzed 40 patients with esophageal cancer who had undergone FDG-PET scanning prior to primary tumor resection without any neoadjuvant treatment. The median SUV in their patients was found to be 4.5. Patients with a higher SUV had a significantly worse prognosis than patients with a SUV of less than 4.5 (Rizk et al. 2006). The survival advantage of the SUVmax 4.5 or less group was also seen in clinically early-stage patients (defined as no adenopathy on CT and PET, and by EUS (T1-2 N0)), as well as in patients with pathologically early-stage disease (T1-2 N0). This publication indicates that PET may help to identify patients who are usually no candidates for perioperative treatment because their tumor stage is considered as “early” but who might need neoadjuvant chemotherapy or chemoradiation, because their prognosis is worse than expected. This hypothesis would merit to be tested in a prospective trial.
5 PET and Treatment Response
Conventional imaging techniques like CT and endoscopy are of limited value in assessing response to preoperative treatment in esophageal cancer, especially following chemoradiation. Particularly, the discrimination of vital tumor tissue from scar is difficult. Clinical evaluation of dysphagia scores was shown to be meaningless with regard to histopathologic response (Ribi et al. 2009). Even post-treatment cytology and biopsies failed to accurately assess response to preoperative treatment, because residual tumor is often located at the outward areas of the tumor and not within its accessible luminal parts (Peng et al. 2009; Sarkaria et al. 2009).
Recently, PET response criteria in solid tumors (PERCIST 1.0) have been advocated (Wahl et al. 2009). The authors argued that anatomic imaging alone using standard World Health Organization (WHO) criteria, and response criteria in solid tumors (RECIST) have important limitations, particularly in assessing the activity of newer cancer therapies that stabilize disease rather than shrink it. FDG-PET appears particularly valuable in such cases. The proposed PERCIST 1.0 criteria should serve as a starting point for use in clinical trials and in structured quantitative clinical reporting. According to the authors, subsequent revisions and enhancements are to be expected as validation studies are ongoing in several diseases and during different forms of treatment.
5.1 Post-Therapeutic Response Assessment
The value of resection has been called into question in squamous cell cancer of the cervical and intrathoracic esophagus. Being able to predict the true response and prognosis following chemoradiation would be of major importance in order to refine the selection of patients who require surgery.
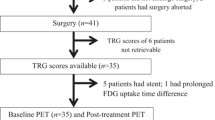
Numerous studies have investigated post-therapeutic PET scanning in order to define the predictive and prognostic value of the test (Table 1). In summary, most studies show a clear correlation of metabolic response as assessed by FDG-PET on the one hand and response and survival on the other hand. One recent study even indicated a relatively strong concordance of 71 % between histopathologic and metabolic complete response (Kim et al. 2007). However, cut-off values that may indicate a correlation with histopathologic complete response have never been validated in prospective studies. Multicenter experience from prospective studies is lacking. Finally, the positive predictive value of the test (i.e., the ability of PET to predict complete histopathologic response) does not seem to be high enough to justify treatment decisions against surgery.
5.2 Pre-Therapeutic Assessment
In an ideal scenario, we would use one pre-therapeutic PET to complement staging and to predict response to any preoperative treatment (chemotherapy or chemoradiation). Some investigators examined the value of pre-therapeutic FDG tumor uptake and treatment response (Table 2). In summary, results are conflicting. While some investigators found a correlation between higher SUV’s and response to subsequent chemo- or chemoradiotherapy, some others did not. Prospective validation studies confirming specific techniques and cut-offs are lacking.
5.3 Early Metabolic Response
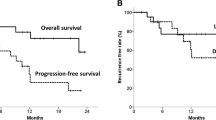
Early metabolic response assessment during neoadjuvant chemotherapy of AEG has been intensively studied; cut-offs have been prospectively validated and have also been used in an interventional clinical study (Fig. 2). In consecutive phase II studies the metabolic tumor activity was quantified, defined by the SUV before and during chemotherapy. It was observed that only after 2 weeks of induction chemotherapy significant decreases of SUV were measured. A drop of ≥35 % measured after 2 weeks of chemotherapy revealed as the most accurate cut-off value to predict the clinical and histopathological response that was found after 12 weeks of preoperative chemotherapy. Weber et al. first established the cut-off decrease in a retrospective study. Ott et al. performed a prospective validation study of this cut-off (Weber et al. 2001; Ott et al. 2006). The validated cut-off was used in subsequent studies. It was further noticed that the metabolic response to induction chemotherapy was an independent and important prognostic factor in case of locally advanced adenocarcinoma of the oesophago-gastric junction (Ott et al. 2006). Metabolic changes measured by PET were shown to be much more sensitive in detecting response early in the course of chemotherapy as compared to morphologic changes measured by high resolution CT (Wieder et al. 2005). This suggested that PET could be used to tailor treatment according to the chemo-responsiveness of tumors. This concept was realized in the MUNICON trial (Lordick et al. 2007) (Fig. 3). This trial prospectively confirmed that responders to induction chemotherapy can be identified by early metabolic imaging using FDG-PET. The rate of major histopathologic remissions in PET responders was 58 %. The continuation of chemotherapy in the responding population resulted in a favorable outcome: after a follow-up 28 months the median overall survival was not reached in PET responders as compared to 26 months in nonresponders. In patients with metabolic nonresponse, chemotherapy could be discontinued at an early stage, thereby saving time, and reducing side effects and costs. Compared to patients from previous studies one can delineate that the outcome of metabolic nonresponders was at least not compromised by the early discontinuation of preoperative treatment. Investigators from the United States validated the −35 % SUV cut-off for patients receiving neoadjuvant chemotherapy. In contrast to the German investigators they did a second PET after having finished induction chemotherapy (which is 6 weeks after its start) and before commencing neoadjuvant chemoradiation (Ilson et al. 2011).
Schema of the explorative and validation studies for the early metabolic response assessment by PET during neoadjuvant chemotherapy of AEG (Lordick et al. 2007; Ott et al. 2006; Weber et al. 2001). CT computed tomography, CTx chemotherapy, EGD esophago-gastro-duodenoscopy, EUS endoscopic ultrasound, PET positron emission tomography
Design of the MUNICON study (Lordick et al. 2007), AEG adenocarcinoma of the esophago-gastric junction, CTx chemotherapy, n number, PET positron emission tomography
Of note, the concept of early response evaluation was successfully studied in patient receiving chemotherapy without radiation. In contrast, in patients treated with chemotherapy plus radiation therapy, metabolic response assessment during treatment failed to accurately predict tumor response (Gillham et al. 2006; Klaeser et al. 2009; van Heijl et al. 2011). This indicates that cell death induced by radiation therapy may follow different mechanisms and time lines than chemotherapy-induced apoptosis. In addition, radiation induces inflammatory reactions and other phenomena leading to false-positive and false-negative features. Therefore, step-by-step implementation of cut-off values is required when metabolic thresholds for response monitoring are implemented into clinical practice.
6 Conclusions
Current data indicate that FDG-PET ameliorates the staging accuracy in esophageal cancer. The main indication is the exclusion of distant metastases which has an important impact on treatment decisions. Whether PET may serve as a basis for tailoring radiation volumes or defining the extent of surgery should be further studied. In the light of the limited sensitivity of PET in detecting locoregional lymph nodes, the risk of reducing treatment radicality must be carefully weighed against the increased morbidity and mortality associated with surgery and large radiation volumes in the preoperative setting.
High FDG uptake values may indicate a critical prognosis of patients presenting with localized esophageal cancer. This finding may guide the decision for multimodality treatment. This is even more true, as some studies show that patients with FDG-avid tumors have a better response and benefit more from neoadjuvant chemo- or chemoradiotherapy. But cut-off values are not clear at this stage and prospective multicenter studies need to be performed.
Post-therapeutic FDG uptake values have a prognostic impact and correlate with histologic response. However, the limited positive predictive value for complete pathologic response does not allow to taking decisions against surgical resection. But this point certainly merits further investigation, especially in patients presenting with proximal esophageal squamous cell cancer, where the operative risk following chemoradiation is high.
The most exciting use of FDG-PET in the management of esophageal cancer is the early assessment of metabolic response during neoadjuvant chemotherapy. This approach may allow for modifications of the treatment plan in patients who do not respond to chemotherapy. However, it must be taken into account that all data are derived from single-center studies, many data have been gathered with older generations of PET machines (before the era of combined PET-CT) and therefore the multicenter validation of cut-off values and quality control is of major importance. The European organization of research and treatment of cancer (EORTC) is currently planning an international validation trial of the MUNICON findings, using a central imaging platform and central quality assurance of PET and pathologic response criteria (Lordick et al. 2008).
References
Boellaard R, Krak NC, Hoekstra OS et al (2004) Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 45:1519–1527
Boellaard R, Oyen WJ, Hoekstra CJ et al (2008) The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging 35:2320–2333
Brücher BL, Weber W, Bauer M et al (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233:300–309
Downey RJ, Akhurst T, Ilson D et al (2003) Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 21:428–432
Flamen P, Lerut A, Van Cutsem E et al (2000) Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18:3202–3210
Flamen P, Van Cutsem E, Lerut A et al (2002) Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 13:361–368
Fletcher JW, Djulbegovic B, Soares HP et al (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49:480–508
Gillham CM, Lucey JA, Keogan M et al (2006) (18)FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br J Cancer 95:1174–1179
Herrmann K, Ott K, Buck AK et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48:1945–1950
Ilson DH, Minsky BD, Ku GY et al (2011) Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer. doi: 10.1002/cncr.26591. (Epub ahead of print)
Javeri H, Xiao L, Rohren E et al (2009) The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer 115:5184–5192
Kim MK, Ryu JS, Kim SB et al (2007) Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer 43:1385–1391
Klaeser B, Nitzsche E, Schuller JC et al (2009) Limited predictive value of FDG-PET for response assessment in the preoperative treatment of esophageal cancer: results of a prospective multi-center trial (SAKK 75/02). Onkologie 32:724–730
Levine EA, Farmer MR, Clark P et al (2006) Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg 243:472–478
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
Lordick F, Ruers T, Aust DE et al (2008) European organisation of research and treatment of cancer (EORTC) gastrointestinal group: workshop on the role of metabolic imaging in the neoadjuvant treatment of gastrointestinal cancer. Eur J Cancer 44:1807–1819
Monjazeb AM, Riedlinger G, Aklilu M et al (2010) Outcomes of patients with esophageal cancer staged with [18F] fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection. J Clin Oncol 28(31):4714–4721
Ott K, Weber WA, Lordick F et al (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 24:4692–4698
Peng HQ, Halsey K, Sun CC et al (2009) Clinical utility of postchemoradiation endoscopic brush cytology and biopsy in predicting residual esophageal adenocarcinoma. Cancer Cytopathol 117:463–472
Räsänen JV, Sihvo EI, Knuuti MJ et al (2003) Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 10:954–960
Ribi K, Koeberle D, Schuller JC et al (2009) Is a change in patient-reported dysphagia after induction chemotherapy in locally advanced esophageal cancer a predictive factor for pathological response to neoadjuvant chemoradiation? Support Care Cancer 17:1109–1116
Rizk N, Downey RJ, Akhurst T et al (2006) Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg 81:1076–1081
Rizk NP, Tang L, Adusumilli PS et al (2009) Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol 4:875–879
Sarkaria IS, Rizk NP, Bains MS et al (2009) Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg 249:764–767
Schomburg A, Bender H, Reichel C et al (1996) Standardized uptake values of fluorine-18 fluorodeoxyglucose: the value of different normalization procedures. Eur J Nucl Med 23:571–574
Shields AF (2012) PET imaging of tumor growth: not as easy as it looks. Clin Cancer Res 18:1189–1191
Shields AF, Grierson JR, Dohmen BM et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Swisher SG, Erasmus J, Maish M et al (2004) 2-Fluoro-2-deoxy-d-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer 101:1776–1785
Vallböhmer D, Hölscher AH, Dietlein M et al (2009) [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 250:888–894
van Heijl M, Omloo JM, van Berge Henegouwen MI (2011) Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg 253(1):56–63
van Vliet EP, Heijenbrok-Kal MH, Hunink MG et al (2008) Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98:547–557
van Westreenen HL, Cobben DC, Jager PL et al (2005) Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. J Nucl Med 46:400–404
van Westreenen HL, Westerterp M, Bossuyt PM et al (2004) Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22:3805–3812
Vrieze O, Haustermans K, De Wever W et al (2004) Is there a role for FGD-PET in radiotherapy planning in esophageal carcinoma? Radiother Oncol 73:269–275
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Weber WA, Ott K, Becker K et al (2001) Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19:3058–3065
Wieder HA, Beer AJ, Lordick F et al (2005) Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med 46:2029–2034
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Lordick, F. (2012). Optimizing Neoadjuvant Chemotherapy Through the Use of Early Response Evaluation by Positron Emission Tomography. In: Otto, F., Lutz, M. (eds) Early Gastrointestinal Cancers. Recent Results in Cancer Research, vol 196. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-31629-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-31629-6_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31628-9
Online ISBN: 978-3-642-31629-6
eBook Packages: MedicineMedicine (R0)