Abstract
BNCT confers a tumoricidal effect that is heavily influenced not by the type of irradiation but by the biological distribution of boronated substrate injected into the body. Therefore, an important step in the planning for optimal BNCT for malignant tumor is to estimate the ratio of the boron concentration in tumor to surrounding normal tissue. One of the authors (KI) have first reported the radiosynthesis of the PET imaging probe [18F]FBPA and have confirmed its efficacy in estimating boron concentrations in animal experiments. A clinical PET application using [18F]FBPA have been started in clinical protocols in Japan for the selection of candidates for BNCT. Our comparative clinical imaging studies have revealed that [18F]FBPA PET images are almost identical to the images obtained with another amino acid probe, 11C methionine (MET). Static images of FBPA or MET-PET can be used for the planning of BNCT. PET imaging with amino acid probes may contribute significantly to the establishment of an appropriate BNCT application for patients with malignant tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Boron Concentration
- Boron Neutron Capture Therapy
- Positron Emission Tomography Tracer
- Amino Acid Tracer
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Positron emission tomography (PET) is a useful medical imaging modality for monitoring biological events inside the human body. PET can quantitate the distribution of positron-labeled molecules in living tissue by coincidence detection of gamma rays and attenuation correction with an external positron-emitting source [32]. Thus, the tomographic image obtained by PET serves well as an in vivo analogue of an autoradiograph [31, 33].
Another important advantage of PET is the existence of the positron-emitting isotopes 11C and 15O, two major constituents of organic molecules, and 18F, a useful analogue of hydrogen. These isotopes have been used for the development of diagnostic probes for both clinical and experimental use, as their rates of uptake express various biological processes of the living body.
With these diagnostic probes, PET has served as one of the most powerful tools for investigating human brain function noninvasively [5]. Recently, however, the applications of PET have spread beyond mainly research settings into clinical settings with practical targets. Two of the major practical uses for PET are in the fields of oncology and pharmacology. In the former, the whole-body PET scan with 2-deoxy-2-[18F]fluoro-d-glucose (FDG) is now accepted as a modality for routine use. Oncologists, meanwhile, await newer probes for the imaging of tumors, as FDG imaging lacks sufficient sensitivity in the organs in which FDG accumulates under normal conditions, such as the brain. Pharmacologists use PET as a tool to monitor pharmacokinetics in drug development [3, 35]. By injecting a small amount of a positron-labeled drug, researchers can noninvasively monitor the dynamics of the drug or the occupancy of the drug at target sites in human. The application of PET for boron neutron capture therapy (BNCT) amply demonstrates the benefits of PET in both oncology (i.e., tumor imaging with PET) and pharmacology (i.e., monitoring the pharmacokinetics of treatment substances).
In contrast to the other types of radiotherapy, BNCT confers a tumoricidal effect that is heavily influenced not by the type of irradiation but by the biological distribution of boronated substrate injected into the body. Thus, oncologists await the establishment of an imaging method capable of quantifying boron uptake into tumors and surrounding normal tissue [38]. PET will serve as just such a tool, provided that an appropriate positron-labeled tracer is developed.
4-Borono-2-[18F]fluorophenylalanine ([18F]FBPA) is a positron label of 4-boronophenylalanine (BPA), a boron carrier for BNCT. Since its synthesis in the early nineties by one of the authors (KI) [13–16], [18F]FBPA has been the only PET tracer capable of monitoring boron concentrations in vivo in human. The clinical protocol of [18F]FBPA PET for BNCT was validated by two groups [10–12, 19] in the late nineties. Today, [18F]FBPA PET is considered an inevitable screening tool for candidates for BNCT in Japanese clinical trials [1, 25, 39].
This chapter will define PET imaging with [18F]FBPA and describe its radiopharmaceutical synthesis, experimental use, clinical use as a tumor-imaging agent, and use for BNCT.
2 Radiosynthesis of [18F]FBPA
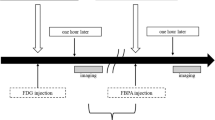
[18F]FBPA is synthesized by direct fluorination of BPA with [18F]acetyl hypofluorite ([18F]AcOF) or [18F]F2, as shown in Fig. 11.1 [14, 19, 36, 37]. The radiolabeled [18F]F2 used is produced by deuteron irradiation of a high-pressure Ne gas containing a low-percentage (0.05–0.2 %) F2, via the 20Ne(d,α)18F reaction. By passing a target Ne gas containing [18F]F2 through a column filled with potassium/sodium acetate, the [18F]F2 is converted to [18F]AcOF. The effluent containing [18F]AcOF is then bubbled into BPA in trifluoroacetic acid, and the [18F]FBPA product is purified by high-performance liquid chromatography. Ishiwata et al. first prepared [18F]FBPA using a mixture of d- and l-isomer of 4-boronophenylalanine as a precursor [14]. Later, they and other groups prepared [18F]FBPA using a pure l-isomer, 4-[10B]borono-l-phenylalanine. Ishiwata et al. used [18F]FBPA for animal studies. The other groups decided to further composite [18F]FBPA with fructose to produce [18F]FBPA-fructose ([18F]FBPA-Fr), as BPA conjugated with fructose has been proven to increase the solubility of the boron carrier [19, 37].
Because the [18F]F2 produced in pure Ne is very active and chemically adsorbed to the target holder, the presence of carrier F2 is essential to recover [18F]F2. Thus, the radiosynthesis using carrier-added [18F]F2 via the 20Ne(d,α)18F reaction provides [18F]FBPA with low specific activity (35–60 GBq/mmol [14] and 130 GBq/mmol [10]). Välhätalo et al., on the other hand, have produced [18F]F2 by an alternative procedure [36]. By applying the 18O(p,n)18F reaction, they produced a high specific activity [18F]F− and then carried out a post-target conversion of [18F]F− to [18F]F2 via [18F]CH3F. Though only a small amount of carrier F2 (1.2 μmol) was used in the conversion, [18F]FBPA with relatively high specific activity was produced (850–1,500 GBq/mmol). With higher levels of initial activity for clinical studies, it becomes possible to increase the specific activity of [18F]FBPA up to 3,700 GBq/mmol.
3 Experimental Studies of [18F]FBPA in Animal Models
3.1 Tumor Accumulation
The potential of [18F]FBPA for tumor imaging has been investigated in the following tumor models: FM3A mammary carcinoma in mice [13, 23], B16 melanoma in mice [15, 16, 23] or melanotic Greene’s melanoma no. 179 and amelanotic Greene’s melanoma no. 178 in hamsters [15, 16], and F98 glioma in rats [4, 37]. All of the reports demonstrate that [18F]FBPA accumulates in tumors for the first 1–2 h, while decreasing in all other tissues. These results, particularly those in hamsters with Greene’s melanomas [16], clearly confirm the potential of [18F]FBPA as a PET tracer for tumor imaging.
Intriguingly, tumors with melanogenic capability have an enhanced uptake of [18F]FBPA. In hamster models, Greene’s melanoma no. 179, a melanotic cell line, showed an [18F]FBPA uptake at 1.7 times higher than that in amelanotic Greene’s melanoma no. 178 [15, 16]. Yet the same two melanomas exhibited similar metabolic activities in a tracer uptake study using l-[14C]methionine, 2-deoxy-d-[14C]glucose, and [3H]thymidine, markers of mainly protein synthesis, glucose metabolism, and DNA synthesis, respectively [15, 16]. In a mouse model, the uptake of [18F]FBPA was higher in a B16-F1 melanoma than in a B16-F10 melanoma, a tumor which grew faster and had a more highly metastatic potential (confirmed by uptake of FDG) but whose melanin content was lower. These findings stand to reason, as [18F]FBPA is partially incorporated into the melanogenic cells [15, 16]. The studies by Ishiwata et al. on the topic were animal studies using a mixture of d- and l-isomer of [18F]FBPA. Later, Ishiwata’s group demonstrated that the tumor uptake of the l-isomer is higher than that of d-isomer and that both isomers are incorporated into the melanogenic cells to a similar extent [16].
3.2 Cellular Distribution
Kubota et al. investigated the cellular distribution of [18F]FBPA in murine B16 melanoma sublines and FM3A mammary carcinoma by double-tracer microautoradiography in vivo [23]. According to their results, the greatest amount of [18F]FBPA was found in S phase melanocytes and the lowest amount was found in non-S phase nonmelanocytes. The [18F]FBPA accumulation is primarily related to the activity of DNA synthesis and secondarily related to the degree of pigmentation in melanocytes. The therapeutic efficacy of BNCT with BPA may be greater in melanomas with higher DNA synthesis activity and higher melanin content.
3.3 Metabolism
The artificial amino acid [18F]FBPA is generally thought to be taken up by tumors and other tissues via the amino acid transport system, without being incorporated into proteins. In mice with FM3A mammary carcinoma, [18F]FBPA was found to be stable for metabolic alteration [13]. In an experiment with FM3A mammary carcinoma tissues, most radioactivity (>94 %) was detected as [18F]FBPA over a 6-h postinjection, and the acid-insoluble fraction was present at levels of less than 2 %. In an experiment with B16 melanoma tissue, considerable amounts of the radioactivity were detected in the acid-insoluble fraction (27 % by 6 h). This suggests that [18F]FBPA is involved in melanogenesis [15], as described above. On the other hand, the percentages of the acid-insoluble fraction in plasma were found to increase with time after injection of [18F]FBPA (10 % by 2 h) [13]. This implies that a deboronation of the [18F]FBPA takes place in vivo. Liver phenylalanine 4-monooxygenase may convert [18F]FBPA to 2-[18F]fluoro-l-tyrosine, a molecule used in the synthesis of plasma proteins recirculated into the bloodstream. If 2-[18F]fluoro-l-tyrosine is recirculated into the bloodstream, it may contribute slightly to the total radioactivity of the tumor tissues. These findings suggest that there may be some discrepancy between the concentrations of 18F radioactivity and 10B in vivo.
3.4 Relationship Between Concentrations of 18F Radioactivity and 10B
Ishiwata et al. evaluated whether the concentration of 10B can be measured by PET signals [16]. After injecting a mixture of [18F]FBPA and an excess amount of BPA in B16 melanoma-bearing mice and Greene’s melanoma-bearing hamsters, they estimated the concentrations of 10B from the levels of radioactive uptake and specific activity of the [18F]FBPA, then directly measured the concentrations of 10B by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) in the same tissues and blood from the test animals. In the B16-bearing mice, the ratios of the estimated concentration by the 18F signal to the measured concentration by ICP-AES (18F/ICP-AES ratios) were small in blood (0.24) and muscle (0.21) but relatively large in B16 (3.7) at 6 h after the injection. In the hamsters, the 18F/ICP-AES ratios were 0.92 in blood, 0.70 in muscle, 1.00 in Greene’s melanoma no. 179, and 0.96 in Greene’s melanoma no. 178 at 6 h after the injection. Thus, the 18F/ICP-AES ratios differed between the two animal species and also between the tissues. Wang et al. injected [18F]FBPA-Fr and BPA separately into F98 glioma-bearing rats and then used ICP-AES to measure the concentrations of 10B in normal brain hemispheres and in the brain hemispheres implanted with the glioma [36]. According to their findings, the uptake characteristics of [18F]FBPA-Fr and BPA were similar [37].
3.5 Kinetic Analysis
Chen et al. performed a kinetic analysis of [18F]FBPA-Fr in glioma-bearing rats by dynamic scanning with a high-resolution PET scanner for tracer kinetic modeling in trying to examine if such model analysis is applicable for a clinical use [4]. According to an estimation of the rate constants of BPA using a three-compartment model, the optimal irradiation time for BNCT was 4 h after the BPA-Fr injection.
4 Clinical Use of [18F]FBPA
4.1 Clinical PET Imaging of Malignant Tumors with [18F]FBPA
Tumor imaging with FDG, a glucose analogue, is an established clinical imaging tool. Whole-body imaging with FDG-PET is routinely used to diagnose cancer [6, 7]. As the sensitivity of PET tumor diagnosis depends on the uptake contrast of tracer into the tumor versus that into the surrounding normal tissue (T/N), FDG-PET can only be used to good effect in organs in which FDG do not accumulate in abundant levels under normal conditions. Thus, the method is not suitable for application for the brain and genitourinary system, and therefore, PET tracers based on principles other than FDG have also been clinically used, including amino acid probes such as l-[methyl-11C]methionine (MET) for brain tumor [28, 29] and [11C]choline for prostate cancer [8, 9].
BPA and its positron-labeled substance, [18F]FBPA, are analogues of the amino acid phenylalanine and are taken up into tumor cells through a large neural amino acid transporter located at the luminal membranes of microvessels and cell membranes [24, 34]. Given that MET and all of the other positron-labeled amino acid probes are taken up into the tumor cells through the same transporter system, the [18F]FBPA method can be regarded as an imaging method which uses amino acid PET tracers. Our comparison between [18F]FBPA PET and MET-PET for malignant tumors of the brain and skull indicates that the two probes provide almost identical tumor images (Fig. 11.2) [30]. In another comparative study between MET-PET and the amino acid analogue O-[11C]methyl-l-tyrosine, the PET tumor images using the two probes were also identical [17, 18]. It thus seems that the PET tumor images obtained by different amino acid probes, including [18F]FBPA, are quite similar.
PET images of four patients with glioblastoma. The [11C]methionine (MET) images and [18F]fluoroboronophenylalanine (FBPA) images are almost identical (Cited from [30])
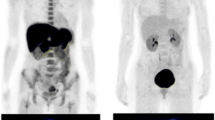
Artificial amino acid probes such as [18F]FBPA or O-[11C]methyl-l-tyrosine differ from nutritional amino acids such as MET in their uptake into normal tissue. As the former play no part in protein synthesis, they accumulate into tumor tissue selectively, as well as in excretion sites such as the kidney and bladder (Fig. 11.3). The latter, meanwhile, take part in protein synthesis and thus accumulate extensively in the liver and glandular organs such as the pancreas and salivary glands [22]. On this basis, [18F]FBPA seems to have better potential as an imaging tool for malignant tumors in most parts of the body other than the urinary system. And by extension, we can assess BPA as a superior pharmaceutical for sending boron into tumors wherever the contrast with surrounding tissue is high.
Comparison of whole-body PET images obtained with [18F]FBPA (a patient with low-grade cerebellar tumor), MET (a normal control), and FDG (a normal control with adenoids). Note the difference in the accumulation of these tracers into normal tissue. The image of MET was provided by Dr. Kazuo Kubota from the Division of Nuclear Medicine in the Department of Radiology at the International Medical Center of Japan. Ad adenoids, Bl bladder, Bo bone marrow, Br brain, G glioma, H heart, K kidney, L liver, P pancreas, Sal salivary glands, Sp spleen
4.2 PET Imaging with [18F]FBPA for BNCT
Imahori et al. [12] and Kabalka et al. [19] established and validated an [18F]FBPA method to estimate the boron concentrations of malignant brain tumors in patients who received BNCT. First, they used dynamic PET scans to examine patients with glioblastoma multiforme before BNCT. Next, they constructed a three-compartment model to estimate the tumor concentration of boron administered via an i.v. injection of BPA. Finally, they measured the boron concentrations after the BPA injections directly from surgical specimens (from seven patients in the former study and from two patients in the latter). Both groups concluded that the estimation achieved by PET examination was close enough for practical use.
In practice, it is sufficient to determine the ratio of the boron concentration in the tumor to that in the surrounding normal tissue. To do so, it may be sufficient to compare a static scan of the activity of the tumor [18F]FBPA with a static scan of the activity of the normal tissue (T/N of [18F]FBPA). A recent analysis by our group has indicated that the estimated T/N of the boron concentration after a slow infusion of BPA had a significant linear correlation with the T/N of radioactivity after a bolus injection of [18F]FBPA (Fig. 11.4) [30].
A graph to indicate the relationship between the T/N ratio of [18F]FBPA on a static PET scan (x-axis) and the T/N ratio of the tissue boron concentration after a 1-h constant infusion of BPA estimated by a pharmacokinetic analysis of a dynamic [18F]FBPA PET scan (y-axis) (Cited from [30])
Researchers in Japan have conducted several BNCT series by setting certain thresholds on the T/N of [18F]FBPA obtained by PET studies for patient selection. Three categories of tumor have been covered in these series: malignant glioma [25], malignant meningioma [26], and head and neck malignancies [1, 2, 21]. Kabalka et al. have reported a case of metastatic malignant melanoma in brain in whom [18F]FBPA was useful to make decision to perform BNCT[20]. The dearth of comparative studies has made it difficult to verify the superiority of PET-based BNCT over non-PET-based BNCT or other radiotherapies. Findings have suggested, however, that PET-based BNCT for glioblastoma is achieving progressively better results than the former protocol [39].
4.3 Practical Use of PET for BNCT
As long as BNCT is based on the transfer of the boron molecule to the tumor tissue, PET boron imaging will continue to play a key role in successful treatment. Of the various in vivo measurement methods, PET sensitivity is maximum with high-powered irradiation from a minimal quantity of molecular probe. To extend the use of BNCT on a practical basis, parallel efforts must be made to extend the use of PET clinically. As this chapter has described, [18F]FBPA PET is categorizable as one of PET methods using amino acid tracers. Thus, PET studies with other amino acid tracers can be applied for the screening of tumor types or of individual patients who may benefit from BNCT, as long as BPA is used as the boron carrier agent. MET-PET may be suitable for this type of screening, in light of the ease with which it can be synthesized and the many years it has been used at PET institutes around the world.
The clinical application of PET for the posttreatment evaluation of patients who have received BNCT is also inevitable. PET should be useful to differentiate tumor regrowth from pseudoexpansion by radiation injury [27]. Without PET, the treatment effects of BNCT cannot be precisely evaluated. FDG-PET is also suited for evaluating the treatment effects of patients with whole-body malignancies, while amino acid PET is the better choice for patients with brain tumors. We are convinced that the routine use of PET tumor imaging with amino acid tracers, MET, [18F]FBPA, and the like will support the widespread and beneficial use of BNCT.
5 Summary
An important step in the planning for optimal BNCT for malignant tumor is to estimate the T/N of the boron concentration. Investigators have developed the PET imaging probe [18F]FBPA and have confirmed its efficacy in estimating boron concentrations in animal experiments. Others have established a clinical PET application using [18F]FBPA and have started to use it for the selection of candidates for BNCT in clinical protocols in Japan. Comparative clinical imaging studies have revealed that [18F]FBPA PET images are almost identical to the images obtained with another amino acid probe, MET. Static images of FBPA or MET-PET can be used for the planning of BNCT. PET imaging with amino acid probes may contribute significantly to the establishment of an appropriate BNCT application for patients with malignant tumors.
Abbreviations
- BNCT:
-
Boron neutron capture therapy
- BPA:
-
4-boronophenylalanine
- [18F]FBPA:
-
4-borono-2-[18F]fluorophenylalanine
- FDG:
-
2-deoxy-2-[18F]fluoro-D-glucose
- ICP-AES:
-
Inductively coupled plasma-atomic emission spectroscopy
- MET:
-
l-[methyl-11C]methionine
- PET:
-
Positron emission tomography
- T/N :
-
Tumor-to-normal-tissue ratio
References
Aihara T, Hiratsuka J, Morita N, Uno M, Sakurai Y, Maruhashi A, Ono K, Harada T (2006) First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 28:850–855
Ariyoshi Y, Miyatake S, Kimura Y, Shimahara T, Kawabata S, Nagata K, Suzuki M, Maruhashi A, Ono K, Shimahara M (2007) Boron neuron capture therapy using epithermal neutrons for recurrent cancer in the oral cavity and cervical lymph node metastasis. Oncol Rep 18:861–866
Bauer M, Wagner CC, Langer O (2008) Microdosing studies in humans: the role of positron emission tomography. Drugs R&D 9:73–81
Chen JC, Chang SM, Hsu FY, Wang HE, Liu RS (2004) MicroPET-based pharmacokinetic analysis of the radiolabeled boron compound [18F]FBPA-F in rats with F98 glioma. Appl Radiat Isot 61:887–891
Cherry S, Phelps M (1996) Imaging brain function with positron emission tomography. In: Toga A, Mazziotta J (eds) Brain mapping: the methods. Academic, San Diego, pp 191–221
Coleman RE (2002) Value of FDG-PET scanning in management of lung cancer. Lancet 359:1361–1362
Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK (2001) Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 285:914–924
Groves AM, Win T, Haim SB, Ell PJ (2007) Non-[18F]FDG PET in clinical oncology. Lancet Oncol 8:822–830
Hara T, Kosaka N, Kishi H (1998) PET imaging of prostate cancer using carbon-11-choline. J Nucl Med 39:990–995
Imahori Y, Ueda S, Ohmori Y, Kusuki T, Ono K, Fujii R, Ido T (1998) Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med 39:325–333
Imahori Y, Ueda S, Ohmori Y, Sakae K, Kusuki T, Kobayashi T, Takagaki M, Ono K, Ido T, Fujii R (1998) Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part I. Clin Cancer Res 4:1825–1832
Imahori Y, Ueda S, Ohmori Y, Sakae K, Kusuki T, Kobayashi T, Takagaki M, Ono K, Ido T, Fujii R (1998) Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part II. Clin Cancer Res 4:1833–1841
Ishiwata K, Ido T, Kawamura M, Kubota K, Ichihashi M, Mishima Y (1991) 4-Borono-2-[18F]fluoro-D, L-phenylalanine as a target compound for boron neutron capture therapy: tumor imaging potential with positron emission tomography. Int J Rad Appl Instrum B 18:745–751
Ishiwata K, Ido T, Mejia AA, Ichihashi M, Mishima Y (1991) Synthesis and radiation dosimetry of 4-borono-2-[18F]fluoro-D, L-phenylalanine: a target compound for PET and boron neutron capture therapy. Int J Rad Appl Instrum A 42:325–328
Ishiwata K, Ido T, Honda C, Kawamura M, Ichihashi M, Mishima Y (1992) 4-Borono-2-[18F]fluoro-D, L-phenylalanine: a possible tracer for melanoma diagnosis with PET. Int J Rad Appl Instrum B 19:311–318
Ishiwata K, Shiono M, Kubota K, Yoshino K, Hatazawa J, Ido T, Honda C, Ichihashi M, Mishima Y (1992) A unique in vivo assessment of 4-[10B]borono-L-phenylalanine in tumour tissues for boron neutron capture therapy of malignant melanomas using positron emission tomography and 4-borono-2-[18F]fluoro-L-phenylalanine. Melanoma Res 2:171–179
Ishiwata K, Tsukada H, Kubota K, Nariai T, Harada N, Kawamura K, Kimura Y, Oda K, Iwata R, Ishii K (2005) Preclinical and clinical evaluation of O-[11C]methyl-L-tyrosine for tumor imaging by positron emission tomography. Nucl Med Biol 32:253–262
Ishiwata K, Kubota K, Nariai T, Iwata R (2008) Whole-body tumor imaging: [O-11C]methyl-L-tyrosine/positron emission tomography. In: Hayat M (ed) Cancer imaging: instrument and application, vol 2. Elsevier, Amsterdam, pp 175–179
Kabalka GW, Smith GT, Dyke JP, Reid WS, Longford CP, Roberts TG, Reddy NK, Buonocore E, Hubner KF (1997) Evaluation of fluorine-18-BPA-fructose for boron neutron capture treatment planning. J Nucl Med 38:1762–1767
Kabalka GW, Nichols TL, Smith GT, Miller LF, Khan MK, Busse PM (2003) The use of positron emission tomography to develop boron neutron capture therapy treatment plans for metastatic malignant melanoma. J Neurooncol 62:187–195
Kato I, Ono K, Sakurai Y, Ohmae M, Maruhashi A, Imahori Y, Kirihata M, Nakazawa M, Yura Y (2004) Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 61:1069–1073
Kubota K (2001) From tumor biology to clinical PET: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 15:471–486
Kubota R, Yamada S, Ishiwata K, Tada M, Ido T, Kubota K (1993) Cellular accumulation of 18F-labelled boronophenylalanine depending on DNA synthesis and melanin incorporation: a double-tracer microautoradiographic study of B16 melanomas in vivo. Br J Cancer 67:701–705
Langen KJ, Muhlensiepen H, Holschbach M, Hautzel H, Jansen P, Coenen HH (2000) Transport mechanisms of 3-[123I]iodo-alpha-methyl-L-tyrosine in a human glioma cell line: comparison with [3H]methyl]-L-methionine. J Nucl Med 41:1250–1255
Miyatake S, Kawabata S, Kajimoto Y, Aoki A, Yokoyama K, Yamada M, Kuroiwa T, Tsuji M, Imahori Y, Kirihata M, Sakurai Y, Masunaga S, Nagata K, Maruhashi A, Ono K (2005) Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg 103:1000–1009
Miyatake S, Tamura Y, Kawabata S, Iida K, Kuroiwa T, Ono K (2007) Boron neutron capture therapy for malignant tumors related to meningiomas. Neurosurgery 61:82–90; discussion 90–81
Miyatake SI, Kawabata S, Nonoguchi N, Yokoyama K, Kuroiwa T, Ono K (2009) Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol 11(4):430–436
Nariai T, Senda M, Ishii K, Maehara T, Wakabayashi S, Toyama H, Ishiwata K, Hirakawa K (1997) Three-dimensional imaging of cortical structure, function and glioma for tumor resection. J Nucl Med 38:1563–1568
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, Senda M, Ishii K, Hirakawa K, Ohno K (2005) Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 103:498–507
Nariai T, Ishiwata K, Kimura Y, Inaji M, Momose T, Yamamoto T, Matsumura A, Ishii K, Ohno K (2009) PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl Radiat Isot 67:S348–S350
Phelps ME, Mazziotta JC (1985) Positron emission tomography: human brain function and biochemistry. Science 228:799–809
Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM (1975) Application of annihilation coincidence detection to transaxial reconstruction tomography. J Nucl Med 16:210–224
Raichle ME (1983) Positron emission tomography. Annu Rev Neurosci 6:249–267
Sanchez del Pino MM, Peterson DR, Hawkins RA (1995) Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood–brain barrier. J Biol Chem 270:14913–14918
Suhara T, Takano A, Sudo Y, Ichimiya T, Inoue M, Yasuno F, Ikoma Y, Okubo Y (2003) High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry 60:386–391
Vahatalo JK, Eskola O, Bergman J, Forsback S, Lehikoinen P, Jaaskelainen J, Solin O (2002) Synthesis of 4-dihydroxyboryl-2-[F-18] fluorophenylalanine with relatively high-specific activity. J Label Compd Radiopharm 45:697–704
Wang HE, Liao AH, Deng WP, Chang PF, Chen JC, Chen FD, Liu RS, Lee JS, Hwang JJ (2004) Evaluation of 4-borono-2-18F-fluoro-L-phenylalanine-fructose as a probe for boron neutron capture therapy in a glioma-bearing rat model. J Nucl Med 45:302–308
Wittig A, Michel J, Moss RL, Stecher-Rasmussen F, Arlinghaus HF, Bendel P, Mauri PL, Altieri S, Hilger R, Salvadori PA, Menichetti L, Zamenhof R, Sauerwein WA (2008) Boron analysis and boron imaging in biological materials for boron neutron capture therapy (BNCT). Crit Rev Oncol Hematol 68:66–90
Yamamoto T, Nakai K, Kageji T, Kumada H, Endo K, Matsuda M, Shibata Y, Matsumura A (2009) Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol 91:80–84
Acknowledgement
We thank Dr. Kazuo Kubota from the Division of Nuclear Medicine in the Department of Radiology at the International Medical Center of Japan for kindly offering us whole-body PET images of MET uptake.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Nariai, T., Ishiwata, K. (2012). Analysis and Imaging: PET. In: Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y. (eds) Neutron Capture Therapy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-31334-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-31334-9_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31333-2
Online ISBN: 978-3-642-31334-9
eBook Packages: MedicineMedicine (R0)