Abstract
High-dose-rate (HDR) brachytherapy is an established curative treatment option for patients diagnosed with prostate cancer. In contrast to low-dose-rate (LDR) brachytherapy, that involves the permanent implant of several radioactive seeds in the prostate, HDR uses a single, high-intensity radioactive source of 192Iridium which is sent and retracted sequentially into each of the needles (or catheters) temporarily implanted in the gland. Advances in mechanical and planning systems and in computer technology result in a sophisticated treatment technique that reliably delivers a very high-quality radiation dose distribution to the target in a very short period of time while minimizing the irradiation of the surrounding critical organs. Several advantages over other radiotherapy techniques have been identified, namely, (1) the accurate positioning of the sources by first implanting nonactive guide needles, (2) the possibility to exactly choose the position of the source over the entire length of every single needle, (3) the absence of any target movement during irradiation, (4) the possibility of “online” adjustments of dwell source locations according to 3-D planning based on individual dose prescription, (5) the absence of any need for preimplant needles preparation, and finally (6) a safer radioprotection profile as compared to permanent seeds implant procedure. HDR brachytherapy is generally proposed in combination with external beam irradiation for patients having intermediate- to high-risk cancers, but protocols have been recently developed in order to use it as monotherapy for low-risk cases. In this chapter, the rational, indications, technique, and clinical results (both in terms of efficacy and toxicity) of HDR brachytherapy for prostate cancer will be presented and discussed. Finally, some future directions together with critical issues regarding this technique will be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dose Distribution
- Androgen Deprivation Therapy
- Clinical Target Volume
- Urethral Stricture
- Dose Constraint
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Background and Rationale
11.1.1 Dose–Response Relationship for Prostate Cancer
Convincing experimental and clinical data have been published in recent years, clearly demonstrating that high doses are needed in order to optimize clinical and biochemical outcomes when irradiating men with localized prostate cancer (Cahlon et al. 2008; Dearnaley et al. 2007; Peeters et al. 2006). It is widely accepted that a dose–response relationship exists for prostate cancer, and conformal and intensity-modulated external beam radiotherapy techniques have been developed to achieve dose escalation and to allow a better sparing of radiosensitive dose-limiting adjacent normal structures such as rectum and bladder. However, inter- and intrafractions organ motion together with variations in daily setup represent a serious challenge to external beam radiotherapy even when image-guided technology is employed (De Crevoisier et al. 2005). Brachytherapy on the contrary is not limited by positioning uncertainties as the target is immobilized by the implanted needles (or catheters) and treated within very short treatment times; therefore there is no need for an extramargin expanding the clinical target volume (CTV) to the planning target (PTV).
11.1.2 Radiobiology
Extensive literature supports the concept of a low α/β ratio describing the radiobiological response of prostate cancer cells to irradiation. Although this value is still debated, a general consensus estimate it around 1.5–3, well below 5 (Brenner et al. 2002). This implies that an enhanced cell kill is to be expected when larger than standard dose per fraction is used, and furthermore, assuming that the α/β ratio for prostate cell is lower than the one for the rectal mucosa, an increase in the therapeutic ratio regarding rectal toxicity is also to be expected, and in this respect, HDR brachytherapy may be considered as an extreme form of hypofractionated irradiation. HDR brachytherapy thus combines the advantages of being one of the most efficient methods to perform dose escalation by means of the most favorable fractionation schedule. In fact, when comparing HDR brachytherapy regimens to standard external beam radiotherapy regimens (2–3 Gy per fraction), equivalent dose in 2 Gy fractions (EQD2) or biologically equivalent dose (BED) formula is often used, indicating invariably that HDR regimens deliver 25–50% higher doses in the prostate as compared to conventional fractionated EBRT (Joiner and Bentzen 2009; Hoskin 2008). Interestingly, several authors have compared different radiotherapy techniques (HDR brachytherapy, IMRT, TomoTherapy, CyberKnife) in terms of their capabilities of obtaining the best dose distribution both for target coverage and of organs at risk sparing (Hermesse et al. 2009; Nickers et al. 2006). Invariably, HDR has been associated to the highest dosimetric selectivity.
11.1.3 HDR Versus LDR Brachytherapy
Moreover, high dose rate presents several advantages as compared to LDR brachytherapy (Kovacs et al. 2005; Martinez et al. 2005a; Hoskin 2008). By implanting at first nonactive afterloading guide needles or catheters, the spatial source position may be accurately modulated and the source dwell time efficiently adapted according to a three-dimensional-imaging-based individual dose prescription, and only once the most suitable dose distribution is obtained the irradiation is started. This means that small inaccuracies in needles/catheters placement can be corrected by adjustments of treatment parameters before irradiation while accurate seeds implant within the gland is technically challenging with limited possibility for live adjustments. In addition, HDR needles/catheters may be placed not only inside the prostatic capsule but also in the extraprostatic tissue or even in the seminal vesicles, making it possible to treat more advanced cases as compared to a strictly intraprostatic technique as LDR brachytherapy. No source preparation is necessary before or during HDR treatment, and no free radioactive materials are used during the implant, thus minimizing the risk of source loss and the need for radioprotection procedures. Finally, costs of temporary HDR brachytherapy are limited as many radiotherapy centers are equipped with an afterloading unit for other brachytherapy treatments.
11.2 Indications and Patients Selection
Originally, HDR brachytherapy has been reserved for patients with locally advanced prostate cancers in the intermediate-to-high-risk groups (Gleason Score >6, PSA at diagnosis >10 ng/ml) as a boost to the prostatic volume combined with external beam RT. Recently, HDR monotherapy schedules have been proposed for patients having favorable risk cancers with HDR brachytherapy delivering the entire radiation treatment. The GEC/ESTRO-EAU group has published guidelines for patients selection for HDR brachytherapy (Kovacs et al. 2005): classical exclusion criteria for any transrectal-ultrasound (TRUS)-guided transperineal implant technique are suggested also for HDR (Table 11.1), but it should be emphasized that large gland size (>60 cc) should not be regarded as an absolute contraindication when considering the potential for geometrical downsizing of 3–6 months of androgen deprivation therapy (ADT). Probably, no absolute cutoff volume exists since what really matters is the relationship between the gland volume and the pelvic anatomy of the patient with hip positioning and TRUS-probe angle adjustments, playing a major role in making the implant feasible even for large glands. When a pubic arch interference (PAI) is suspected on digital rectal examination or on imaging, it is preferable to check it with a TRUS with the patient in the implant position in order to decide on the need for hormonal cytoreduction knowing that preimplantation ADT reduces prostate size by about 30% (Stone et al. 2010) to the price, at least for some authors, of a higher postimplant retention rate (Crook et al. 2002). Likewise, a prior history of transurethral resection of the prostate (TURP) is clearly associated with a somewhat higher risk of developing postimplant grades 2–3 late genitourinary toxicity (mostly incontinence), but this probably holds true only when a large central defect is present in the gland, and in general, a lower dose to the urethra may be administered without compromising the peripheral zone dose coverage. Again a preimplant TRUS may help in evaluating the TURP central defect. Low urinary tract obstructive symptoms (LUTS) should be carefully investigated prior to the implant using a validated scoring scale such as the International Prostate Symptom Score (1991) or the American Urological Association (AUA) criteria (Barry et al. 1992), and preferably, a voiding study by uroflowmetry should be carried on to evaluate the urinary flow rate and the postvoiding residual volume (Martens et al. 2006) knowing that a substantial urinary obstruction represents a possible exclusion criteria due to the accrued risk of developing postimplant bladder retention. The GEC/ESTRO-EAU recommendations also mention tumor invasion of bladder neck and a rectum-prostate distance at TRUS inferior to 5 mm as exclusion criteria. Finally, the role of imaging in the local staging of prostate cancer for patient’s selection for HDR brachytherapy should be emphasized. Magnetic resonance (MR) is the single most sensitive imaging investigation in assessing the local extent of prostatic adenocarcinoma. The precise knowledge of the extent and the location of extracapsular disease and/or seminal vesicles infiltration may guide the brachytherapist for further treatment decision (Cornud et al. 2002). Independently, of the technique adopted for needles/catheters placement and for treatment planning (TRUS, CT, or MR based), it is thus advisable to perform a staging MR before planning the implant (Fuchsjager et al. 2008; Heidenreich et al. 2011; Kovacs et al. 2005).
11.3 Technique
11.3.1 Procedure
The technique of HDR brachytherapy is similar to LDR one, and the equipment needed is not very different. Obviously, a treatment room with adequate shielding is necessary (where, if possible, also the needles implantation should take place in order to avoid additional patient transportation and potential needles displacement) together with an afterloading HDR unit with a 192Iridium stepping source and a camera system to monitor the patient during the irradiation (Kovacs et al. 2005). Slightly different methods are proposed by the teams performing HDR brachytherapy, depending on the adoption of a two-step procedure (preplanning a few days before implantation) or an intraoperative online planning, on the imaging used for needles guidance and for dose planning (TRUS, CT, or MR based), and on the clinical protocol adopted (single fraction versus multifractionated HDR), but some general steps are common. (1) The procedure requires general or spinal anesthesia with the patient in the dorsal lithotomy position: particular care should be reserved to patient positioning especially when a PAI is suspected (= more acute spine-hip angle). (2) Modern brachytherapy implants are performed transperineally, and needles placement is generally made under TRUS control. The TRUS device is secured to a stepper unit, and adequate fixation (to the floor or to the patient table) is needed in order to avoid movements during the procedure. (3) A Foley catheter is placed in the bladder in order to better visualize the transprostatic urethra and the bladder neck during the entire procedure, and the gland is localized by TRUS on the axial, coronal, and sagittal planes and positioned as symmetrical as possible in relation to the urethra by adequate probe inclination. If the treatment planning is performed using three-dimensional TRUS reconstructed volumes, a set of images using the stepper unit is acquired in 3–5 mm steps from 1 cm above the base to 1 cm below the apex of the prostate and recorded in the treatment planning unit. (4) TRUS-based contouring of the prostate (CTV), rectum adjacent to the gland, urethra (following the Foley catheter images and eventually using aerated gel to better visualize it), and bladder neck is performed, and commercially available software will integrate ultrasound images to provide a three-dimensional reconstruction of the CTV and organs at risk for intraoperative online planning purposes (see Sect. 3.2). Alternatively, when CT and/or MR images are used for contouring and treatment planning, the patient should be transferred after the implant in the supine position with a Foley catheter in place to the CT-MR scan unit for the acquisition of the set of images needed for organ contouring. Generally, no margins are added to the CTV to obtain a PTV (= the CTV and the PTV are identical), but some authors advocate the need to contour, beside a whole gland CTV (= CTV1), a CTV2 encompassing the peripheral posterior zone of the prostate (where a highest tumor load is presumed) and even a CTV3 if tumor infiltration areas are detectable by classical imaging techniques or by functional ones (Kovacs et al. 2005). (5) A template with a detachable perineal portion and holes with 0.5 cm spacing is used for needles guidance. It is fixed to the stepper unit and positioned parallel and close to the perineal skin plane. Needles are implanted under direct TRUS control as parallel as possible to each other and to the probe with the largest prostate cross section seen on sonography used as reference view for needle distribution (Fig. 11.1). Different philosophies exist in the literature about the best needles implant distribution strategy. Some authors prefer a homogenous intraprostatic needle distribution with fixed interneedle spacing, while others start by implanting peripheral needles at 8–10 mm spacing (Fig. 11.2): in this case, the central needles are implanted at a later stage according to the actualized dosimetry, and for eventual real time, better tuning of the final dose distribution (Edmundson et al. 1995; Slessinger 2010). The prostatic base-plane is regarded as the planned position of the needles tips which are all inserted at the same depth, but if needed and especially in the posterior aspect of the gland when seminal vesicles infiltration is suspected, a few needles may be pushed at different depths to better adapt to the prostate shape. It is important to remember that the first source position available for treatment is some millimeters backward from the tip of the needle/catheters: this implies that, using the sagittal TRUS view, all needles should be inserted deep enough in order to obtain an optimal dose coverage particularly at the level of the prostate base without piercing the bladder and/or the urethral wall. The tenting of the bladder mucosa by needles’ tips may be checked by a flexible cystoscopy if available once the implant is completed. On average, 12–22 needles/catheters are needed for whole prostate coverage depending on the volume and shape of the prostate. (6) Finally, when several fractions are planned and/or when CT-/MR-based treatment planning is performed, the detachable perineal part of the template is unscrewed and sutured to the skin, and the patient is transferred to the CT/MR unit for treatment planning scan. Transferring the patient from the operating room table to the CT/MR table and then to the treatment one, or when several fractions are planned, implies that a nonnegligible source of error is introduced in the procedure due to needle retraction in the craniocaudal direction associated with repositioning (Foster et al. 2011; Simnor et al. 2009). The needles/catheters shift has been reported in the range of 5–7 mm and may translate into suboptimal dose coverage especially at the base of the prostate but also into a higher than planned urethral dose. As a consequence, the regular control of needle geometry is strictly recommended (by visual inspection and catheters measurements, by fluoroscopy, and by repeated scanning before each fraction), and any displacement of more than 3 mm should be corrected (Seppenwoolde et al. 2008; Tiong et al. 2010).
11.3.2 Treatment Planning and Delivery
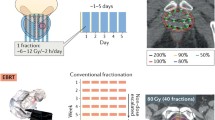
As already mentioned, treatment planning can be based on three-dimensional TRUS, CT, or MR imaging: in this latter case, flexible plastic catheters will have to replace metallic needles to allow for CT planning. Intraoperative, TRUS-based, real-time planning is time sparing and certainly advantageous for patient comfort while CT- and/or MR-based postimplant procedures will benefit from a better visibility of prostate and catheters contours as compared to sonography. Once the contouring is completed, planning of the treatment can start: intraoperative real-time treatment planning optimization software’s are available to perform multiple iteration of the 192Iridium source position (= dwell positions, every 2.5–5 mm) and the duration (= dwell time) that the source will remain in any particular position within each single needle/catheter in order to provide the requested dose distribution to the target and organs at risk. Once the optimized treatment plan is approved by the brachytherapist, each catheter/needle is connected by means of transfer tubes to the HDR afterloading apparatus and dose delivery begins (Fig. 11.3). Large variations in terms of number of implants and/or fractions, planning parameters (= dose constraints for the target and the organs at risk), dose per fraction, and timing of the implant, both for HDR as a boost combined to EBRT or as a monotherapy, are reported in the literature with patterns of practice having undergone significant evolution across institutions. Tables 11.2 and 11.3 report the HDR brachytherapy schedules adopted in some of the series recently published for, respectively, combined HDR + EBRT treatment and HDR alone. For combined HDR-EBRT, the prescribed brachytherapy dose to the prostate varies from 5.5 to 15 Gy per fraction for a total dose of 15–21 Gy in 1–4 fractions (1–3 implants), while the total EBRT dose is reported between 37.5 and 55 Gy in 1.8–2.75 Gy/fr. When HDR brachytherapy is employed alone, 3–6 fractions of 6–10.5 Gy each are used in 1–2 implants (total dose of 31.5–54 Gy). Due to differences in protocols (HDR given before, after, or during EBRT) and techniques (a new implant for each fraction or one implant with several loadings), the reported interfraction times vary from a few hours to 21 days. The clinical relevance of these differences (timing of HDR, overall treatment time, interfraction gap) is not clearly understood. For comparison with full EBRT course at standard fractionation, Tables 11.2 and 11.3 also report the EQD at 2 Gy for α/β values of 1.5 and 3 knowing the limits of applicability of the linear-quadratic model for dose per fraction beyond 5–6 Gy (Joiner and Bentzen 2009).
11.3.3 Dose Constraints
The optimization process for treatment planning is based on dose prescription to the prostate volume and on dose constraints for organs at risk. It has correctly been pointed out that, in contrast to EBRT, the exclusive use of dose-volume histograms (DVH) is of limited value in brachytherapy when evaluating a treatment plan due to the nonhomogenous dose distribution obtained within the target by temporary (or permanent) implants (Kovacs et al. 2005). Tables 11.4 and 11.5 report the dose constraints suggested in the literature for HDR brachytherapy combined with EBRT or when used alone. There is no general agreement on which parameters to use and which dose level to recommend: it is in general advisable to adopt maximum dose (D max), dose to fixed volume levels (D2 cc, D0.1 cc,…), or volumes in cc receiving certain dose levels (V100, V125,…) since dose to percent of the organ (D10, D30,…) depends on contouring protocols (typically for rectum and bladder). Urethral D max should not be higher than 120–125% of the prostate prescribed dose, while rectum (and bladder) D max should be kept lower than 75–80%. The volume of the CTV-PTV receiving 100%, 125%, and/or 150% of the prescribed dose (V100, V125, V150) should be respectively >90–95%, <60%, and <35–40%. When using HDR combined to EBRT, no predetermined dose constraints are suggested for the external irradiation part of the treatment. Finally, for reporting purposes, it is suitable to “translate” the dose limits adopted into absolute dose levels in Gray in order to make the comparisons with other treatment schedules easier.
11.4 Clinical Results
11.4.1 Efficacy
The greatest clinical experience with HDR for prostate cancer involves its combination with EBRT. A recent systematic review of available literature has compared EBRT alone (at doses >75 Gy), EBRT combined with HDR brachytherapy boost, and EBRT combined with LDR permanent seeds boost in terms of efficacy endpoints (Pieters et al. 2009). More than 180 papers published from 1980 to 2007 have been analyzed, and despite the fact that patients treated with EBRT + HDR boost had more advanced disease, both biochemical-disease-free survival (biochemical Not Evidence of Disease, bNED) and overall survival rates were significantly better with this combination. Moreover, Hoskin et al. have published the early results of the only phase III randomized trial available in this field comparing EBRT alone (55 Gy in 20 fractions) versus a combined EBRT (35.75 Gy in 13 fractions) + an HDR boost of 2 fractions of 8.5 Gy (Hoskin et al. 2007). A significant advantage in bNED is reported favoring the combined arm (mean bNED at a median follow-up of 30 months is 5.1 years in the HDR arm versus 4.3 years in the EBRT arm), but not in overall survival. The principal limitations of this study pertain to the control arm of EBRT alone: the hypofractionated regime chosen cannot be considered as standard practice today, and furthermore, the EQD2 of this regimen is clearly lower than the one of the EBRT + HDR boost arm (66.8 vs. 92 Gy for an α/β of 1.5): not surprisingly, the bNED results of the EBRT alone arm are suboptimal as compared to other series. Table 11.6 reports the efficacy results of the most relevant published series of combined HDR brachytherapy and EBRT. Comparisons between series and with other therapeutic options for the same patients risk groups are complicated by inherent methodological difficulties in terms of dissimilar HDR and EBRT schedules, varying risk categories treated, use of different irradiated volumes and dose, use of ADT, reported endpoints and biochemical-relapse-free survival definitions adopted, and length of follow-up. Since intermediate- to high-risk patients are often well represented in HDR + EBRT series, the use of ADT in association to irradiation is frequently considered. Interestingly, ADT has not always been shown to improve outcomes in this setting with some authors even reporting a detrimental effect of ADT on overall survival and metastatic failure rates (Krauss et al. 2011; Martinez et al. 2005a, b). HDR as monotherapy for patients diagnosed with low-to-intermediate prostate cancer is not yet widely established, and few series with mature survival data have been published so far (Table 11.7). It is worthwhile mentioning that the phenomena of PSA bounce after HDR monotherapy or combined HDR-brachytherapy-EBRT has been described. In a comparative, nonrandomized study, patients treated with HDR monotherapy showed higher rates of PSA bounce as compared to patients irradiated with EBRT alone or with combined protocols with significant differences identified for bounce definitions ≥0.3 and >0.5 ng/ml (McGrath et al. 2010).
11.4.2 Toxicity and Quality of Life
11.4.2.1 Combined HDR-Brachytherapy-EBRT
The only phase III randomized clinical trial published so far and comparing EBRT to EBRT combined with a HDR boost has also reported treatment toxicities and quality of life data according to the FACT-P summary score, a validated patient-reported questionnaire (Hoskin et al. 2007). The RTOG acute and grade 2 and greater late toxicity scores were similar in the two arms of the study while a significant difference favoring the combined HDR-EBRT arm was present at 12 weeks after irradiation as far as quality of life was concerned. In all published series, acute toxicity primarily consists of mild LUTS (dysuria, urinary frequency, urgency) in 40–60% of patients, but grade 3 genitourinary (GU) symptoms are only presents in 1–5%. Morton has prospectively measured in a cohort of 125 patients treated with EBRT (45 Gy in 25 fractions) with an HDR brachytherapy boost of 15 Gy in a single fraction without ADT, the evolution of acute GU toxicity by means of the IPS-Score: the return to baseline values was obtained at the third month postimplant, much earlier than after LDR brachytherapy (Morton et al. 2010). In the immediate postimplant hours, hematuria is also relatively common but resolves rapidly without special intervention. Urethral stricture is the most frequent nontrivial late toxicity reported after combined HDR and EBRT (Table 11.8) occurring in the bulbomembranous urethra in more than 90% of the cases (Sullivan et al. 2009). Overall, urethral strictures develop in 5–15% of patients with several patient-related predictors being identified such as a prior history of TURP, an elevated preimplant IPS-Score, older age, prostate volume (and use of neoadjuvant ADT for preimplant downsizing), and hypertension but also with a number of treatment-related ones (HDR dose per fraction, number of midline needles implanted, a long Z-axis of the CTV). Incontinence is less common and typically related to postimplant need of a TURP. Gastrointestinal (GI) toxicity is frequently dependent on EBRT protocol adopted (irradiated volumes, prostate alone versus pelvis +/− prostate CTV, dose/fraction to the pelvis) with grade 3 toxicity reported occasionally and proctitis, anal pain, and rectal bleeding occurring in less than 5% of patients in all published papers. Data on erectile dysfunction after combined HDR-brachytherapy-EBRT irradiation have been rarely reported with a variety of scales at different time frame from implant which makes it extremely difficult to derive a meaningful global picture. Duchesne et al. have prospectively evaluated the erectile function in 55 patients irradiated with combined EBRT (46 Gy in 23 fractions) and HDR boost (16–20 Gy in 4 fractions) without ADT and potent before treatment with an “in-house” scale (Duchesne et al. 2007). The 5-year actual incidence of insufficient erection for intercourse (grade 2) or no erection at all (grade 3) was 77%. The International Index of Erectile Function (IIEF) (Rosen et al. 1997) has been prospectively used by Morton: the median baseline IIEF Score (=19) decreased to 6 one-year posttreatment and among patients reporting good baseline erectile function (IIEF >21), 35% developed moderate-to-severe erectile dysfunction (Morton et al. 2010).
11.4.2.2 HDR Monotherapy (Table 11.9)
It has been correctly observed that the pattern of toxicity after HDR monotherapy is different from that after LDR brachytherapy (Hoskin 2008; Crook 2011). GU symptoms after HDR alone peak in the first 2 weeks after the implant with IPS-Score increasing at that time but rapidly falling to baseline in 2–3 months. As already mentioned, hematuria may be present in the immediate postimplant hours due to bruising of the bladder wall during the procedure but generally resolves spontaneously within 2 days. So far, no randomized trial has ever compared the two techniques, but nonrandom evaluations have confirmed that both acute (dysuria, urinary frequency, and urgency) and late toxicity are significantly less frequent after HDR than LDR monotherapy (Martinez et al. 2010). In contrast, the rates of urinary retention or incontinence and of erectile dysfunction do not seem to be different between HDR and LDR monotherapies.
11.5 Future Directions
Current data have established HDR brachytherapy, both as a boost combined with EBRT or alone for patients with low-to-intermediate-risk disease, as an effective form of local treatment for prostate cancer. However, several areas remain for further investigations and should be explored in future trials. (1)The optimal dosing regimens is still unclear: the first published series (both for HDR as a boost and as monotherapy) adopted HDR schedules of two or more fractions, while recently, protocols proposing a single fraction/implant have been developed with encouraging early results, thus minimizing the risk of potential needles/catheters displacement between fractions. (2)Therapeutic options for local salvage (re)treatment after biopsy-proven intraprostatic relapse of irradiated prostate cancers are currently limited to ADT (continuous or intermittent), salvage prostatectomy, cryotherapy, or high-intensity-focused ultrasound. HDR (together with LDR) brachytherapy has also been proposed in this setting with encouraging results both in terms of efficacy and of toxicity, but larger series with longer follow-up are needed to fully validate this strategy (Lee et al. 2007; Tharp et al. 2008). (3) The typical inhomogeneous dose distribution obtained with brachytherapy techniques can be exploited in view of a “focal irradiation” of the prostate for carefully selected patients harboring limited unilateral cancers at the diagnostic biopsy confirmed by imaging techniques such as functional MRI. HDR is probably the best technique to create intraprostatic dose gradients that will give the opportunity to target limited regions of the gland to very high doses, while treating to more conventional doses the biopsy-negative subvolumes of the CTV (Ares et al. 2009; Zaider et al. 2000). (4) We have already mentioned that costs of temporary HDR brachytherapy are limited. Rigorous cost analysis and comparisons between therapeutic alternatives have so far never been attempted, but if HDR brachytherapy techniques are able to convincingly demonstrate a cost-effective advantage as compared to other therapeutic options for localized prostate cancer, the procedure is likely to be offered in the near future to increasing numbers of patients and to gain popularity even in developing countries.
References
Ares C, PopowskiY PS et al (2009) Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int J Radiat Oncol Biol Phys 75:656–673
Arruda-Viani GA, Pellizzon AC, Guimaraes FS et al (2009) High dose rate and external beam radiotherapy in locally advanced prostate cancer. Am J Clin Oncol 32:187–190
Astrom L, Pedersen D, Mercke C et al (2005) Long-term outcome of high dose rate brachytherapy in radiotherapy of localized prostate cancer. Radiother Oncol 74:157–161
Barry MJ, Fowler FJ, O’Leary MP et al (1992) Measurement committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 148:1549–1557
Brenner DJ, Martinez A, Edmundson GK et al (2002) Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late responding normal tissue. Int J Radiat Oncol Biol Phys 52:6–13
Cahlon O, Zelefsky MJ, Shippy A et al (2008) Ultra high dose, 86.4 Gy, IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 71:330–337
Corner C, Rojas AM, Bryant L et al (2008) A phase II study of high-dose-rate afterloading brachytherapy as monotherapy for the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys 72:441–446
Cornud F, Flam T, Chauveinc L et al (2002) Extraprostatic spread of clinically localized prostate cancer: factors predictive of pT3 tumors and of MR imaging examination results. Radiology 224:203–210
Crook J (2011) The role of brachytherapy in the definitive management of prostate cancer. Cancer Radiother 15:230–237
Crook J, McLean M, Catton C et al (2002) Factors influencing risk of acute urinary retention after TRUS-guided permanent prostate seed implantation. Int J Radiat Oncol Biol Phys 52:453–460
De Crevoisier R, Tucker SL, Dong L et al (2005) Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 62:965–973
Dearnaley DP, Sydes MR, Graham JD et al (2007) Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RTO1 randomised trial. Lancet Oncol 6:475–487
Demanes DJ, Rodriguez RR, Schour L et al (2005) High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California Endocurietherapy’s 10 year results. Int J Radiat Oncol Biol Phys 61:1306–1316
Demanes DJ, Martinez A, Ghilezan M et al. (2011) High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 81:1286–1292
Deutsch I, Zelefsky MJ, Zhang Z et al (2010) Comparison of PSA relapse-free survival in patients treated with ultra-high-dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy 9:313–318
Duchesne GM, Williams SG, Das R et al (2007) Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer: mature prospective phase I-II study results. Radiother Oncol 84:128–134
Edmundson GK, Di Yan RT, Martinez A et al (1995) Intraoperative optimization of needle placement and dwell times for conformal prostate brachytherapy. Int J Radiat Oncol Biol Phys 33:1257–1263
Foster W, Cunha JAM, Chow-Hsu I et al (2011) Dosimetric impact of catheters interfractions movement in high dose rate prostate brachytherapy. Int J Radiat Oncol Biol Phys 80:85–90
Fuchsjager M, Shukla-Dave A, Akin O et al (2008) Prostate cancer imaging. Acta Radiol 49:107–120
Galalae RM, Kovacs G, Schultze J et al (2002) Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high dose rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 52:81–90
Galalae RM, Martinez A, Nuernberg N et al (2006) Hypofractionated conformal HDR brachytherapy in hormone naïve men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol 182:135–141
Ghadjar N, Keller T, Rentsch CA et al (2009) Toxicity and early treatment outcomes in low and intermediate-risk prostate cancer managed by high dose rate brachytherapy as a monotherapy. Brachytherapy 8:45–51
Heidenreich A, Bellmunt J, Bolla M et al (2011) EAU guidelines on prostate cancer. Part 1: screening, diagnosis and treatment of clinically localized disease. Eur Urol 59:61–71
Hermesse J, Biver S, Jansen N et al (2009) A dosimetric selectivity intercomparison of HDR brachytherapy, IMRT and helical tomotherapy in prostate cancer radiotherapy. Strahlenther Onkol 185:736–742
Hoskin P (2008) High dose rate brachytherapy for prostate cancer. Cancer Radiother 12:512–514
Hoskin P, Motohashi K, Bownes P et al (2007) High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomized phase three trial. Radiat Oncol 84:114–120
Hsu IC, Bae K, Shinohara K et al (2010) Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int J Radiat Oncol Biol Phys 78:751–758
Joiner MC, Bentzen SM (2009) Fractionation: the linear-quadratic approach. In: Joiner MC, Van der Kogel A (eds) Basic clinical radiobiology, 4th edn. Hodder Arnold, London
Kalkner KM, Wahlgren T, Ryberg M et al (2007) Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dos-rate iridium 192 brachytherapy boost: a 6 year follow-up. Acta Oncol 46:909–917
Kovacs G, Potter R, Loch T et al (2005) GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localized prostate cancer. Radiother Oncol 74:137–148
Krauss D, Kestin L, Ye H et al (2011) Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-dose prostate cancer. Int J Radiat Oncol Biol Phys 80:1064–1071
Lee B, Shinohara K, Weinberg V et al (2007) Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco experience. Int J Radiat Oncol Biol Phys 67:1106–1112
Mark RJ, Anderson PJ, Akins RS et al (2010) Interstitial high-dose-rate brachytherapy as monotherapy for early stage prostate cancer: median 8 years results in 301 patients. Brachytherapy 9:s76
Martens C, Pond G, Webster D et al (2006) Relationship of the IPS score with urinary flow study, and catheterization rates following 125I prostate brachytherapy. Brachytherapy 5:9–13
Martinez A, Demanes J, Galalae R (2005a) High dose rate afterloading 192Ir prostate brachytherapy. In: Dicker AP, Merrick GS, Waterman FM et al (eds) Basics and advanced techniques in prostate brachytherapy. Taylor Francis Group, Abingdon
Martinez A, Demanes J, Galalae R (2005b) Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regimen. Int J Radiat Oncol Biol Phys 62:1322–1331
Martinez A, Demanes J, Vargas C et al (2010) High-dose-rate-prostate brachytherapy. An excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol 33:481–488
Martinez A, Gonzalez J, Ye H et al (2011) Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys 79:363–370
McGrath SD, Antonucci JV, Fitch DL et al (2010) PSA bounce after prostate brachytherapy with or without neoadjuvant androgen deprivation. Brachytherapy 9:137–144
Mohammed N, Kestin L, Ghilezan M et al. (2012) Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 82:204–212
Morton GC, Loblaw DA, Sankreacha R et al (2010) Single-fraction High-Dose-Rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate-risk prostate cancer: analysis of short- and medium-term toxicity and quality of life. Int J Radiat Oncol Biol Phys 77:811–817
Neviani CB, Miziara MA, De Andrade CH (2011) Results of high dose-rate brachytherapy boost after 2D or 3D external beam irradiation for prostate cancer. Radiother Oncol 98:169–174
Nickers P, Thissen B, Jansen N et al (2006) 192Ir Or 125I prostate brachytherapy as a boost to external beam radiotherapy in locally advanced prostatic cancer: a dosimetric point of view. Radiother Oncol 78:47–52
Peeters ST, Heembergen WD, Koper PC et al (2006) Dose–response in radiotherapy for localized prostate cancer. Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24:1990–1996
Pieters BR, De Back DZ, Koning CCE et al (2009) Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: a systematic review. Radiother Oncol 93:168–173
Rogers CL, Alder SC, Rogers RL et al (2012) High dose brachytherapy as Monotherapy for Intermediate risk prostate cancer. J Urol 187:109–116
Rosen RC, Riley A, Wagner G et al (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49:822–830
Seppenwoolde Y, Kolkman-Deurloo IK, Sipkema D et al (2008) HDR prostate monotherapy-dosimetric effects of implant deformation due to posture change between TRUS- and CT-imaging. Radiother Oncol 86:114–119
Simnor T, Li S, Lowe G et al (2009) Justification for inter-fraction correction of catheter movement in fractionated high dose-rate brachytherapy treatment of prostate cancer. Radiother Oncol 93:253–258
Slessinger ED (2010) Technical note practical consideration for prostate HDR brachytherapy. Brachytherapy 9:282–287
Stone NN, Marshall DT, Stone JJ et al (2010) Does neo-adjuvant hormonal therapy improve urinary function when given to men with large prostates undergoing prostate brachytherapy? J Urol 183:634–640
Sullivan L, Williams SG, Thai KH et al (2009) Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 91:232–236
Tharp M, Hardacre M, Bennett R et al (2008) Prostate high-dose-rate brachytherapy as salvage treatment of local failure after previous external or permanent seed irradiation for prostate cancer. Brachytherapy 7:231–236
The International Prostate Symptom Score (I-PSS) and Quality of Life assessment (1991) Proceedings of the international consultation on benign prostatic hyperplasia (BPH), Paris, 280–281
Tiong A, Bydder S, Ebert M et al (2010) A small tolerance for catheters displacement in high dose rate prostate brachytherapy is necessary and feasible. Int J Radiat Oncol Biol Phys 76:1066–1072
Yoshioka Y, Konishi K, Sumida I et al (2011) Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five years results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys 80:469–475
Zaider M, Zelefsky M, Lee EK et al (2000) Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys 47:1085–1096
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bossi, A., Verstraet, R., Calmels, L., Blanchard, P. (2012). High-Dose-Rate Brachytherapy: Indications, Technique, and Results. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27597-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-27597-5_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-27596-8
Online ISBN: 978-3-642-27597-5
eBook Packages: MedicineMedicine (R0)