Abstract
In recent years, research and the use of nanomaterials has attracted much interest due to their small size (1–100 nm) and novel structures that exhibit significantly improved physical, chemical, and biological properties compared to their bulk or molecular precursors. In this context, a new branch of multidisciplinary science integrating engineering with biology, chemistry and physics has emerged as nanosciences or nanotechnology, due to their existence and potential applications in a wide variety of fields such as electronics, ceramics, catalysis, magnetic data storage, structural components, food, cosmetics, biological and medical [1–3]. Metal oxides, in particular the transition metal oxides, have profound applications in various fields due to their excellent optical, magnetic, electrical and chemical properties. As the size decreases from the micrometer to the nanometer range, the materials exhibit enhanced diffusivity, increased mechanical strength and chemical reactivity, higher specific heat and electrical resistivity, and enhanced biological properties. This is in part because as particles become smaller, the proportion of atom found at the surface increases relative to the proportion inside its volume, which means that composite materials containing nanoparticles can be more reactive and have enhanced chemical properties. Nanostructure metal oxides are more interesting in that they can be synthesized with a very high surface-to-volume ratio and with unusual morphologies that contain numerous edge/corner and other reactive surface sites, which can be easily functionalized with different groups for the desired applications. An increasing use of nanomaterials has been reported in biological- and medical-related applications such as imaging, sensing, target drug delivery, fighting human pathogens, healthcare products, cosmetics, and food preservative agents due to better safety and stability compared to bulk precursors or their organic counterparts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In recent years, research and the use of nanomaterials has attracted much interest due to their small size (1–100 nm) and novel structures that exhibit significantly improved physical, chemical, and biological properties compared to their bulk or molecular precursors. In this context, a new branch of multidisciplinary science integrating engineering with biology, chemistry, and physics has emerged as nanosciences or nanotechnology, due to their existence and potential applications in a wide variety of fields such as electronics, ceramics, catalysis, magnetic data storage, structural components, food, cosmetics, biological, and medical [1–3]. Metal oxides, in particular the transition metal oxides, have profound applications in various fields due to their excellent optical, magnetic, electrical, and chemical properties. As the size decreases from the micrometer to the nanometer range, the materials exhibit enhanced diffusivity, increased mechanical strength and chemical reactivity, higher specific heat and electrical resistivity, and enhanced biological properties. This is in part because as particles become smaller, the proportion of atom found at the surface increases relative to the proportion inside its volume, which means that composite materials containing nanoparticles can be more reactive and have enhanced chemical properties. Nanostructure metal oxides are more interesting in that they can be synthesized with a very high surface-to-volume ratio and with unusual morphologies that contain numerous edge/corner and other reactive surface sites, which can be easily functionalized with different groups for the desired applications. An increasing use of nanomaterials has been reported in biological- and medical-related applications such as imaging, sensing, target drug delivery, fighting human pathogens, healthcare products, cosmetics, and food preservative agents due to better safety and stability compared to bulk precursors or their organic counterparts.
The development of agents with antimicrobial activity and surface coatings has been attracting increased interest among various researchers in recent years due to the continuous emergence and spread of multiple or pan-antibiotic-resistant strains of various infectious organisms worldwide. In addition, microbial contamination is a serious health issue in both hospital- and community-associated settings and in the food industries. The antimicrobial activity of nanoparticles has been studied for several metal and metal oxide nanoparticles and bulk powders with different pathogenic and nonpathogenic bacteria [3–19]. Inorganic materials with antibacterial activity can be used in different forms such as powders, coated on the surface of hospital or medical devices, and/or as a part of organic/inorganic nanocomposite coatings in numerous industrial sectors including environmental, food, textiles, packaging, husbandry, healthcare, and medical care, as well as construction and decoration [1–3]. The main advantages of using inorganic oxides when compared with organic antimicrobial agents are their stability at higher temperatures and/or pressures, their ability to withstand harsh processes, and their robustness and long shelf life [3, 4]. Some of the inorganic or bulk oxide powders that have been tested for their antimicrobial activity are TiO2, ZnO, MgO, CaO, CuO, Al2O3, AgO, and CeO2 under different conditions [4, 6–8, 10–19]. Interestingly, several metal oxides (e.g., CaO, MgO, and ZnO) showed antimicrobial activity without photo-activation in contrast to others like TiO2 that required photo-activation [4, 8, 9, 20, 21]. This has attracted much more attention toward developing an alternative compound to substitute for the conventional organic compounds like quaternary ammonium salt and chlorine disinfectant. Among the inorganic materials, metal oxides such as TiO2, ZnO, MgO, and CaO are not only stable under harsh conditions but also regarded as safe materials for human beings and animals, and are part of essential minerals for human health [1, 4, 20]. Furthermore, TiO2 and ZnO have been used extensively in the formulation of various personal care products [22].
ZnO is a wurtzite-type semiconductor and piezo-electrical material exhibiting excellent electrical, optical and chemical properties with band-gap energy of 3.1–3.4 eV. It has a very large excitation binding energy of 60 meV at room temperature, which is very close to that of TiO2 [14]. It is considered to be more suitable for photocatalysis applications due to its high photosensitivity, and chemical stability. Recently, special interest has been shown in its morphology, as ZnO can form various nanostructures suitable for a wide variety of applications in UV-shielding materials, gas sensors, biosensors, semiconductors, piezoelectric devices, field emission displays, photocatalytic degradation of pollutants, and antimicrobial treatments [1–3]. Several physical parameters such as surface area, particle size, surface charge, and zeta potential of a material are very important for its applications and function. These physical factors of nanoparticles very often govern the stability, uptake, persistence, and chemical or biological activities inside the living cells. ZnO nanoparticles are considered to be non-toxic, biosafe, and biocompatible and have been found in many biological applications in daily life such as drug carriers, and in cosmetics, and as fillings in medical materials or devices [3, 4, 20]. Discovery of antimicrobial properties of metal oxides either powders or nanoscale materials has gained increased interest over the past decade largely due to the increasing emergence and spread of multiple antibiotic-resistant strains against organic antimicrobial agents. The majority of research and interest in the antimicrobial properties of metal oxides regards their use as antimicrobial coatings of the surfaces of various devices to eliminate survival of microorganisms on surfaces in the environment, community and health care settings that will eventually stop the spread of the diseases. In addition, coatings are expected to have better stability and safety, although nanoscale materials may pose a health-related hazard upon inhalation [10, 22]. It has been recognized that nanoscale materials pose more cytotoxicity than larger particles of the same material [20]. In this context, ZnO has advantages over other metal oxides such as TiO2 for developing metal oxide-based antimicrobial agents at ambient conditions as its activity does not require photo-activation [10]. Antimicrobial activity and stability of ZnO nanoparticles can be enhanced by incorporating agents during synthesis. Because of the multifunctional nature of ZnO nanoparticles, it would be difficult to cover all aspects of interest. In this chapter, we will focus on the following aspects: synthesis, characterization, mechanism of antimicrobial activity, and biological applications.
2 Zinc Oxide Crystal Structure and Band Structure
Zinc oxide normally crystallizes in a hexagonal wurtzite structure, which is its most thermodynamically stable phase. This phase is where each anion is surrounded by four cations at the corners of a tetrahedron with a typical sp 3 covalent bonding [3, 23, 24]. It exhibits partial polar characteristics with lattice parameters a o = 0.32495 nm, c o = 0.52069 nm and a o /c o = 1.602–1.633. As shown in Fig. 5.1, the ZnO structure can be described as number of alternative unit cells composed of tetrahedrally coordinated O2− and Zn2+ stacked alternatively along the c-axis, resulting in the absence of inversion symmetry structure. For nanoparticle ZnO, the concentration of zinc and oxygen atoms located on the surface is greatly increased due to the very large surface area, which affects its density and renders it with novel physical, chemical, and optoelectronic properties or responses. The most interesting characteristic of ZnO is that its polar surface is induced by its strong sensitivity to normal light at room temperature. Specifically, once a ZnO crystal is exposed to normal light, it readily deforms by the basal plane or growth direction {0001} and forms polar surfaces. One end of the basal polar plane terminates with partially positive Zn lattice sites and the other end terminates with partially negative oxygen sites, resulting in a normal dipole moment and spontaneous polarization along the c-axis as well as variation in surface energy. To maintain a stable structure, the polar surfaces generally have facets or exhibit surface deformations, but nano-ZnO surfaces are exceptions as they are atomically flat, stable and exhibit no deformations. However, for nano-ZnO, there have been many conflicting views regarding the superior stability, electron transport, band structure and other characteristics compared to the bulk ZnO, although discussions are ongoing among the theoretical physicists and chemists [1, 3, 4]. In addition to growth surface or facet {0001}, two more commonly observed facets for ZnO are the top surface {2110} and side surface {0110}, which are non-polar and have lower energy. ZnO exhibits various novel structures which can be grown by turning the growth rates along different directions of the facets.
The hexagonal wurtzite structure model of ZnO. The lattice constants are a = 0.325 nm and c = 0.5207 nm. All atoms are tetrahedrally coordinated. The fundamental band gap is direct with a value of 3.3 eV at room temperature (Adapted from http://en.wikipedia.org/wiki/ Wurtzite_ crystal_ structure)
The crystal structure, size, and shape of the synthesized ZnO nanoparticles can be investigated using several methods such as nitrogen physisorption (N2), powder X-ray diffraction (XRD), and scanning and transmission electron microscopies (SEM and TEM) [3, 4, 23–31]. The crystallographic orientation of ZnO nanoparticles synthesized using hydrothermal methods is determined by XRD. A typical XRD pattern of a 12-nm-diameter particle synthesized ZnO nanoparticles by a hydrothermal method is shown in Fig. 5.2a. Nine peaks appeared at the positions of 2θ = 31.63°, 34.50°, 36.25°, 47.50°, 56.50°, 62.80°, 66.35°, 67.92°, and 68.91°, which correspond to {100}, {002}, {101}, {102}, {110}, {103}, {200}, and {201}, respectively, which are in good agreement with the assigned standard wurtzite-type ZnO structure (JCPDS 36–1,451) [31]. The specific surface areas, pore volumes, and diameters of ZnO nanoparticles can be determined using nitrogen physisorption studies in a Quantachrome Nova 2200e series surface area analyzer or similar instruments. For example, the surface areas of ZnO nanoparticles can be calculated using the Brunauer–Emmett–Teller equation in the relative pressure range (P/P o ) of 0.05–0.30. The average particle diameter (D) of the zinc oxide particles can also be estimated under the assumption that all particles are spherical in shape, and using the equation (D = 6/S ρ), where S is specific surface area per unit gram of the sample and ρ is the density of zinc oxide. The morphology and the size of the synthesized nanoparticles can be determined using various high resolution microscopes such as SEM or TEM [3, 19, 31]. A typical TEM pattern for ZnO nanoparticles synthesized through a hydrothermal method is shown in Fig. 5.2b. Lattice spacing of 0.28 and 0.16 nm indicate the presence of the {100} and {110} planes. In addition, electron diffraction studies can be performed to determine the crystal structure and the growth orientation of the ZnO nanoparticles.
(a) Typical X-ray diffraction (XRD) pattern for a 25-nm-diameter ZnO nanoparticle prepared using hydrothermal method. (b) TEM image of a 13-nm-diameter ZnO nanoparticle synthesized by force hydrolysis of zinc acetate at 160°C. The inset shows a selected area electron diffraction pattern confirming the crystalline ZnO phase (TEM image reproduced from [47]. With permission of the American Institute of Physics)
3 Synthesis of ZnO Nanoparticles
Significant progress has been made in understanding fundamental aspects of the synthesis of nanoparticles in different forms. Various routes have been developed for the small- and large-scale production of nanoparticles. Several methods have been employed for the preparation of different forms of ZnO nanoparticles to investigate various functions and further improvements; however, a more fundamental approach of the exact growth mechanism of nanoparticles synthesis remains largely unknown. The different synthesis methods with different parameters and stringent growth conditions like temperature, pressure, hydrolysis ratio, and precursors has been adopted to achieve different forms of ZnO nanoparticles such as nanospheres, nanotubes, nanorods, nanobelts, nanowires, nanocombs, nanorings, nanoloops, nanobows, and nanoribbons [24–29]. These methods include metal–organic chemical vapor deposition, hydrothermal synthesis, thermal evaporation via a vapor liquid solid process assisted by a catalyst, and oxidation of metallic zinc powder without metal [24–26]. In addition, synthesis of ZnO nanoparticles largely depends on whether they will be used in biological and/or non-biological related systems. The relatively simple room temperature synthesis is more important than other methods for either coatings or biological applications. In low temperatures, synthesis is primarily focused on redox–reaction processes for metal chalcogenides or limited precipitation and/or crystallization for some metal salts and hydroxides. Overall, these methods are broadly classified into two main routes of nanoparticles synthesis: vapor phase and solution phase synthesis.
3.1 Vapor or Gas Phase Synthesis of ZnO
Various approaches have been developed for the synthesis of metal and ceramic nanoparticles with well-defined and narrow size distribution in the gas or vapor phase synthesis route using an inert gaseous environment in closed chambers. Normally, the synthesis of ZnO nanoparticles is carried out at higher temperatures from 500°C to 1,500°C, which is below the melting point of 1,975°C. Some of the commonly used gas phase methods are vapor phase transport that includes vapor solid and vapor liquid solid growth, physical vapor deposition, chemical vapor deposition, metal organic chemical vapor deposition, thermal oxidation of pure Zn and condensation, and microwave-assisted thermal decomposition [24]. Different sources such as evaporation, sputtering and laser are used to evaporate materials from the surface of solids into clusters, which are then condensed, transported, and collected on a cold finger to form the nanoparticles. Various types of ZnO nanoparticles such as nanowires, sheets, and tetrapods can be synthesized by physical vaporization of zinc powder with or without the presence of catalysts and exposure to air or N2/O2 mixture at relatively high temperature ranging from 800°C to 850°C [27]. One-dimensional high purity ZnO nanobelts have been synthesized using thermal evaporation of zinc sulfate (ZnS) powders in a hydrogen–oxygen mixture gas at 1,050°C [28] for potential application in optoelectronic devices. Generally, to synthesize ZnO nanoparticles, the metal bulk of zinc is placed inside the vacuum chamber and then, by setting the heating current, vacuum pressure and vaporized temperature, it melts and vaporizes into gas. Inside the chamber, the material vapor collides with insert gas to cool off, which then flows into a low temperature collector and forms nanoparticles. This simple principle has been used to synthesize different structures of ZnO or metallic Zn nanoparticles for fabrication on the surface of potential applications. Several methods which have been described for growing the different ZnO nanostructures or nanoparticles in the solution phase are also part of the gas phase synthesis.
3.2 Solution Phase Synthesis
Solution phase synthesis is often known as the hydrothermal growth process because the synthesis is carried out in aqueous solutions at a relatively low temperature. Some of the solution phase synthesis methods are the zinc acetate hydrate-derived nanocolloidal sol–gel route, zinc acetate hydrate in alcoholic solution with hydroxide (s), temperature-assisted synthesis, spray pyrolysis for growth of thin film, and electrophoresis [24, 25]. Numerous methods have been reported which are beyond the scope of description in this chapter; instead, we will present several of the most frequent methods used.
3.2.1 Sol–Gel Synthesis of ZnO Nanoparticles
The sol–gel processing method has been used for producing metal oxide and ceramic powders with high purity and high homogeneity [24, 29]. The sol–gel offers a degree of control of composition, shape, and morphologies of nanoparticles at the molecular level. The process involves the preparation of a colloidal suspension (“sol”), which is subsequently converted to viscous gels and solid materials using the principles of hydrolization, condensation, and polymerization reactions. Templates or precursors such as zinc acetate hydrates in an alcohol or ethanolic suspension are refluxed and distilled to form a transparent sol to remove the solvents and followed by drying. Small ZnO nanoparticles (>5 nm) can be formed under high concentration conditions by addition of hydroxides (e.g., LiOH, NaOH, etc.) and subsequent condensation and polymerization reactions. There are several factors, such as the nature of the alkyl group and the solvent, the concentration of each species in the solvent, the temperature, the water to alkoxide molar ratio, and the presence of acid or base catalysts, known to affect the different steps for the growth of ZnO nanoparticles. When zinc acetate, Zn(Ac)2, is heated in alcohol, the following reaction initially occurs:
The tetrahedral oxy-acetate, Zn4O(Ac)6, is also known as basic zinc acetate and is a well-designed molecular model of ZnO. Zinc acetate can form larger homologues like ethoxy acetate, [Zn10O4(Ac)12] and hydroxy-double salt, [(Zn-HDS)Zn5(OH)8 (Ac)2 (H2O)2] from Zn4O(Ac)6. The by-products such as H2O, acetic acid, etc. are removed by distillation. ZnO nanoparticles are formed by continuous refluxing from these sol substrates. It has been observed that Zn4O(Ac)6 is more stable than zinc acetate hydrate, while the stability of ethoxy acetate is enhanced in the presence of H2O and ethanol. There is spontaneous formation of zinc hydroxyl double salts due to the presence of water and the higher stability of Zn-HDS monomer with respect to the oxy-acetate clusters. The zinc ethoxy-acetate is most the stable precursor because free Zn2+ ions do not exist in alcoholic zinc acetate hydrate solutions due to a strong chemical bond exists between Zn2+ ions and Ac ligands. The sol–gel process has been useful for synthesizing metal oxides as a result of the presence of metal–oxygen bonds. The process has distinct advantages over other methods for preparing metal oxide nanoparticles that include faster nucleation and growth, the formation of high purity powders as a result of homogenous mixing of the raw materials and the large-scale industrial production of nanopowders. The disadvantage of the process is the high cost for the precursors of the metal materials.
3.2.2 Hydrothermal Synthesis of ZnO Nanoparticles
Hydrothermal or wet chemical synthesis processes are solution-based processing routes used for the synthesis of nanoparticles. These include precipitation of solids from a supersaturated solutions, homogeneous liquid phase chemical reduction, and ultrasonic decompositions of chemical precursors [24, 29, 30]. These methods are more attractive due to their simplicity, versatility, and availability of low cost precursors.
3.2.2.1 Room Temperature Synthesis
The simplest route used to synthesize ZnO nanoparticles is room temperature synthesis using an acid-base precipitation method [22, 25, 29, 31]. In a typical synthesis, the precursor solution of zinc such as zinc nitrate, zinc acetate, or zinc sulfate, and an aqueous solution of base like NaOH, KOH, trimethyl (or ethyl) ammonium hydroxide, or NH4OH are prepared in nanopure water. The acid solution is mixed with the base by varying the molar hydrolysis ratio of Zn2+/OH− from 1 to 10, and the precipitate is harvested, washed, and dried in a static air oven at 80–90°C for several hours. The following reactions occur during the formation of ZnO nanoparticles:
Liu and Zeng [17] used similar room temperature solution synthesis to synthesize ZnO nanorods using the precursor solution of zinc nitrate [Zn(NO3)2.6H2O] and a NaOH solution in deionized water with different molar ratios of Zn2+ to OH− varying from 1:3 to 1:40 at room temperature (25 ± 2°C) under constant stirring for 1–12 days. In addition, several reports of hydrothermal synthesis in aqueous solution have been reported with modification of the reaction conditions and the precursor materials used to achieve desired sizes and morphologies of the synthesized ZnO nanostructures/nanoparticles [24, 31]. For example, zinc acetate hexahydrate was mixed with sodium hydroxide in water with varying molar ratios of Zn2+ and OH− at room temperature for 2 h, followed by sonication for 30 min to impart uniformity. Synthesis of ZnO nanoparticles can be carried out in an autoclave at different temperatures from 80°C to 180°C with varying reaction times from 12 to 48 h. For more simplicity of hydrothermal synthesis, the mixture of zinc salts (e.g., zinc acetate) and base (e.g., NaOH or NH4OH) with adjustment in pH (>7.0) and/or heat (100–200°C) for 2 h is also very effective in forming a crystalline ZnO nanopowder.
Conveniently, the particle size and morphology can be manipulated during the growth of ZnO nanoparticles by using different precursors such as zinc nitrate, zinc sulfate, zinc chloride; bases like potassium hydroxide, ammonium hydroxide, tetramethylammonium hydroxide (TMAH), tetraethylammonium hydroxide (TEAH), diethanolamine; growth temperature; pressure; pH; and addition of macromolecules such as different surfactants such as polyvinyl alcohol (PVA), polyethylene glycol (PEG), sodium dodecyl sulpfate (SDS), and cetyltrimethyl ammonium bromide (CTAB) [24]. Similar modification followed a significant change in morphology from rod-like to polyhedral, which was observed using ZnCl2 and NaOH in hydrothermal synthesis with different organic compounds. In this example, crystalline nanoparticles of 10–20 nm were synthesized at room temperature by adding TMAH to an ethanolic solution of zinc acetate dehydrate, whereas addition of water to the ethanolic solution prior to adding TMAH produced ZnO nanoflakes [24]. Spherical ZnO nanoparticles of diameters ranging from 39 to 320 nm were synthesized by oxidation of zinc acetate in supercritical water [32]. Thus, particle size and morphology can be modulated by varying conditions like temperature, pressure and/or the reaction atmosphere.
3.2.2.2 Synthesis of Different Structures ZnO Nanostructures
The synthesis of nanoparticles can be divided into four steps: precursor formation, nucleation, growth, and aging. The kinetics of these processes determines the properties of the final product. The chemistry of precursor formation depends on the solvent and reactants present in the solution. The presence of water helps speed up the nucleation process during synthesis. Condensation reactions lead to nucleation that determines the detailed structure of the solid material. Faster nucleation processes combined with relatively slow growth and aging kinetics, is desirable for monodispersed colloids. The growth process does not significantly alter the particle size and size distribution; rather, aging can influence the types of aggregation or coarseness of the final product. Most of the nanostructures of ZnO such as nanowires, nanorods, nanobelts, etc., are important for various applications during fabrication and can be easily synthesized using different controlling agents such as surfactants, doping agents, or changing solvents. ZnO nanowires can be formed on glass surfaces by thermal decomposition of highly water soluble methenamine and zinc nitrate. These probably act as shape-inducing polymer surfactants, thereby blocking the growth of ZnO to other faces (e.g., {2110} and {0110}) and leaving only the polar {0001} face. There are numerous investigations regarding variation in the morphology of ZnO nanostructures using different surfactants, growth on substrates, and the control of reaction conditions. Tang et al. [33] synthesized ZnO nanorods using single precursor, zinc acetylacetonate hydratein, and the presence of four different surfactants (PVA, PEG, SDS, and CTAB). Similarly, carbamide was used as a surfactant for the synthesis of ZnO nanobelts in a hydrothermal growth process with ZnSO4 and NaOH. Zhang et al. [34] has been able to synthesize bundles of ZnO nanostructures through a macromolecular surfactant (L64 and F68) using a hydrothermal growth route. Nanoflowers of ZnO structure with hexagonal nanorod petals as long as 2–4 μm were synthesized at a temperature of 90°C in 30 min using zinc acetate dehydrate and sodium hydroxide through the hydrothermal method [24]. The growth of flower-like and cabbage-like nanostructures was also achieved using the hydrothermal growth method and the surfactant CTAB at temperatures of 120°C, 150°C, and 180°C. The flower-like micro- and nanostructures of ZnO nanorods are preferentially formed at a temperature of 120°C, whereas cabbage-like nanostructures are formed at higher temperatures of 150°C and 180°C due to repeated growth of two-dimensional ZnO sheets of 50 m or 100 nm. Overall, the nanoparticle size and morphologies can be modulated during the hydrothermal growth process of ZnO by changing different precursors, solvents, molar ratio, temperature, pressure, atmosphere of reaction, and the presence of catalysts or surfactants.
3.2.2.3 Other Hydrothermal Processes for the Synthesis of ZnO Nanoparticles or Nanostructures
At present, numerous processes have either been developed or are under development for producing nanoparticles in a manner that reduces both synthesis time and cost. Metal nanoparticles can be generated using either ultrasonic waves or microwave irradiation of metal salts or chemical precursors. Power ultrasonic waves can stimulate certain novel chemical processes such as nucleation, growth, and collapse of cavitation bubbles formed in liquid through localized hot spots in the liquid of extremely high temperature (~2,700°C) and pressure (~1,000 atm). Increased use of microwaves mediated the synthesis of nanostructures or nanoparticles have been reported in comparing to this conventional oven. Microwave-assisted decomposition of zinc acetate precursor in the presence of an ionic liquid, 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide, [bmim][NTf2], was used to synthesized ZnO nanoparticles [35]. Ma et al. [36] also synthesized ZnO micro- and nanoparticles using zinc acetate hexahydrate and pyridine in a hydrothermal process through microwave irritation at 90°C for 10 min. Adjusting the concentration of pyridine in the reaction, synthesis of various ZnO structures such as hexagonal columns, needles, nanorings, and hollow structures was reported. ZnO nanocrystallites synthesized using microwave irradiation were found to have more defects and were capable of exhibiting visible light photocatalysis without any doping with transition metals [24].
Several reports detailing the synthesis of ZnO nanostructures using simple routes have been published [24, 37]. Large arrays of ZnO nanorods on zinc foil have been synthesized in the absence of zinc salts, oxidant or coating of metal oxide layers by simply dipping the zinc foil into a 25% aqueous ammonium solution and heating at 80°C [24]. Vijayan et al. [37] successfully synthesized thin films of ZnO using a double dip method where sodium zincate served as the first dipping solution prior to dipping in hot water. The parameters of ZnO microstructures or nanoparticles can vary based on the temperature of the hot water, the pH, and the amount of zinc sulfate used for preparation of the sodium zincate solution.
4 Antimicrobial Activity of ZnO Nanoparticles
Zinc oxide nanoparticles have attracted much attention due to their versatile and promising applications in biological sciences (antibacterial, antifungal, antifouling, and biosensors), ultraviolet applications (catalysis, sunscreen, paint, polymer nanocomposite, and rubber), and opto-electronics (light-emitting diodes, field effect transistors, field emitters, solar cells, toners, and sensors) [3, 38]. In addition, there are other applications such as in ferrite, varistor, pigment, ceramic flux, animal food, pollutant filter, dental fillings, hydrogen fuel, and nanotextiles. The main advantages of using ZnO nanoparticles compared with organic or bulk oxide is their chemical stability, thermal resistance, robustness, and long shelf life [3, 4, 39]. This is important for harsh conditions such as high temperatures and pressures that occur during product manufacturing, storage, and transportation. Considering the continuous emergence of multiple drugs and antibiotic-resistant microorganisms, in particular, seen amongst pathogenic bacteria, it has become an emerging public health issue. The morbidity and mortality associated with bacterial diseases as well as the cost associated with treatment remains high, due to continuous emergence of multiple antibiotic-resistant pathogenic strains that either acquire resistance readily or are naturally resistant [40–42]. Some of these emerging bacterial diseases that occur frequently include large outbreaks of food- and water-borne infections, hospital-acquired (nosocomial) infections, bioterrorism-associated infections, microbiota shift diseases and antibiotic resistance. In addition, several pathogens are capable of forming biofilms, typically on either host organs or medical and non-medical devices, which makes treatment difficult due to reduced drug penetration [42, 43]. The capacity of various bacterial pathogens to cause a multitude of animal diseases is due to the organisms’ ability to produce multiple factors associated with survival and virulence when they are in their environmental settings [40–43]. Many of these factors are associated with colonization, neutralization of host immune systems, host tissue damage, and spreading of pathogens in their environmental settings. Therefore, alternative therapeutics that control the spread of pathogens and eliminate resistant pathogenic bacteria from different environmental settings such as community, hospital, and various food industries are being sought.
A literature survey revealed that over the last 20 years, efforts have been carried out to develop nanomaterial agents with novel properties that have specific antibacterial activities to combat the growing threat of infectious diseases [1–3]. Some of the inorganic nanoparticles or the bulk oxide powders that have been tested for their antibacterial activity are TiO2, ZnO, MgO, CaO, CuO, Al2O3, AgO, and CeO2 [4–19]. Among these metallic oxide nanoparticles, photocatalytic inactivation of bacteria by TiO2 has been studied over the last 20 years [6, 7]. TiO2 can kill both Gram-negative (Escherichia coli), and Gram-positive (Bacillus subtilis, Staphylococcus aureus) organisms as well as viruses including poliovirus 1, hepatitis B virus, Herpes simplex virus and MS2 bacteriophage with different sensitivity [44]. Similar to TiO2, various structures of ZnO have attracted much interest due to extensive applications in sunscreens, coating, and paints [10], and also due to their piezoelectricity and band gap in the near ultraviolet range and large excitation binding energy at room temperature. ZnO has been shown to have different modes of action towards microorganisms from other metal oxides [5, 8, 9, 11, 19, 46, 47]. Specifically, studies have demonstrated the antimicrobial activity of bulk or larger particle-sized ZnO in the range of 0.1–1.0 μm under visible light [21], whereas similar studies on ZnO nanoparticles showed higher antibacterial activities against E. coli, S. aureus, and B. subtilis [8, 9, 11, 21, 30, 39, 45–49]. Studies by several groups have shown that functional activities and consequent toxicity of ZnO nanoparticles may be influenced by particle size and concentration which is inversely proportional to the size and concentration of ZnO nanoparticles used against S. aureus and E. coli [9, 21, 31, 46]. Specifically, studies on E. coli with particle sizes ranging from microns (2 μm) to nanometers (45 and 12 nm) showed more effective antibacterial effect with smaller nanoparticles compared with larger sized particles [46]. Size-dependent growth inhibition and viability of S. aureus were demonstrated in the presence of 6 mM concentration of hydrothermally synthesized ZnO particle sizes ranging from 302 to 12 nm as shown in Fig. 5.3 [31]. This is due to the fact that smaller particles will have both a higher number and a higher surface area to volume ratio to cover target microorganisms and attribute higher surface reactivity compared to larger particles. Contradictory results have been reported where size-dependent effects were not found to influence the antimicrobial activity of ZnO [50]. In addition to the particle size-dependent antibacterial effect of ZnO nanoparticles, most of the studies in the literature strongly indicate that dose-dependent antibacterial activity exists for various forms of ZnO nanoparticles against both Gram-positive and Gram-negative bacteria. Interestingly, the antibacterial effect of the nanoparticles was significantly more pronounced on the Gram-positive bacteria than on the Gram-negative bacteria probably due to the complexity of the cell membrane structure that exhibits significant changes in membrane permeability and other surface properties in the presence of metal oxide nanoparticles. It has been reported that more than 95% inhibition of S. aureus growth could be seen at ZnO nanoparticle (diameter ~13 or 8 nm) concentration of ≥1 or 1 mM, whereas complete inhibition of E. coli growth was observed with ≥3 mM of ZnO nanoparticles of about 11 nm diameter[9, 19, 47, 51].
Effect of different sizes ZnO nanoparticles on the growth of S. aureus. (a) Growth analysis curves measured by monitoring the culture turbidity as a qualitative measure of cell growth using the optical density (OD) at 600 nm. (b) The percentage of growth inhibition as counted the viable S. aureus colonies recovered from TSA (tryptic soy broth agar) plates and plotted against ZnO particle sizes. The results demonstrate that size-dependent bacterial growth inhibition of S. aureus exists in the presence of 6 mM of different sizes of ZnO nanoparticles (Adapted from [31]; American Chemical Society)
Although most of the studies on the antibacterial activity of either ZnO bulk or nanoparticles has been performed using Gram-negative E. coli and Gram-positive S. aureus, very few studies has been performed using other bacterial species. Some of the bacterial species that have been tested for various sizes ZnO nanoparticles or bulk are Streptococcus (mutans, pyogenes, and agalactiae), Enterococcus (faecalis), Staphylococcus epidermidis, Bacillus (subtilis and atrophaeus), Lactobacillus (casei and helveticus), Vibrio fischer, Salmonella typhimurium, Shigella (dysenteriae and flexinari), Pseudomonas (aeruginosa, chlororaphis, and alcaligenes), Campylobacter jejuni, and Proteus vulgaris [8, 9, 13, 31, 48, 51–54]. Figure 5.4 represents a typical growth inhibition assay for several bacterial pathogens with various concentrations of ZnO nanoparticles (particle size ~ 12 nm) under normal ambient lighting conditions [31]. These results demonstrate that most of the bacterial growth (>95%) could be inhibited for most of the bacteria, except for a select few such as S. typhimurium. In addition to the growth inhibition assays, viable cell counts of the ZnO nanoparticle-treated cultures demonstrated that the number of recovered bacteria was significantly fewer compared to the untreated control, which correlates with growth inhibition patterns. The antibacterial activity of ZnO nanoparticles varies from species to species due to the characteristic difference of the organisms tested. For example, among the Gram-negative bacteria, the minimal inhibitory concentration (MIC) of ZnO nanoparticles (~30 nm) for C. jejuni (0.05–0.25 mg/mL) is 8- to 16-fold lower than Salmonella enterica and E. coli O157:H7 (0.4 mg/mL) strains [54]. It has also been reported that strains within a species vary significantly in terms of infectivity and tolerance to various agents including antibiotics. Analysis of the antibacterial activity of various clinical isolates of S. aureus suggest that there is a variation in antibacterial activity of ZnO nanoparticles to some of the isolates, although majority of the isolates showed similar patterns of inhibition [9, 31]. In addition to these microorganisms, efficient growth inhibition or killing activity of both bulk and nanoscale ZnO has been demonstrated for fungi (Candida albicans, Saccharomyces cerevisiae, Neurospora crassa, and Aspergillus oryzea) and algae (Nitzschiapallea and Crustaceans daphnia magna) and nematodes (C. elegans) [50, 52, 55, 56]. Overall, published reports clearly suggest that ZnO nanoparticles have significantly higher antibacterial or antifungal activity compared to the bulk ZnO, target a wide range of microorganisms, and that their activity does not require any photo-activation.
Growth inhibition assays of various microorganisms in the presence of different concentrations of ZnO nanoparticles (~12 nm particle diameter). The different strains of microorganisms are: (a) S. aureus strain MW2 (community-associated MRSA). (b) S. aureus strain Newman (MSSA). (c) S. aureus strain Cowan (hospital-associated MRSA). (d) Proteus vulgaris. (e) Salmonella typhimurium. (f) Shigella flexinari (left) and Bacillus cereus (right). The results demonstrate that the concentration range of 4–7 mM colloidal suspension of ZnO nanoparticles could inhibit more than 95% of growth for most of the microorganisms, except S. typhimurium (Adapted from [31]; American Chemical Society)
The properties of regular spherical or different shapes nanoparticles depend strongly on their dimensions and morphologies [57–60]. The relationship between the different forms of ZnO with the antibacterial activity is not clear, in spite of the fact that different structures and morphologies of ZnO can be efficiently synthesized using various synthesis methods. Studies have demonstrated that antibacterial activity increases with the increase of the lattice constant value of co in the hexagonal structure of ZnO powder and is also due to enhanced generation of hydrogen peroxide [60]. The relationship between antibacterial activity and various orientations of ZnO nanowire arrays indicate differential antibacterial activity among ZnO nanoarrays, in spite of having similar average diameter. Randomly oriented ZnO nanoarrays exhibited superior antibacterial activity compared with less or well-defined oriented ZnO nanoarrays in E.coli [57]. Although the exact mechanism of the antibacterial actions of different orientations of ZnO nanoarrays is not known, the probable mechanism is considered to be due to the generation of hydrogen peroxide as well as surface abrasiveness [46]. The aggregation of ZnO nanoparticles does not greatly affect the antibacterial activity, rather the size of the nanoparticles greatly influences the antibacterial activity against various microorganisms. The dimension and morphology of ZnO nanoparticles play important roles in determining the antibacterial activity, although it has not been well investigated compared to other non-biological applications.
In summary, both bulk and nanoparticle preparation of ZnO can effectively inhibit both Gram-positive and Gram-negative bacteria as well as algae and fungi under normal ambient lighting conditions. The antibacterial activity of ZnO can be observed against various states of bacteria such as vegetative and spores that naturally resist both high temperature and high pressure treatments. Antibacterial activity of ZnO nanoparticles is size dependent, as smaller particle sizes have more efficient antibacterial activity than the larger size nanoparticles or bulk particles. The antibacterial activity of ZnO depends on the surface area and concentration of the particles, while the crystalline structure and particle shape have little effect. Thus, increasing the concentration and surface area improves the effectiveness of ZnO particles against most of the tested microorganisms. High temperature treatment of ZnO nanoparticles (calcination) does not have a significant effect on antibacterial activity [31], whereas high temperature treatment of ZnO bulk decreases its antibacterial properties [60].
5 Mechanism of Antibacterial Activity of ZnO Nanoparticles
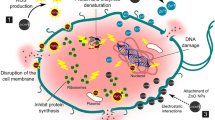
To date, a number of mechanisms have been proposed to interpret the antibacterial or cytotoxic activity of ZnO powders or nanoparticles. These include toxicity based on chemical composition (e.g., release of toxic ions); production of reactive oxygen species (ROS) due to the presence of nanoparticles; stress of stimuli caused by the surface, size, and shape of particles; damage to membrane cell wall through adhesion on the cell membrane; penetration through the membrane cell wall; and cellular internalization of nanoparticles [4, 5, 19, 46, 55, 61, 62]. Although there are numerous studies regarding the antibacterial effect of ZnO mostly using E. coli and S. aureus, little is known regarding ZnO interactions with other bacteria and the mechanism underlying the antimicrobial effects. It is clear from numerous studies that interactions of ZnO nanoparticles or powders in water produce various reactive oxygen species, predominantly hydroxyl radicals (OH−), singlet oxygen or superoxide anion (O −2 ), and hydrogen peroxide (H2O2), which play a crucial role in nanoparticle-induced antimicrobial activity. As direct proof, the formation of hydroxyl radicals and singlet oxygen species of a suspension of ZnO was determined by electron spin resonance (ESR) [46, 63], while hydrogen peroxide formation was evident by direct quantification [5, 11, 12]. It has been shown that, under ordinary room light with a light intensity of 10 μW/cm2 (or the intensity of UV light ~1 μW/cm2), is sufficient to induce ROS production in suspension of ZnO in water [63]. However, the level of ROS production increases significantly when the suspension was subjected to irradiation with visible light at the range of 400–500 nm or UV light, which subsequently increased either the antimicrobial activity or ecotoxicity toward various microorganisms or environmental species [55, 63]. As a basic principle, ZnO with defects can be activated by both UV and visible light, and electron hole pairs (e−h+) can be created. The holes split H2O molecules into OH− and H+. Consequently, oxygen molecules are transformed to superoxide radical anions (O −2 ), which in turn react with H+ to generate (HO2 .) radicals that subsequently collide with free electrons to produce hydrogen peroxide anions (HO −2 ). Transient hydrogen peroxide anions react with hydrogen ions to produce molecules of hydrogen peroxide (H2O2). The hydroxyl radicals and superoxides are negatively charged particles, which are unable to penetrate into the cell membrane and remain in direct contact with the outer surface of the microorganisms, whereas hydrogen peroxide can easily penetrate into the cell [46]. The generation of active oxygen species and the disruption of cell membranes caused by ZnO nanoparticles may actually be bacteriocidal. The generation of hydrogen peroxide depends on the surface area of ZnO as the smaller size of the nanoparticles means higher the surface area yielding increases in the production of ROS and higher antimicrobial activity [9, 46].
A comparative study of the effect of particle size on ROS production in TiO2 suggested that a sharp increase in ROS generation per unit surface area of dry samples occurs only for particles with a size range of 10–30 nm, and a relatively constant ROS generation per unit surface area were observed for particles below 10 nm and above 30 nm [64]. In addition to particle size, aggregation of nanoparticles in solution or medium may strongly impact their interaction with biological systems, activity, and toxicity [64]. For example, nanoparticles and bulk ZnO formed similar-sized aggregates in solution, yet the primary size appears to be more important than aggregate size in determining the toxicity of ZnO particles, probably due to the increased surface area and ROS generation [45]. Contradicting this is the finding that aggregate size determines both the uptake and response of immortalized brain microglia to nano-TiO2 and the bioavailability of nanoparticles to plant roots, algae, and fungi [65]. It is interesting that ZnO nanoparticles decreased the ability of growth of bacteria such as E. coli [66] and S. aureus [9] even in the dark compared to ambient light, which is probably due to the generation of super oxide anion (O −2 ) or alternative modes of ZnO nanoparticles activity. Photo-activation and antibacterial properties of ZnO nanoparticles are similar to TiO2 and are receiving increasing application in numerous areas. However, information describing phototoxicity of ZnO nanoparticles has been very limited. Increases in the antibacterial activity of ZnO nanoparticles were demonstrated when S. aureus and ZnO nanoparticles were exposed to UV light [31]. Similarly, phototoxicity of nano-ZnO and bulk-ZnO was dramatically enhanced under natural sunlight illumination as compared to artificial laboratory light illumination, which is due to increased generation of ROS in C. elegans [67]. Generation of ROS by nanoparticles such as TiO2 and ZnO interacting with environmental agents (e.g., UV) can be enhanced significantly to cause oxidative stress and can eventually elicit toxicity to bacteria [68]. Overall, the literature suggests that ZnO powder or nanoparticles produce greater amounts of hydrogen peroxide which mediate its antibacterial effect, whereas hydrogen peroxide production was not detected using similar CaO and MgO powders [5].
Although the detailed mechanism for the antimicrobial activity of ZnO is still under debate, several studies have shown that the production of ROS is not the only contributing factor [19, 46]. One of the several alternative mechanisms suggested is that ZnO nanoparticles have abrasive surface defects as confirmed by the photoluminescence spectrum of ZnO [4, 46]. The abrasiveness of nanoparticles compared with the bulk is caused by uneven surface texture due to rough edges and corners, which contributes to mechanical damage of the cell membrane by electrostatic interaction [4]. It has been shown that increased antibacterial effect of ZnO nanoparticles against E. coli can be observed as the particle diameter is reduced from 2 μm to 45 nm to 12 nm, and that this is attributed to the enhanced effect of the greater surface area to volume ratio and mechanical damage to cells due to increase abrasiveness of the smaller nanoparticles [46]. Upon interaction with bacterial cells, ZnO nanoparticles can either be transported into the cytoplasm, be deposited on the surface, penetrate into the cell wall or membranes, and/or a combination of all of these scenarios can occur. In fact, it has been reported that all these scenarios can occur in both Gram-negative (e.g., E. coli) and Gram-positive (e.g., S. aureus) bacteria [19, 31, 68]. It has been reported that ZnO nanoparticles cause damage to the membrane of E. coli, which yielded as accumulation of ZnO and cellular internalization [19]. Figure 5.5 represents the cell membrane disorganization and cellular internalization of ZnO nanoparticles in E. coli as a probable mechanism of ZnO nanoparticles-mediated antibacterial activity [19]. These scenarios, along with ROS generation, lead to extensive disorganization of the cell membranes or wall and cell death. It has also been suggested that the orientation of ZnO can affect bioactivity, as randomly oriented ZnO nanowires demonstrated higher antimicrobial activity than regularly oriented ZnO nanowires [57]. Thus, the mechanism of antibacterial activity of ZnO nanoparticles is complex and not fully understood. Furthermore, the role of Zn2+ ions released from dissolution of ZnO is not very clear [11, 20, 31, 68]. Zinc ions are known to inhibit multiple activities in bacteria, such as glycolysis, transmembrane proton translocation and acid tolerance. Thus, the presence of zinc ions is likely to inhibit proliferation by binding to the membrane of bacteria, which can prolong the lag phase of growth, and contribute to the antimicrobial activity of ZnO [68]. It is estimated that the minimal inhibitory concentration (MIC) of zinc ions is 1,917 (P. aeruginosa), 9 (S. aureus), and 39 (Candida albicans) μg/mL, varying for different organisms [68], yet the antibacterial effects of ZnO are similar to these organisms, although zinc ions are reported to be bacteriostatic rather than bacteriocidal [69]. However, the release of Zn2+ ions from ZnO nanoparticles suspension compared to the precursor is not significantly high enough, about 354-fold less [31] or five to ten times less [47, 63], to impart its cytotoxicity. Zinc is a ubiquitous essential metal ion and plays a role in catalysis, protein structure and perhaps as signaling molecules in living systems [70]; therefore, low concentration of zinc ions will be metabolized and may not have any antimicrobial or ecotoxicity effect. In addition, the pH of the ZnO nanoparticle suspension in water, saline or medium does not change in a neutral region (pH 7), and thus its suitability for bacterial survival and antibacterial activity [59]. It should be noted that the solubility of ZnO increases sharply when pH is below 6 or above 11 and it becomes completely dissolved around pH 6 and 12 [38], thus using ZnO for the antibacterial agent or the biological applications will be more suitable.
Transmission electron microscopic micrographs of E. coli thin section under various conditions: (a, b) E. coli grown in Luria Broth (LB) medium without any ZnO nanoparticles treatment. (c, d) E. coli grown with diethylene glycol (DEG) in LB medium (used for ZnO nanoparticles synthesis). (e, f) E. coli cellular division when cells are grown in LB and DEG media, respectively. (g–j) E. coli grown in the presence of 1 mM concentration of ZnO nanoparticles (~14 nm average diameter). The results showed that the E. coli grown with DEG in LB medium indicates changes in the morphology of the cells (c, d). Damage and disorganization in the cell wall as well as cell morphology were prominent in case of E. coli grown the presence of DEG (f) compared with in the absence in (c). Micrographs in (g, h) of E. coli grown in the presence of ZnO nanoparticles demonstrated that more cellular internalization of ZnO nanoparticles and cell wall disorganization. The results also showed more extensive damage of the cell membrane and the leakage of intracellular contents. NP ZnO nanoparticles (TEM images reprinted from [19]. With permission of the American Chemical Society)
Several factors, in particular host-specific factors, play crucial roles in the antibacterial effect as well as differential activity of ZnO nanoparticles. Similarly, differential antibacterial effect among the Gram-negative (E. coli) and Gram-positive (S. aureus) microorganisms against ZnO nanoparticles has been observed [9, 54], probably due to, firstly, the nature and difference of the cell wall or outer layer structures of Gram-negative and Gram-positive bacteria. Specifically, in Gram-positive bacteria (S. aureus) the cell wall is thick and lacks any periplasmic region, consisting of a large amount of peptidoglycans as well as other components such as lipoteichoic acids (LTA). On the other hand, Gram-negative bacteria (E. coli) have relatively thin cell walls, but with inner and outer membranes in-between the periplasmic region. Secondly, there is differential resistance to oxidant and UV due to production of carotenoid pigments as S. aureus produces more pigments than E. coli. And, thirdly, S. aureus has developed efficient pathways to defend against oxidative stresses by increasing the expression of oxidative stress-responsive gene products such as superoxide dismutase (the sodA and sodM gene products converts O −2 to H2O2), catalase (the katA gene product converts H2O2 to H2O and O2), thioredoxin reductase (trxB; maintains thioredoxin in reduced form to protect cells against toxic oxygen species), thioredoxin (encoded by trxA), alkyl hydroperoxide reductase sub C and F (ahpCF, having activity against organic peroxides, peroxynitrate, and H2O2) and endopeptidase (clpC) in combating oxidative stress [71, 72]. In contrast, E. coli has less efficient pathways to eliminate oxidative stresses. Quantitative PCR analysis (RT-qPCR) for various genes suggests a significant increase in the expression levels of two oxidative genes, katA (52-fold) and ahpC (7-fold), and a general stress response gene, dnaK (17-fold), under ZnO nanoparticle treatment conditions, which demonstrate that the antibacterial activity of ZnO nanoparticles is most likely due to disruption of the cell membrane and oxidative stress in Camplobacter jejuni [54]. A similar transcriptional analysis for various oxidative stress-responsive genes of S. aureus strains treated with bulk, 8- or 12-nm-diameter ZnO nanoparticles suggest that there was no significant variation in the expression levels of the katA, sodA, sodM, trxA, trxB, and perR (hydrogen peroxide regulator) genes, except for the ahpCF genes [31]. The results suggest that the production of ROS by ZnO nanoparticles or powder may not be high enough to induce the expression of most of the ROS-specific genes in S. aureus, although the antibacterial activity of these ZnO agents has been investigated.
In addition, other factors, such as differences in the mode of association of the particles to the cell surface, capacity of solubilizing and metabolizing ZnO, cell permeability, and the various pump and transporter systems for transporting and exporting ZnO nanoparticles, may also play important roles for the effectiveness of ZnO against a particular bacterium. It is also important that, within the same species such as Staphylococcus, different clinical strains may have different effectiveness towards the same ZnO nanoparticles, as has been demonstrated in the antibiotic resistance or sensitivity profiles of different isolates [42, 43]. Therefore, the evidence suggests that different forms of ZnO yield different degrees of antimicrobial activity towards the same or different bacteria, and presumably microorganisms are unlikely to develop resistance against zinc or ZnO nanoparticles, as they do against conventional and narrow-target antibiotics, because the metal oxide or metal attack a broad range of targets within the bacteria. This means that bacteria would have to develop a large number of mutations simultaneously to protect themselves; therefore, ZnO nanoparticles may demonstrate improved effectiveness with an ability to target more microorganisms than conventional organic antibiotics.
6 Enhancement of ZnO Antibacterial Activity by Surface Modification
Surface coating or modification of nanomaterials has been recognized as one of the most advanced and intriguing approaches to increasing their affinity or activity and improving their applicability. Since surface area and surface defects play an important role in the photocatalytic activities of metal oxides, therefore the doping of metal oxides and/or transient metals increases the surface defects leading to higher biological activity and also affects the optical and electronic properties, probably shifting the optical absorption towards the visible region. Doping is a widely used method for modification of nanoparticles, in particular for ZnO, to enhance their electrical, optical and biological properties. Although different approaches have been adopted to synthesize ZnO nanoparticles, doping and fabrication of ZnO nanoparticles is done using the wet chemical synthesis method which offers many advantages such as ease to synthesis, reduced reaction temperature, increased yield, and formation of well-defined nanostructures or nanoparticles. Work performed in the area of transient metal doping of ZnO single crystals and thin films indicates that ZnO nanorods doped with manganese (Mn), chromium (Cr), and cobalt (Co) can be formed using hydrothermal synthesis, and the morphology of the doped ZnO nanorods was different from that of the undoped ZnO [73]. Doping of Mn into the ZnO offers an interesting method to alter various properties such as the band gap of ZnO (3.37–3.70 eV), which can alter the emission properties by providing an efficient channel for the recombination of electron and hole. ZnO doped with Mn has also been synthesized using the co-precipitation method. It has been demonstrated that Mn-doped ZnO nanoparticles have increased antibacterial activity against both Gram-negative and Gram-positive bacteria than undoped ZnO nanoparticles [40]. Enhanced antibacterial activity against E. coli was reported with hydrothermally synthesized ZnO doped with transient metals such as Fe and Co [74]. It has also been reported that tyrosine-assisted addition of silver (Ag) metal to ZnO nanoparticles enhanced photo-degradation of organic dye pollutants and destruction of bacteria [75]. Similarly, ZnO-Al2O3 or MgO-ZnO composites have been prepared using zinc–aluminum layered double hydroxides [76] or mixing MgO/ZnO solid solution powders and heating at 1,400°C for 3 h in air [77]. In this instance, higher antibacterial properties were observed with decreasing amount of doped ZnO nanoparticles. A different type of doping of ZnO nanoparticles with non-metal nitrogen has been reported using the mechano-chemical method, which exhibits high photocatalytic activity under visible light irradiation [78].
Antimicrobial activity can be enhanced by antibiotic–nanoparticle interaction or by the combination of antibiotics and ZnO nanoparticles against different antibiotic-resistant bacteria. Although there are no reports on antibiotics coupled with ZnO nanoparticles, several studies have been described on antibiotics–nanoparticles interaction and their antibacterial activity. It has been demonstrated that when amoxicillin and silver nanoparticles are combined, its efficiency was enhanced against E. coli compared to amoxicillin and silver nanoparticles alone. Similarly, when vancomycin was covalently attached to TiO2 nanoparticles, its antibacterial activity against S. aureus increased and was sustained for a longer period of time [79]. Combination of ZnO nanoparticles ranging from 20 to 45 nm with ciprofloxacin-enhanced antibacterial activity against E. coli and S. aureus [49]. A probable cause of the synergistic effect may be due the action of nanoparticles on the surface of the bacteria complementing the action of the antibiotic. The advantage of using double agents to kill or inhibit bacterial growth will be more beneficial as the mode of inhibition or killing for each individual agent are different. For example, resistant strains can be targeted using antibiotics–nanoparticles, as a particular antibiotic may be ineffective to kill bacteria, but nanoparticles will restrict growth or eliminate the bacteria. Several potential applications with respect to the biological activity of ZnO nanoparticles has been demonstrated using thin layer coating on different surfaces such as cotton, fabric, glass or paper, which showed significant bacterial growth inhibition or protection from UV light [79–81]. ZnO or other nanoparticles have been successfully used for water disinfection and purification [73]. Functionalized ZnO with sepiolite (ZnO-Sepiolite) has been used in weaning piglet diets to fulfill the Zn requirement as well as the health requirement to protect from bacterial infection [82]. Therefore, observations in the published literature indicate that ZnO nanoparticles or functionalized ZnO nanoparticles have extensive biological applications and benefits and can be further developed to suit desired applications.
7 Potential Toxicity of ZnO Nanoparticles
During the last few years, research on toxicologically relevant properties of nanoparticles, in particular engineered nanoparticles, has increased substantially. Most of the experimental toxicological work on nanoparticles has been based on a small set of nanoparticles such as carbon black, TiO2, iron oxides and amorphous silica, while little is known related to ZnO nanoparticles [83, 84]. Because a wide range of applications can lead to large-scale production and finally release to the environment, it is still uncertain whether engineered nanoparticles are inherently toxic or safe to health and environment. It should be stressed that natural nanoparticles including metal oxides existing in all ecosystems play an important role in biogeochemical processes and thus living systems have adapted to their presence in the environment. The greatest current risk is to the occupational health of workers involved in research and manufacture of nanoparticles and nanofabrics. In addition, various nanostructures of ZnO, including particles, rods, wires, belts, tubes, and rings, have attracted a great deal of attention because of their useful electronic and opto-electronic properties and novel applications. Therefore, significant increases in production and demand in the products could lead to unintended exposure via possible various routes such as inhalation, dermal absorption, and oral absorption of various nanoparticles including ZnO by the living systems. Due to their unique properties, including small size and corresponding large surface area, it is believed different degrees of biological effects are imposed related to their bulk [62, 83, 84].
Several studies have been directed toward the potential toxicity of ZnO nanoparticles or powders in various animal systems such as in rats [85, 86], mice [87–89], guinea pigs [90], human skin [91], and zebra fish [92], as well as C. elegans [55] and Daphnia [93]. In addition, the cytotoxicity of both bulk and nanoparticles of ZnO has also been performed in several cell culture-based studies including mouse embryo fibroblast cells [94], human bronchoalveolar carcinoma-derived cells [76], mouse neural stem cells [95], phagocytic cells and bronchial epithelial cells [61], and NIH 3T3 fibroblast, epithelial, and endothelial cells [96]. Nanoparticles can deposit in the respiratory tract after inhalation of 0.5, 2.5 and 12.5 mg/m3, which can induce inflammatory reactions or oxidative-stress responses in the respiratory tracts and lungs [86]. Oral exposure of experimental mice with two different sizes (20 and 120 nm) of ZnO nanoparticles and with the doses of – 5 g/kg body weight demonstrated that the liver, spleen, heart, pancreas, and bone are the target organs for oral exposure [89]. Exposure to human skin with 20–30 nm ZnO nanoparticles suggested that ZnO nanoparticles stayed in the stratum corneum and accumulated into skin folds and/or hair follicle roots, which indicate that the form of ZnO nanoparticles studied is unlikely to result in safety concerns [91]. There is no doubt that ZnO nanoparticles impart cytotoxicity against different culture cells, mostly due to the induction of oxidative and inflammatory responses, and that the mechanism is often misleading owing to the small number of reported studies. Cell viability assays with 10-, 30-, 60-, and 200-nm particles indicated that ZnO nanoparticles manifested dose-dependent, rather than size-dependent, toxic effects on mouse neural stem cells, where Zn ions from ZnO nanoparticles in the culture or inside cells caused cell damage at 12 ppm or higher for 24-h treatment [95]. Similar studies in human bronchoalveolar carcinoma-derived cells with 70- and 120-nm ZnO particles suggest that size- and dose-dependent cytotoxicity as reflected in oxidative stress, lipid peroxidation, cell membrane damage, and oxidative DNA damage, which is mediated by neither Zn ions nor metal impurity in the ZnO particle samples, and rather the cytotoxicity is caused by localization of ZnO in vesicles [76]. A comparative study on the effect of three nanoparticles, ZnO, TiO2, and CeO2, in macrophage and epithelial cells suggested that non-activated TiO2 did not cause any toxicological injury to these cells, whereas CeO2 exerted a cytoprotective effect due to its antioxidant properties. Interestingly, ZnO nanoparticles exhibited major toxicity toward these cells due to particle dissolution and the release of Zn2+ that engaged in oxidant-mediated injury of cells [63]. The cytotoxicity of ZnO nanoparticles depends on the availability or the concentration as zinc is an essential element. Thus, low concentrations are not cytotoxic, rather living systems utilize it for growth. Several studies have demonstrated efficient growth inhibition or killing activity of both bulk and nanostructures ZnO or TiO2 for algae (Nitzschiapallea, and Crustaceans daphnia magna) and nematodes (C. elegans) in environmental systems [50, 52, 55]. The genotoxicity of ZnO nanoparticles to human sperm and lymphocytes can be enhanced by UV irradiation [97]. The control of pathogenic bacteria by antimicrobial nanoparticles is a promising approach to limit the spread of multiple drug-resistant pathogens such as S. aureus; however, it is expected that the same agent will affect the population of the microbes that play beneficial roles in the environment. Toxicity studies on manufactured metal oxide nanoparticles such as TiO2 and ZnO are expanding rapidly, but the effectiveness is not sufficient to draw a conclusion due to the lack of environmentally relevant conditions used in the experiments [50]. It should also be noted that, for the environmental conditions, it is known that the type and amount of natural organic matter in the ecosystem or environment affects nanoparticle stability and bioavailability, because the interaction of nanoparticles with environmental organic and inorganic colloids may strongly influence their behavior and potential cytotoxicity. Thus, there is currently little evidence from skin penetration studies that dermal applications of metal oxide nanoparticles, in particular TiO2 and ZnO2 used in sunscreens, lead to systemic exposure, nor for inhalation of nanoparticles over longer periods or uptake of nanoparticles in the gastrointestinal tract after oral intake of various designs of food and pharmacological components. Therefore, it is safe to conclude that ZnO powders or nanoparticles are biosafe and environmentally safe up to a certain amount, but may become hazardous at higher concentrations as it has been reported that ZnO nanoparticles have reduced the viability of human T cells at an elevated concentration (≥5 mM) [47]. Zinc oxide is listed as “generally recognized as safe” by the U.S. Food and Drug Administration (21CFR1828991). As a food additive, it is the most commonly used zinc source in the fortification of cereal-based foods. Due to its antimicrobial properties, ultrafine ZnO has been incorporated into the lining of food cans in packages for meat, fish, corn, and peas to preserve colors and to prevent spoilage. Although, presently, there are no set threshold limit values for various forms of ZnO to the community members due to lack sufficient experimental studies and data, the recommended threshold limit values of ZnO for welders and others in the workplace have been set at 5 mg/m3 [98].
8 Conclusion and Future Perspective
Metal oxide, in particular ZnO, is an environmentally friendly wide band gap semiconductor (3.37 eV) with a large excitation binding energy, and high mechanical and thermal stability. This makes its suitable for applications in opto-electronic and piezo-electrical fields. Moreover, ZnO can be manipulated easily to change its size and morphology, so that the physical and chemical properties can be modulated to suit its applications. Extensive research in term of synthesis and modification such as coatings, and doping has been performed for several decades to improve and enhance its novel properties, which opens up opportunities for new technological applications mostly in solar cells, sensors, detectors, and energy generators. ZnO nanoparticles are being increasingly used as a photocatalyst for degrading harmful contaminants like pesticides from ground water. A wide variety of synthetic approaches have been employed to prepare various forms of ZnO colloids and films, which are generally classified as gas and liquid phase synthesis. Some of them are sol–gel synthesis, hydrothermal synthesis, spray pyrolysis, metal–organic chemical vapor deposition, sonochemical processing, and pulsed laser deposition. The choice of synthesis methods is purely dependent upon the application, cost effectiveness, purity, and downstream fabrication. Simple methods, such as wet chemical synthesis, and in particular hydrothermal methods, have gained more popularity for their simplicity and their further applications such as thin layer coatings and surface modification due to synthesis temperature and other synthesis conditions.
The antimicrobial activity of ZnO has been known for a long time and it has been used as an active ingredient in antibacterial creams, lotions, and ointments. The last decade has seen a logarithmic growth in the field of antimicrobial properties of ZnO nanoparticles and potential applications in various forms that leads to substantial progress in the development, process, functionalization and applications of nanoparticles. Studies of antimicrobial activity of ZnO nanoparticles suggested several important points such as antimicrobial activity can be achieved under normal ambient light condition and can be enhanced with UV irradiation; activity is size-dependent, the smaller-sized ZnO nanoparticles exhibit higher activity; and there is activity against wide variety of both Gram-positive and Gram-negative bacteria as well as fungi, algae, and nematodes. The mechanism of antimicrobial activity of ZnO is not well defined; numerous studies demonstrate that the production of reactive oxygen species (ROS), in particular H2O2, from ZnO is proportional to ZnO concentration and surface area that may contribute to cell death or inhibition of bacterial growth. However, ROS-mediated killing is not the main mechanism of antibacterial activity of ZnO, several other probable interactions between bacteria-ZnO nanoparticles also play important roles. Interactions between bacteria-ZnO nanoparticles or the antibacterial activity of ZnO nanoparticles can be enhanced by engineering ZnO with a transient metal such as Mn, Ag, and Mg. Since ZnO dissolves easily and both ZnO and Zn2+ have antibacterial activity, aquatic organisms can be highly sensitive to zinc; therefore, its application in drinking water treatment is limited. One possible application could be in combination with existing disinfection technologies such as UV disinfection systems, although ZnO nanoparticles in water treatment is not very reasonable because loss of zinc to water may have potential impacts on human health and ecosystems.
Application of ZnO nanoparticles in controlling the spread and colonization of potential pathogens is very a promising finding that has motivated many investigations into the application and fundamental knowledge of nanoparticles and improvement in the techniques for synthesis and fabrication of new nanomaterials with controlled properties and dimensions. The use of ZnO nanoparticles or other nanomaterial coatings is still limited because the application requires control of the synthesis of nanoparticles. Several methods have been developed to fabricate nanoparticles that can be routinely prepared using conventional fabrication processes, but their application is limited to the small scale. It would be interesting to determine if any derivate of ZnO nanoparticles with chemical groups or bioagents are more effective at eliminating various microorganisms. Several reports have suggested that modification of nanoparticle surfaces can efficiently target and kill both Gram-positive and Gram-negative bacteria. Therefore, in the future, ZnO nanoparticle-containing formulations may be utilized as antibacterial agents in ointments, lotions, mouthwashes, and surface coatings on various substrates to prevent microorganisms from attaching, colonizing, spreading, and forming biofilms on surfaces in the community, health care and environmental settings. In summary, more research is needed to achieve better technologies to formulate and retain nanomaterials in order to reduce costs associated with premature material loss and to prevent potential human health and environmental impacts.
References
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562.
Rotello V (2003) Nanoparticles: Building Blocks for Nanotechnology, Kluwer Academia, Boston.
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys: Condens Matter 16:R829.
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal Oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686.
Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, Kojima H (1998) Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment Bioengg 86:521–522.
Matsunaga T, Tomoda R, Nakajima T, Wake H (1985) Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett 29:211–214.
Wei C, Lin WY, Zainal Z, William NE, Zhu K, Kruzic AP, Smith RL, Rajeshwar K (1994) Bactericidal activity of TiO2 photocatalyst in aqueous media: toward s solar-assisted water disinfection system. Environ Sci Tech 28:934–938.
Fang M, Chen J-H, Xu X-L, Yang P-H, Hildebrand HF (2006) Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int J Antimicrobiol Agents 27:513–517.
Jones N, Ray B, Koodali RT, Manna AC (2008) Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76.
Fu G, Vary PS, Lin CT (2005) Anatase TiO2 nanocomposites for antimicrobial coating. J Phys Chem B 109:8889–8898.
Sawai J (2003) Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Method 54:177–182.
Sawai J, Yoshikawa T (2004) Quantative evalution of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J Appl Microbiol 96:803–809.
Liu H-L, Yang TC-K (2003) Photocatalytic inactivation of Escherichia coli and Lactobacillus helveticus by ZnO and TiO2 activated with ultraviolet light. Proc Biochem 39:475–481.
Sondi I, Salopak-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182.
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D’Alessio M, Zambonin, PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17:5255–5262.
Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli, physicochemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6156.
Sunada K, Kikuchi Y, Hashimoto K, Fujishima K (1998) Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ Sci Technol 32:726–728.
Bellantone M, Williams HD, Hench LL (2002) Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob Agents Chemother 46:1940–1945.
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870.
Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E (2003) Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr 133:4077–4082.
Yamamoto O (2001) Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorgan Mater 3:643–646.
Li Y, Leung P, Yao L, Song QW, Newton E (2006) Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect 62:58–63.
Campo EJA, Peiteado M, Caballero AC, Villegas M, Modriguez-Paez JE (2009) Room temperature synthesis of high purity 2D ZnO nanoneedles. J Ceramic Proc Res 10:477–481.
Baruah S, Dutta J (2009) Hydrothermal growth of ZnO nanostructures. Sci Technol Adv Mater 10:013001 (18pp).
Liu B, Zeng HC (2004) Room temperature solution synthesis of monodispersed single-crystalline ZnO nanorods and derived hierarchical nanostructures. Langmuir 20:4196–4204.
Xu F, Zhang P, Navrotsky A, Yuan ZT, Ren TZ, Halasa M, Su B-L (2007) Hierarchically assembled porous ZnO nanoparticles: synthesis, surface energy, and photocatalytic activity. Chem Mater 19:5680–5686.
Kim H, Sigmund W (2004) ZnO nanocrystals synthesized by physical deposition. J Nanosci Nanotechnol 4:275–278.
Chen ZG, Li F, Liu G, Tang Y, Cong H, Lu GQ, Cheng HM (2006) Preparation of high purity ZnO nanobelts by thermal evaporation of ZnS. J Nanosci Nanotechnol 6:704–707.
Meulenkamp EA (1998) Synthesis and growth of ZnO nanoparticles. J Phys Chem B 102:5566–5572.
Tam KH, Djurisic AB, Chan CMN, Xi YY, Tse CW, Leung YH, Chan WK, Leung FCC, Au DWT (2008) Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 516:6167–6174.
Raghupati RK, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028.
Vishwanathan R, Gupta RB (2003) Formation of zinc oxide nanoparticles in supercritical water. J Supercritical Fluids 27:187–193.
Tang L, Bao X-B, Zhou H, Yuan A-H (2008) Synthesis and characterization of ZnO nanorods by a simple single-source hydrothermal method. Physica E 40:924–928.
Zhang Z, Mu J (2007) Hydrothermal synthesis of ZnO nanobundles controlled by PEO–PPO–PEO block copolymers. J Colloid Interface Sci 307:79–82.
Jalal R, Goharshadi EK, Abareshi M, Moosavi M, Yousefi A, Nancarrow P (2010) ZnO nanofluids: green synthesis, characterization, and antibacterial activity. Matters Chem Phys 121:198–201.
Ma M-G, Zhu YJ, Cheng GF, Huang YH (2007) Microwave synthesis and characterization of ZnO with various morphologies. Mater Lett 62:507–510.
Vijayan TA, Chandramohan R, Valanarasu S, Thirumalai J, Venkateswaran S, Mahalingam T, Srkumar SR (2008) Optimization of growth conditions of ZnO nano thin films by chemical double dip technique. Sci Technol Adv Mater 9:035007.
Dange C, Phan T, Andre V, Rieger J, Persello J, Foissy A (2007) Adsorption mechanism and dispersion efficiency of three anionic additives [poly(acrylic acid), poly(styrene sulfonate) and HEDP] on zinc oxide. J Colloid Interface Sci 315:107–115.
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behaviour of suspension of ZnO nanoparticles (ZnO nanofluids). J Nanoparticles Res 9:479–489.
Desselberger U (2000) Emerging and re-emerging infectious diseases. J Infect 40:3–15.
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R (2009) Bacterial community variation in human body habitats across space and time. Science 326:1694–1697.
Donlan RM, Costerton JW (2002) Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193.
Gilbert P, Collier PJ, Brown MR (1990) Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother 34:1865–1868.
Hajokova P, Spatenka P, Horsky J, Horska I, Kolouch A (2007) Photocatalytic effect of TiO2 films on various virus and bacteria. Plasma Process Polym 4:S397–S401.
Adama LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532.
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles – an antibacterial study. Sci Technol. Adv Mater 9:035004 (7pp).
Reddy KM, Feris K, Bell J, Hanley C, Punnoose A (2007) Selective toxicity of zine oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett 90:213902 (3 pp).
Rekha K, Nirmala M, Nair MG, Anukaliani A (2010) Structure, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Physica B 405:3180–3185.
Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, Moghaddam KM, Shahverdi AR (2005) ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B: Appl Biomater 93B:557–561.
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41:8484–8490.
Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M (2009) Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol 107:1193–1201.
Heinlaan M, Ivask A, Blinova I, Dubourguir HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fisheri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316.
Huang Z, Zheng X, Yan D, Yin G, Liao X, Kang Y, Yao Y, Huang D, Hao B (2008) Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24:4140–4144.
Xie Y, He Y, Irwin PL, Jin T, Shi X (2011) Antibacterial activity and mechanism of zinc oxide nanoparticles on Campylobacter jejuni. Appl Environ Microbiol 77:2325–2331.
Ma H, Bertsch PM, Glenn TC, Kabengi NJ, Williams PL (2009) Toxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegans. Environ Toxicol Chem 28:1324–1330.
Wang H, Wick RL, Xing B (2009) Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut 157:1171–1177.
Wang X, Yang F, Yang W, Yang X (2007) A study on the antibacterial acitivity of one-dimensional ZnO nanowire arrays: Effects of the orientation and plane surface. Chem Commun 4419–4421.
Zhou D, Keller AA (2010) Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res 44:2948–2956.
Ohira T, Yamamoto O, Iida Y, Nakagawa Z (2008) Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Mater Med 19:1407–1412.
Yamamoto O, Komatsu M, Sawai J, Nakagawa Z (2004) Effect of lattice constant of zinc oxide on antibacterial characteristics. J Mater Sci Med 15:847–851.
Xia T, Kovochich M, Liong M, Adler LM, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2:2121–2134.
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolavels. Sciences 311:622–627.
Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R (2009) EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J Phys Chem C 113:15997–16001.
Jiang J, Oberdrster G, Elder A, Gelein R, Mercer P, Biswas P (2008) Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2:33–42.
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386.
Hirota K, Sugimoto M, Kato M, Tsukagoshi K, Tanigawa T, Sugimoto H (2010) Preparation of Zine oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceramics Int 36:497–506.
Ma H, Kabengi NJ, Bertsch PM, Unrine JM, Glenn TC, Williams PL (2011) Comparative phototoxicity of nanoparticle and bulk ZnO to a free-living nematode Caenorhabditis elegans: the importance of illumination mode and primary particles size. Environ Pollut 159:1473–1480.
Applerot G, Perkas N, Amirian G, Girshevitz O, Gedanken A (2009) Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl Surf Sci 2565:53–58.
McCarthy TJ, Zeelie JJ, Krause DJ (1992) The antibacterial action of zinc ion/antioxidant combination. J Clin Pharm Ther 17:51–54.
Blencowe DK, Morby AP (2003) Zn (II) metabolism in prokaryotes. FEMS Microbiol Rev 27:291–311.
Foster TJ (2005) Immune envasion by staphylococci. Nat Rev Microbiol 3:948–958.
Storz G, Imlay JA (1999) Oxidative stress. Curr Opin Microbiol 2:188–194.
Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, Alvarez PJJ (2008) Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res 42:4591–4602.
Dutta RK, Sharma PK, Bhargava R, Kumar N, Pandey AC (2010) Differential susceptibility of Escherichia coli cells towards transition metal-doped and matrix-embeded ZnO nanoparticles. J Phy Chem B 114:5594–5599.
Lu W, Liu G, Gao S, Xing S, Wang J (2008) Tyrosine-assistant preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities. Nanotechnology 19:445711 (10pp).
Lin Y-J, Xu X-Y, Huang L, Evans DG, Li D-Q (2009) Bactericidal properties of ZnO-Al2O3 composites formed from layered double hydroxide precursors. J Mater Sci Mater Med 20:591–595.
Yamamoto O, Sawai J, Sasamoto T (2000) Change in antibacterial characteristic with doping of ZnO in MgO-ZnO solid solution. Int J Inorganic Mater 2:451–454.
Moribe S, Ikoma T, Akiyama K, Zhang Q, Saito F, Tero-Kubota S (2007) EPR study on paramagnetic species in nitrogen-doped ZnO powders prepared by a mechanochemical method. Chem Phys Lett 436:373–377.
Jose B, Antoci V, Zeiger AR, Wickstrom E, Hickok NJ (2005) Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol 12:1041–1048.
Perelshtein I, Applerot G, Perkas N, Wehrschetz E, Hasmann A, Guebitz GM, Gedanken A (2009) Antibacterial properties of an in situ generated and simultaneously deposited nanocrystalline ZnO on Fabrics. Appl Mater Interfaces 1:361–366.
Koga H, Kitaoka T, Wariishi H (2009) In situ synthesis of silver nanoparticles on zinc oxide whiskers incorporated in a paper matrix for antibacterial applications. J Mater Chem 19:2135–2140.
Vila B, Escribano F, Esteban A, Fontgibell A, Esteve-Garcia E, Brufau J (2010) Application of ZnO-functionalised-sepiolite in weaning piglet diets. Livestock Sci 134:232–235.
Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: an emerging disiciple evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839.
Adisehaih P, Hall JB, McNeil SE (2009) Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed Nanobiotechnol 2:99–112.
Hirano S, Higo S, Tsukamota N, Kobayashi E, Suzuki KT (1989) Pilmonary clearance and toxicity of zinc oxide instilled into the rat lung. Arch Toxicol 63:336–342.
Ma-Hock L, Burkhardt S (2008) Inhalation toxicity of nano-scale zinc oxide in comparison with pigmentary zinc oxide using short-term inhalation test protocol. Naunyn-Schmiedebergs Arch Pharmacol 377:354.
Wesselkamper SC, Chen LC, Gordon T (2001) Development of pulmonary tolerance in mice exposed to zinc oxide fumes. Toxicol Sci 60:144–151.
Wesselkamper SC, Chen LC, Gordon T (2005) Quantitative trait analysis of the development of pulmonary tolerance to inhaled zinc oxide in mice. Respir Res 6:73.
Wang B, Feng WY et al (2008) Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res 10:263–276.
Lam HF, Chen LC, Ainsworth D, Peoples S, Amdur MO (1988) Pulmonary function of guinea pigs exposed to freshly generated ultrafine zinc oxide with and without spike concentrations. Am Ind Hyg Assoc J 49:333–341.
Zvyagin AV, Zhao X, Gierden A, Sanchez W, Ross JA, Roberts MS (2008) Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J Biomed Opt 13:064031 doi:10.1117/1.3041492.
Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y (2008) Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health Part A-Toxic/Hazard Subs Environ Engineer 43:278–284.
Zhu X, Zhu S et al (2009) Acute toxicities of six manufactured nanomaterial suspension to Daphnia magna. J Nanoparticle Res 11:67–75.
Yang H, Liu C, Yang D, Zhang H, Xi Z (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J Toxicol Sci 34:119–122.
Deng XY, Luan QX, Chen WT, Wang YL, Jiao Z (2009) Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 20:11510.
Lee J, Kang BS, Hicks B, Chancellor TF, Chu BH, Wang H-T, Keselowsky BG, Ren F, Lele TP (2008) The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 29:3743–3749.
Gopalan RC, Osman IF, Amani A, De Matas M, Anderson D (2009) The effect of zinc oxide and titanium dioxide nanoparticles in the comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology 3:33–39.
Beckett WS, Chalupa DF, Paul-Brown A, Speers DM, Stewart JC, Frampton MW, Well MJ, Haung LS, Cox C, Zareba W, Oberdorster G (2005) Comparing inhaled ultrafine versus fine zinc oxide particles in healthy adults: a human inhalation study. Am J Respir Crit Care Med 171:1129–1135.
Acknowledgements
The author thank Dr. Vector Huber for the critical reading the manuscript. The author would like to thank Dr. R.T. Koodali and Krishna R. Raghupathi for their help in preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Berlin Heidelberg
About this chapter
Cite this chapter
Manna, A.C. (2012). Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles. In: Cioffi, N., Rai, M. (eds) Nano-Antimicrobials. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-24428-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-24428-5_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-24427-8
Online ISBN: 978-3-642-24428-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)