Abstract
Several procedures are currently available for the quantification of DNA and RNA. However, not all these methods are equally useful for the accurate measurement of nucleic acid concentration extracted from fixed and paraffin-embedded samples. This is frequently due to the high degradation levels and low yields of extracted macromolecules, and to the presence of tissue contaminants that may or overestimate the quantity of DNA/RNA suitable for downstream analysis. The methods described in this chapter have been divided into two main categories, according to whether measurement is based on spectrophotometric measurement or on reading in the presence of a fluorescent dye. Each procedure has been illustrated referring to its advantages and/or limitations when used for quantification of nucleic acids extracted from archival samples. This section is focused especially on RNA measurement, for which the majority of the methods relies on detection of ribosomal RNA as an estimated measure of target mRNA quantity. A further section is dedicated to the absolute quantification of specific target sequences by means of the real-time-based amplification that results, particularly suitable when the starting material is extremely low.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Degradation Level
- Ethidium Bromide Solution
- Guanidine Isothiocyanate
- Specific Target Sequence
- Nucleic Acid Concentration
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction and Purpose
Several procedures are available for quantification of nucleic acids. Since the data obtained with different methods are not directly comparable [1], it’s essential to choose the same approach when a comparison of data from different reports has to be done. Some of the following methods give information of both quantity and quality of nucleic acids. All the reported methods are more or less suitable for nucleic acids extracted from formalin-fixed and paraffin-embedded (FFPE) tissues. It should also be taken into account that when RNA concentration is measured, it involves mostly ribosomal RNA that represents 85% of the total RNA in a cell (mRNA is present at 1–5%).
Methods for nucleic acid quantification have been divided according to whether quantification relies on spectrophotometric measurement, reading in presence of a fluorescent dye, or on real-time amplification.
2 Methods
2.1 Spectrophotometric Quantification
The spectrophotometer is the most common device used to determine the quantity of the extracted nucleic acids. Concentration is estimated through absorbance reading. Measurements of the absorbance are recorded at different wavelengths to calculate DNA/RNA concentration and the presence of contaminants. The following absorbance lengths are usually analyzed:
-
A260 nm: Both DNA and RNA concentrations are read at this wavelength, therefore this measurement cannot distinguish between the two nucleic acids. This value is used in the following formula to obtain the nucleic acid concentration (Lambert–Beer law):
$$ {C}_{\mu \text{g/}\mu \text{l}}=A\times \text{dil}\text{. factor}\times \epsilon \times {10}^{-3} $$(16.1)where A is the absorbance, dilution factor (dil. factor) is the ratio between the total volume used for the measurement and the volume of sample, ε (molar extinction coefficient) is a physical constant that is unique for each substance and describes the amount of absorbance at 260 nm (A260) of 1 mole/l of nucleic acid solution measured in a 1 cm path-length cuvette. The molar extinction coefficient is 50 for double-stranded DNA (dsDNA), 40 for RNA, and 33 for oligonucleotides. Estimation of nucleic acid concentration can be affected by phenol contamination. Since phenol strongly absorbs at 260 nm, it can falsely increase DNA yield and purity.
-
A260/230 nm ratio: It gives the level of contamination from copurified organic compounds (sugars, heparin, guanidine isothiocyanate...) that could inhibit downstream experiments. A260/230 ratio >1.8 for both DNA and RNA is indicative of a “pure” nucleic acid preparation. This type of contamination is common in biological samples.
-
A260/280 nm ratio: It gives the level of contamination from proteins, salts, and other copurified reagents. A260/280 nm ratio >1.8 for both DNA and RNA is indicative of a “pure” nucleic acid preparation.
2.1.1 Conventional Spectrophotometer
It requires that the DNA/RNA sample is diluted before quantification. Depending on the expected concentration, 2–3 μl of sample diluted in 100–500 μl of H2O is normally used.
Major disadvantages of this method are the poor sensitivity (the lower limit is generally 0.5–1 μg nucleic acid) and the interferences in signal reading by contaminating components such as nucleotides, proteins, and salts present in the solution.
2.1.2 NanoDrop (e.g., ND-3300, NanoDrop Technologies, USA)
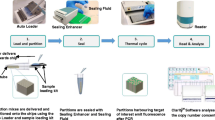
The NanoDrop is a modern spectrophotometer for highly sensitive quantification of DNA and RNA (proteins included). A major advantage of this system is that it requires very low sample consumption, which is very useful when using FFPE, and it is very reliable because no sample dilutions are necessary.Footnote 1 As a conventional spectrophotometer, it gives information about nucleic acid concentration (but it can also distinguish between DNA and RNA) and about the presence of contaminants. The results are acquired on a PC software programme (Fig. 16.1).
2.2 Quantification with a Fluorescent Dye
This approach relies on the measurement of the fluorescence emitted by a dye when it is intercalated in the DNA or RNA filaments. Emitted fluorescence is proportional to DNA/RNA sample concentration. The choice of the method depends on the nature of the material that should be measured.
2.2.1 Ethidium Bromide Gel-Based Assays
This approach can be used for quantification of both PCR product bands and genomic DNA (less commonly for RNA), but it is not the best choice when a precise quantification is required. For PCR product quantification, the assay can be easily performed by electrophoresis of the sample in an agarose gel stained with ethidium bromide (see Sect. 17.2 and Appendix A). The agarose percentage depends on the length of the fragment that has to be resolved.Footnote 2 Quantification can be carried out by acquiring the gel image with a standard gel imaging instrument and calculating the band intensity with dedicated software. The comparison with known reference DNAs allows a relative quantification.
An alternative method, especially for genomic DNA, is to spot the DNA sample directly on an agarose gel prepared in a small Petri capsule together with four or five reference DNAs at known concentration. This gel must be treated with ethidium bromide solution and evaluated at a UV transilluminator by direct comparison.
2.2.2 SYBR Green I Method
SYBR Green I dye is a highly sensitive fluorescent stain for detecting single-stranded DNA (ssDNA) and dsDNA in agarose and polyacrylamide gels. It can also detect RNA, but with much lower sensitivity. This dye is approximately 25–100 times more sensitive than ethidium bromide staining and much less mutagenic.
2.2.3 PicoGreen Method
PicoGreen is an ultrasensitive fluorescent nucleic acid stain for quantification of dsDNA in solution. It enables the quantification of less than 25 ng/ml of dsDNA using a standard spectrofluorometer or a fluorescence microplate. This method is ∼500-fold more sensitive than absorbance measurements at 260 nm and the concentration measurement is not affected by contaminants (nucleotides, single-strand nucleic acids, proteins, organic compounds...).
2.2.4 RiboGreen Method
RiboGreen is an ultrasensitive fluorescent nucleic acid stain for measuring RNA concentration in solution. The use of this reagent requires the availability of a spectrofluorometer or a fluorescence microplate and the preparation of a titration curve before each experiment. The method is ∼1,000-fold more sensitive than absorbance measurements at 260 nm and ∼200-fold more sensitive than ethidium bromide-based assays, allowing detection of 1 ng RNA/ml. Signal intensity of RiboGreen can be affected by several compounds that commonly contaminate nucleic acid preparations, so it should be advisable to make a preliminary quantification by spectrophotometer to ensure the purity of the sample. It does not discriminate between DNA and RNA; for this reason, a treatment with DNase should be performed before RNA quantification. For practical details, follow the manufacturer’s instructions.
2.2.5 Agilent 2100 Bioanalyzer
This approach is more commonly used for the determination of degradation levels in nucleic acid samples (see Chap. 17). However, this microfluidic capillary electrophoresis system is also a very sensitive tool for calculation of DNA and RNA quantity. The instrument uses a laser for excitation of intercalating fluorescent dyes offering a high level of sensitivity.
2.3 Quantification by Real-time PCR
Real-time PCR can be used as an alternative method for the concentration estimation of both DNA and RNA when the starting material is extremely poor. This quantification procedure is based on the absolute quantification of specific target sequences (DNA or cDNA sequence depending on the origin of the starting material) [2]. The absolute quantitation relies on a curve generated from serially diluted standards of known concentrations or number of copies. The standard curve produces a linear relationship between Ct and initial amounts of the standard, allowing the determination of the concentration/number of copies of the unknown target sequences based on their Ct values. This method assumes that all standards and samples have approximately equal amplification efficiencies. The standard used for the curve can be a fragment of dsDNA, ssDNA, or complementary RNA (cRNA) containing the target sequence. The inner standard can be obtained by in vitro transcription (cRNA), by cloning into a plasmid (DNA), or by purification of a PCR product. The primer pair for the target sequence should be designed in order to produce an amplicon no longer than 70 bases (for general information on real-time PCR set up see Chap. 25).
Notes
- 1.
See http://www.nanodrop.com/ for details.
- 2.
The measurement is performed by pipetting 1 μl of nucleic acid solution directly onto the pedestal of the instrument.
- 3.
A 0.7% gel shows a good resolution of large fragments (5–10 kb), while a 2% gel will show a good resolution for small fragments (0.2–1 kb). For smaller fragments a vertical polyacrylamide gel is more appropriate (see Appendix A and Appendix B for more details).
- 4.
See Roche datasheet at the website: https://www.roche-applied-science.com/pack-insert/1988131a.pdf . Equal reagents from other companies may be used.
- 5.
Provided by Molecular Probes. Datasheet is available at the website: http://probes.invitrogen.com/media/pis/mp07581.pdf . Equal reagents from other companies may be used.
- 6.
See Molecular Probes, web site: http://probes.invitrogen.com/media/pis/mp11490.pdf .
- 7.
See http://www.chem.agilent.com/Scripts/PDS.asp?lPage=51 for more details.
References
Bustin SA (2005) Real-time, fluorescence-based quantitative PCR: a snapshot of current procedures and preferences. Expert Rev Mol Diagn 5(4):493–498
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. Biotechniques 39(1):75–85
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dotti, I., Bonin, S. (2011). Quantification of Nucleic Acids. In: Stanta, G. (eds) Guidelines for Molecular Analysis in Archive Tissues. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-17890-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-642-17890-0_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-17889-4
Online ISBN: 978-3-642-17890-0
eBook Packages: MedicineMedicine (R0)