Abstract
The study of novel interstitial cells in the tissues of the urinary tract has defined advances in the field in the last decade. These intriguing cells belong to the same family as the better known interstitial cells of Cajal (ICC) of the gastrointestinal tract, and their discovery has been interpreted to suggest that pacemaker cells may be present in the urinary tract, driving the spontaneous or myogenic activity of the neighboring smooth muscle. This scenario may be true for the urethra where ICC have been described as “loose pacemakers” providing multiple, random inputs to modulate urethral smooth muscle activity. However, there is a paucity of direct evidence available to support this hypothesis in the bladder (where the smooth muscle cells are spontaneously active) or the renal pelvis (where atypical smooth muscle cells are the pacemakers), and it now seems more likely that urinary tract ICC act as modulators of smooth muscle activity.
Interestingly, the literature suggests that the role of urinary tract ICC may be more apparent in pathophysiological conditions such as the overactive bladder. Several reports have indicated that the numbers of ICC present in overactive bladder tissues are greater than those from normal tissues; moreover, the contractility of tissues from overactive bladders in vitro appears to be more sensitive to the Kit antagonist, glivec, than those from normal bladder. Future research on urinary tract ICC in the short to medium term is likely to be dynamic and exciting and will lead to increasing our understanding of the roles of these cells in both normal and dysfunctional bladder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The study of interstitial cells of Cajal (ICC) in the gastrointestinal tract has revolutionized the way that researchers understand gut motility and neurotransmission. ICC were discovered by the Spanish neuroanatomist, Ramon and Cajal, one century ago (Cajal 1911) and are now known to be a specialized class of cells who act as pacemakers, driving peristaltic activity throughout the gut and who play a key role in the transmission of signals from nerves to smooth muscle (Sanders 1996; Horowitz et al. 1999). Outside the gastrointestinal field, ICC have been most widely studied in tissues of the urinary tract with numerous independent laboratories publishing research which points to the seemingly ubiquitous presence of ICC in the urinary tract. The discovery of cells with morphological and physiological properties of ICC in renal pelvis, ureter, bladder, and urethra not only provides new opportunities to advance our knowledge of cellular interactions within these tissues but is also of significant clinical impact in the development of new therapies to treat urinary tract disorders.
1 ICC in the Urinary Tract
The established role of ICC as pacemakers in the gut naturally led to the suspicion that other smooth muscle preparations with properties of spontaneous activity might contain similar cells. While gut ICC provide a useful point of reference, it is unwise to consider that ICC may play similar roles in other tissues in the absence of direct experimental evidence. The urinary bladder has long been shown to display myogenic, low-level, nonvoiding, “background” spontaneous contractions which are thought to underpin bladder tone and shape during filling (Turner and Brading 1997). In vitro tension recordings from renal pelvis preparations showed rhythmic activity in the smooth muscle, implying the existence of a pacemaker mechanism (Lang et al. 1998), although activity in the ureter was less frequently encountered. The ability of urethral strips to develop tone in similar experiments also hinted at a specialized means of modulating contractility of the urethral smooth muscle (Brading 1999).
Transmission electron microscopy (TEM) has been the gold-standard tool for identifying ICC, and there are accepted ultrastructural criteria that a candidate cell must satisfy before being termed an ICC (Komuro 1999; Komuro et al. 1999). While there is some ultrastructural heterogeneity between ICC subtypes in the gut, ICC typically have thin (5 nm) and intermediate filaments (10 nm), abundant mitochondria, caveolae, rough and smooth endoplasmic reticulum, Golgi complexes, a basal lamina which may be discontinuous but do not tend to have thick filaments (15 nm), dense bodies or dense bands which are characteristic of smooth muscle cells (SMC). In TEM, an electron beam is passed through ultrathin sections of tissue, typically 70 nm thickness which enables investigation of the ultrastructure of the cells present within the section at high magnifications (up to 80,000×). The advantage of TEM is that one can establish that ICC are present within the tissue of interest, and with meticulous (and time consuming) serial sectioning, it is possible to study interactions between the cell of interest and neighboring cells. Confocal fluorescent microscopy is perhaps the method of choice to image interactions between cells as this allows the acquisition of optical sections from a “thicker” specimen, up to a volume of 50 μm. Reconstruction of the optical sections in three-dimensions (3D) enables one to analyze the 3D arrangement of a cellular network, and if the sample has been labeled with antibodies and fluorophores of different wavelengths to label, e.g., ICC and nerves, it is possible to visualize relationships between several cell populations. Both of these techniques have been successfully exploited in the study of ICC in the urinary tract and have provided morphological evidence that specialized cells are indeed present.
2 Nomenclature
There has been much debate on the correct nomenclature that should be adopted when describing these novel cells in tissues outside of the gastrointestinal tract, and the literature contains many papers which refers to them as: interstitial cells (IC); ICC; ICC-like cells or myofibroblasts. This issue was debated at the “Vth International Symposium on ICC” held in Ireland, July 2007 and a consensus was reached that these cells should be termed “ICC.” This terminology describes a family of cells found in many disparate tissues including bladder, urethra, ureters, renal pelvis, other genitourinary tissues and blood vessels which possess morphological, ultrastructural, and physiological properties of ICC while not implying that physiological functions of gut ICC would be universal. This consensus was timely and served to consolidate the work of many groups working on similar cells in many smooth muscle preparations. It should be noted, however, that this debate is ongoing and as knowledge in the field continues to advance, there may be changes to this nomenclature in the future. In this chapter, the term ICC will be used, and no disrespect is intended to the original authors who may have chosen an alternative description.
3 Urinary Bladder
3.1 Location and Morphology of Bladder ICC
The traditional view of the bladder considered the organ to be comprised of mucosa, including urothelium and lamina propria, and underlying muscularis of the detrusor, containing smooth muscle. A rich microvasculature was known to be in the lamina propria, along with sensory nerves and connective tissue, and the detrusor’s rich innervation was widely demonstrated with many species exhibiting intramural ganglia. Bladder filling and emptying is well described in text books in terms of nervous control and smooth muscle contractility; however, the field has moved considerably in the last decade with the discovery that previously unknown cell types are also located throughout the bladder wall.
A study of the targets of cGMP signaling in guinea-pig and human bladder (Smet et al. 1996, later confirmed by Gillespie et al. 2004), after nitric oxide stimulation, first indicated that the bladder contained cells which were reminiscent of gut ICC by their morphological appearance (i.e., the cells had lateral processes or branches). The same study also showed a population of cells which were immunopositive for the intermediate filament, vimentin, which is typically found in ICC and other cells of mesenchymal origin, but not smooth muscle, leading the authors to speculate that the bladder may contain cells resembling ICC. Although not a selective ICC marker, vimentin antibodies provide a helpful means of visualizing cell types which may include ICC, within a tissue preparation without labeling SMC. McCloskey and Gurney (2002) later used antibodies to the established ICC marker, c-Kit to demonstrate that the guinea-pig bladder did indeed contain ICC, and this work has since been confirmed by several independent laboratories (Hashitani et al. 2004; Biers et al. 2006; Shafik et al. 2004; Piaseczna Piotrowska et al. 2004; Roosen et al. 2009). c-Kit is a proto-oncogene that encodes the tyrosine kinase receptor, Kit which is expressed by ICC and mast cells but not SMC or fibroblasts (Maeda et al. 1992). The discovery that gastrointestinal ICC could be labeled with anti-c-Kit was a milestone for ICC research, providing a reliable tool for identifying ICC in smooth muscle tissues with light microscopy, moreover, presenting the opportunity to manipulate the function of ICC in tissue preparations or animal models using pharmacological tools or neutralizing Kit antibodies (Sanders et al. 2002; Sanders and Ward 2007).
3.1.1 ICC in the Lamina Propria
A population of ICC has been identified with c-Kit and vimentin antibodies in the lamina propria region (ICC-LP) between the urothelium and the detrusor muscularis (Sui et al. 2002; Davidson and McCloskey 2005; see Figs. 1 and 2). These ICC-LP have a stellate-shaped morphology with several branches emanating from a central cell body (Davidson and McCloskey 2005) and make connections with neighboring ICC-LP to form an interconnected network. Immunohistochemistry and TEM have shown that this network is connected by connexin 43 gap junctions (Sui et al. 2002; Wiseman et al. 2003). The ICC-LP network is closely associated with mucosal nerves as shown by confocal imaging where Kit-positive cells made contacts with anti-PGP9.5 labeled nerves (Davidson and McCloskey 2005) and by TEM which demonstrated close contacts between ICC-LP and nerve endings (Wiseman et al. 2003). Recent work has shown that human bladder ICC-LP make frequent structural associations with a mucosal cholinergic plexus (Johnston et al. 2008; submitted), consistent with the finding that ICC-LP express M2 and M3 muscarinic receptors (Mukerji et al. 2006; Grol et al. 2009). de Jongh et al. (2007, 2009) suggested that cells resembling lCC-LP were immunopositive for cyclooxygenase 1; furthermore, Ost et al. (2002) reported vanilloid receptor immunoreactivity giving further insight into the pharmacological profile of these cells. The finding that purinergic receptors including P2X3, P2Y2, and P2Y4 but predominantly P2Y6 are expressed on ICC-LP (Sui et al. 2006) is particularly interesting as there is now substantial functional evidence for physiological responses to ATP in this cell type (see below).
Kit-positive ICC in guinea-pig and mouse bladder. ICC labeled with anti-c-Kit in guinea-pig bladder lamina propria at low magnification (a) and at higher magnification (b) where nuclei have been counterstained with DAPI (blue). Mouse detrusor Kit-positive ICC are elongated, branched cells orientated in parallel with the muscularis (c). Guinea-pig (d) detrusor ICC (green) are associated with nerves (red) and have similar arrangement to those in mouse detrusor. Images courtesy of Dr RA Davidson and Dr KD McCloskey
Kit-positive ICC in human bladder. ICC labeled with anti-c-Kit (green) in human bladder lamina propria (a) forming a loose network between urothelium and detrusor and making connections with cholinergic nerves (red), labeled with antivesicular acetylcholine transferase. Kit-positive ICC in the human detrusor (c, d) have an elongated, branched morphology and are associated with smooth muscle bundles (red). Images courtesy of Dr L Johnston and Dr KD McCloskey
3.1.2 ICC and the Bladder Microvasculature
A current study has demonstrated a new class of bladder Kit-positive cell, associated with blood vessels in the lamina propria of human bladder (Johnston et al. 2010). These cells are located on the outer surface of the microvessels with the branches of individual cells contacting up to six vascular SMC and may represent pericytes. Cells resembling ICC, associated with vascular smooth muscle, have been reported in guinea-pig gall-bladder (Lavoie et al. 2007) and may represent a local control of perfusion within the tissue in response to metabolic needs. The vascular perfusion of the bladder wall is a key determinant of normal bladder contractility as in vivo ischemia in animal models has been shown to induce bladder overactivity (Azadzoi et al. 1999). Further work is needed to determine whether Kit-positive cells on the bladder microvessels have any physiological role in the regulation of bladder blood flow.
3.1.3 ICC in the Detrusor
The arrangement of ICC in the detrusor region of guinea-pig, mouse, and human bladder is distinctively different from that of the mucosa. Confocal imaging of detrusor whole-mount, flat-sheet preparations, and subsequent 3D reconstruction has revealed that Kit-positive ICC are located on the boundary of detrusor smooth muscle bundles apparently tracking them (McCloskey and Gurney 2002; Hashitani et al. 2004; Davidson and McCloskey 2005; McCloskey et al. 2009; Johnston et al. 2010). These ICC have a distinctive elongated morphology with several lateral branches and appear to be placed as discrete cells with little evidence that they form complex networks (see Figs. 1 and 2). They have previously been termed “intramuscular ICC” (ICC-IM; Brading and McCloskey 2005) and have also been reported to be present within the smooth muscle bundles (Hashitani et al. 2004). These ICC-IM are associated with detrusor nerves as shown in double-labeling experiments with anti-c-Kit and the general neuronal marker, anti-PGP9.5 (Davidson and McCloskey 2005) and cholinergic nerves in particular with anti-vAChT (vesicular acetylcholine transferase, Johnston et al. 2008). Like the ICC-LP, Kit-positive ICC-IM also contain vimentin filaments (Davidson and McCloskey 2005).
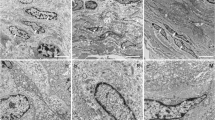
Ultrastructurally, ICC-IM are very similar to gut ICC as shown by Kubota et al. (2008), Rasmussen et al. (2009) and Cunningham et al. (2009). These elongated, branched cells on the boundary of smooth muscle bundles have a basal lamina (or membrane dense bands), extensive rough and smooth endoplasmic reticulum, Golgi complexes, caveolae, mitochondria, thin and intermediate filaments, and a centrally placed nucleus. They are distinct from SMC by the absence of thick filaments and dense bodies and differ from fibroblasts by the absence of dilated rough endoplasmic reticulum which is a defining characteristic of fibroblasts and the presence of a basal lamina. Two studies reported the interesting finding that detrusor ICC contained vesicles or coated pits, perhaps indicative of a secretory function (Rasmussen et al. 2009; Cunningham et al. 2009).
A further ICC subtype is also present in the detrusor which has a stellate morphology and more closely resembles the ICC-LP (Davidson and McCloskey 2005). These so-called ICC-IB (interbundle ICC; Brading and McCloskey 2005) are c-Kit- and vimentin-positive and make connections with each other in the spaces between the detrusor smooth muscle bundles. The TEM work of Rasmussen et al. (2009) demonstrated detrusor ICC–ICC contacts via gap junctions and peg and socket junctions, supporting the existence of interconnected ICC occupying the space between the smooth muscle bundles.
Davidson and McCloskey (2005) proposed that bladder ICC could form a conduit for the relay of information from urothelium to detrusor, incorporating ICC-LP, ICC-IB, and ICC-IM. This view has been shared by others and is consistent with findings from studies of bladder ICC from mice, guinea-pigs, and humans. The morphological evidence suggests that ICC-LP form a network below the urothelium which presumably could respond to chemical transmitters released by urothelial cells, communicate with mucosal nerves, and/or relay information directly to underlying detrusor ICC and/or smooth muscle. Alternatively, ICC-LP could act as stretch-sensors, as proposed by Sui et al. (2004) capable of sensing bladder fullness and relaying information to mucosal sensory afferents.
3.2 Physiological Properties of Bladder ICC
The study of bladder ICC with traditional physiological techniques has been both intriguing and productive, generating a significant body of literature in less than a decade. Several laboratories have used the patch-clamp technique and real-time fluorescent Ca2+-imaging to characterize the physiological properties of bladder ICC, and the overall picture is rather different from urethral ICC or their counterparts in the gastrointestinal tract. Furthermore, ICC-LP and detrusor ICC have been shown to have idiosyncratic differences which may hint at their uniquely different roles in bladder function.
3.2.1 ICC-LP Physiological Properties
Patch-clamp studies of enzymatically dispersed ICC-LP have shown the presence of voltage-dependent Ca2+ currents and TEA-sensitive K+ currents (Sui et al. 2004). Wu et al. (2004) reported spontaneous transient inward currents (STICs) in 45% of cells tested, which reversed close to the chloride equilibrium potential, were associated with increases in intracellular Ca2+-concentration [Ca2+]i and were reduced by the Cl− channel blocker, DIDS. Inward currents, generated in response to ATP application (Sui et al. 2004; Wu et al. 2004), had a similar profile, indicative of a Ca2+-activated Cl− current. The ATP-generated conductance was attenuated by capsaicin (Sui et al. 2008) in keeping with reports that ICC-LP possess vanilloid or TRPV1 receptors (Ost et al. 2002). The original authors recently questioned the reliability of the TRPV1 antibody as nonspecific cellular TRPV1-immunoreactivity was observed in bladders from TRPV1 knockout mice (Everaerts et al. 2009); however, further work with more selective antibodies or molecular techniques should clarify this issue. ICC-LP also fired Ca2+-activated Cl− currents and Ca2+-transients in response to reduction of extracellular pH (Sui et al. 2008) which were similar to those evoked by ATP application.
The mean resting membrane potential (RMP) of ICC-LP from current-clamp studies was found to be around −60 mV (Sui et al. 2004; Wu et al. 2004), and spontaneous depolarizing fluctuations were recorded, demonstrating that ICC-LP are electrically active. The RMP was shown to lie between E K and E Cl as recordings with K+ filled pipettes gave a RMP of −60 mV, whereas recordings with Cs+-filled pipettes gave RMPs of −30 mV. The spontaneous depolarizations were supported by Ca2+-signaling studies of isolated ICC-LP which demonstrated the ability of ICC-LP to undergo spontaneous changes in [Ca2+]i (Sui et al. 2004; Wu et al. 2004).
3.2.2 Detrusor ICC Physiological Properties
Detrusor ICC have also been studied with patch clamp and been shown to possess several ion channels including l-type Ca2+ currents, a nickel-sensitive Ca2+ current which was not a T-type conductance (McCloskey 2006); Ca2+-activated K+ currents (BK) and voltage-dependent K+ currents (McCloskey 2005) including a KCNQ component (Anderson et al. 2009). The urethral pacemaker conductance, a Ca2+-activated Cl− current (Sergeant et al. 2000), similar to that found in bladder ICC-LP (see above), has not yet been clearly demonstrated in detrusor ICC, although spontaneous transient depolarizations (STDs) (of an uncharacterized ionic basis) have been recorded from detrusor ICC in current-clamp mode (Anderson et al. 2009), implying rhythmic electrical firing, consistent with pacemaker-like behavior.
Spontaneous activity was also seen in Ca2+-imaging experiments from detrusor ICC which fired long-duration Ca2+-transients at a rate of approximately three per minute both in isolated cells and in tissue sheets (McCloskey and Gurney 2002; Hashitani et al. 2004; Johnston et al. 2008). This pattern of spontaneous activity was clearly different from SMC which fired Ca2+-transients of comparatively greater frequency and shorter duration. The relationship between ICC and SMC Ca2+-signaling remains rather elusive and is not easily explained by either the views that ICC act as pacemakers or indeed the view that ICC have no meaningful role in bladder spontaneous activity. The observation that the SMC never fire at a rate less than the ICC may suggest that ICC provide a baseline input; moreover, multiple ICC may “pace” a smooth muscle bundle in response to local needs, providing a fine-control on smooth muscle myogenic activity. The situation has similarities to urethral ICC and urethral SMC (see below), where urethral ICC are considered to randomly enhance the activity of neighboring SMC (Hashitani and Suzuki 2007), rather than act as a coordinated pacemaker cellular network.
Muscarinic stimulation by application of carbachol to whole-sheet “in situ” preparations increased the frequency of detrusor ICC Ca2+-transients (Johnston et al. 2008; see Fig. 3), suggesting that detrusor ICC activity can be modulated by parasympathetic nerves. Application of carbachol to enzymatically dispersed detrusor ICC induced an intracellular Ca2+-transient which was not associated with contraction, in contrast to detrusor SMC which were vigorously contractile under identical experimental conditions. The cholinergic signaling pathway in these cells has been shown to be mediated largely via M3 muscarinic receptors and an IP3 and ryanodine receptor-dependent release of Ca2+ from intracellular stores (Johnston et al. 2008). It is interesting that while carbachol generates an increase in [Ca2+]i in detrusor ICC, there is no such response in ICC-LP (Wu et al. 2004) and this difference may represent an important division of labor between two ICC subtypes in the bladder. The physiological consequence of the detrusor ICCs’ Ca2+-response to cholinergic stimulation has not yet been ascertained, although Johnston et al. (2008) suggested that release of transmitter substances may result, consistent with the observation of vesicles in ICC from TEM studies (Rasmussen et al. 2009; Cunningham et al. 2009). de Jongh et al. (2007) suggested that ICC may release prostaglandins and further work is necessary to determine whether detrusor ICC actually exhibit a secretory-type function.
Bladder detrusor ICC physiology. (ai) Typical morphology of bladder ICC. (aii–iii) Time series micrograph showing Ca2+ response to 1 μM carbachol which was blocked by the M3 antagonist 4-DAMP (1 μM). Traces show intensity time series of responses to carbachol and the effects of 4-DAMP and the M2 antagonist methoctramine (1 μM). Fluorescence (F) of an event is expressed as a ratio of background (F0). CCh carbachol. (b) Graph summarizing the effect of 4-DAMP and methoctramine on 7 and 11 cells, respectively. Asterisk denotes statistical significance. Figure taken from Johnston et al. (2008)
3.3 Clinical Significance of Bladder ICC
The morphological and physiological studies of the various subtypes of bladder ICC indicate that these are novel cells, with many properties of classical gut ICC which are ideal candidates to contribute to normal bladder function. However, current research does not adequately resolve the issue of what the actual roles of ICC are in bladder filling and emptying. When these cells were first discovered, several groups considered that ICC could fulfill a pacemaking role, responsible for the origin and propagation of spontaneous activity in the bladder wall during filling. While both ICC-LP and detrusor ICC have been shown to exhibit spontaneous electrical and Ca2+-signaling, a profile consistent with a pacemaking phenotype, direct evidence that this signaling acts to modulate the activity of detrusor smooth muscle has not yet been published.
Pharmacological and animal model-based experimental approaches have been used in the study of ICC function in bladder. The fact that ICC express the tyrosine kinase receptor, Kit, has been exploited using the drug imatinib mesylate (Glivec) to block the Kit receptor in in vitro and in vivo studies. Glivec is used clinically in the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors and targets the tyrosine kinases PDGF receptor, bcr–abl, and c-Kit. Biers et al. (2006) found Glivec to improve capacity, compliance, urinary frequency and to reduce spontaneous activity in cystometric studies in guinea-pigs. This was consistent with the finding that Glivec reduced spontaneous electrical and mechanical activity in isolated guinea-pig detrusor tissues (Kubota et al. 2004, 2006). The study of a mutant mouse strain which had been pivotal in revealing the role of ICC in the gut (Sanders et al. 1999, 2006; Sanders and Ward 2007) was less promising for bladder ICC research. The W/Wv mouse has defects in the W locus which encodes the Kit receptor and does not contain several populations of gut ICC; however, detrusor ICC were apparently little affected by the mutation and were present in comparable numbers and localization to bladders from wild-type animals (McCloskey et al. 2009).
The activity and presence of ICC seems to be less prominent in normal, healthy bladders compared with pathological conditions, and studies of “abnormal” bladder have enhanced our understanding of the implications of ICC in a clinical setting. Biers et al. (2006) demonstrated increased numbers of Kit-positive ICC in overactive human bladder samples compared with normal tissues and interestingly demonstrated a greater inhibitory effect of Glivec on detrusor contractions in samples from overactive bladder. Kubota et al. (2008) reported an increase in the population of ICC in guinea-pig bladder after outlet obstruction. Increased expression of connexin 43 in the bladder lamina propria of rats after spinal cord transection (SCT) was associated with increased coordination of spontaneous activity compared with normal adult rats (Ikeda et al. 2007). Moreover, Glivec was found to have a marked inhibitory effect on the enhanced spontaneous contractions of whole bladders from spinal cord transected rats, whereas there was little effect on control bladders (Sui et al. 2008). Roosen et al. (2009) found increased lamina propria connexin 43-immunostaining in human overactive bladder but noted little change in c-Kit expression perhaps indicating that gap junctions numbers were upregulated rather than the actual numbers of ICC. Piaseczna Piotrowska et al. (2004) compared the presence of ICC in normal bladders and samples from patients with megacystis-microcolon intestinal hypoperistalsis syndrome and demonstrated marked lack of ICC in the MMIHS sample set. This is particularly interesting as the MMIHS bladder is distended, unobstructed, and dysfunctional and supports the finding of increased numbers of ICC in obstructed, overactive bladders.
The literature currently seems to support a more prominent role for ICC in diseased or abnormal bladders, largely explained by an increase in their populations and/or the gap junctions connecting the network. The question remains for the normal bladder, do ICC simply act as bystanders, capable of fine-tuning and regulating SMC activity in response to the needs of the tissue or do they have a primary role as communicators, sensing and relaying information between the complex system of heterogeneous cells (SMC, ICC, nerves, microvessels, and urothelial cells) that make up the bladder wall? The existing body of evidence points to multiple roles for ICC in the bladder, dependent on their location, structural connections with neighboring cells, expression of membrane receptors, and ion channels and appears to be tightly controlled by the physiological/pathophysiological state of the organ. This area of research is dynamic and exciting and is contributing to many areas of inquiry. For example, the multiple and complex functions of the urothelium is a rapidly progressing field in which ICC are clearly involved. The areas of painful bladder syndrome (PBS) and interstitial cystitis are also likely to reveal ICC participation. The work of Mukerji et al. (2006) correlated ICC and M2/M3 receptors to urgency scores in patients with PBS and idiopathic detrusor overactivity. Furthermore, we do not yet know the fate of ICC in diabetic or age-related lower urinary tract symptoms.
Further research should be directed to address the current gaps in our knowledge of bladder ICC with the full complement of techniques available at the level of the gene, protein, cell, tissue, organ, animal model, and translational research in patients. This area has attracted attention from many reputable research groups but remains largely unexploited. Given that present therapies for the treatment of urgency and many of the types of incontinence are effective in only a subset of patients, ICC may present novel opportunities for the development of better treatments.
4 Urethra
The study of urethral ICC is arguably more advanced than our knowledge of ICC in other tissues of the urinary tract, particularly in terms of cellular physiology. Smet et al. (1996) first demonstrated cells morphologically resembling ICC in the guinea-pig and human urethra using cGMP immunohistochemistry. A study of the electrical activity of rabbit urethral smooth muscle with intracellular microelectrode recordings demonstrated STDs which were reminiscent of gut slow waves, normally generated by ICC (Callahan and Creed, 1981; Hashitani et al. 1996). However, the first direct evidence that the urethra contained ICC-like cells was reported by Sergeant et al. (2000), who observed a mixed population of enzymatically dispersed cells from the rabbit urethra muscularis including majority spindle-shaped SMC and a smaller population of branched stellate-shaped cells and elongated cells with lateral branches, both of which were morphologically reminiscent of gut ICC.
4.1 Location and Morphology of ICC in the Urethral Wall
Sergeant et al. (2000) used vimentin immunohistochemistry to distinguish the branched cells from the SMC which contained myosin filaments but not vimentin. In addition, they demonstrated with TEM that the cell dispersal contained branched cells with the defining ultrastructural characteristics of ICC, i.e., abundant mitochondria, intermediate filaments, Golgi complexes, rough and smooth endoplasmic reticulum, caveolae, and a basal lamina. These ICC were clearly distinct from the SMC ultrastructural phenotype, adding support to the hypothesis that the urethra contained specialized ICC. The ICC identified in enzymatic cell dispersals were derived from the muscularis layers and this work was later advanced by Lyons et al. (2007) who carried out a morphological characterization of ICC in whole-mount preparations of rabbit urethra with confocal microscopy (see Fig. 4). Immunohistochemical labeling with anti-c-Kit and anti-vimentin showed that ICC were located within the circular and longitudinal layers of the muscularis and were arranged in parallel with the SMC. Moreover, reconstruction of optical sections demonstrated that ICC were in close proximity to the SMC, consistent with the idea that urethral ICC may have a pacemaker type role. The morphological profiles of the Kit-positive cells described by Lyons et al. (2007) were consistent with those found in the cell dispersals by Sergeant and clearly comprised several subtypes; unipolar, bipolar, stellate, and elongated with several lateral branches. Similar to bladder, several subpopulations of ICC have been reported in the urethral wall; lamina propria ICC, ICC in the muscularis, and ICC associated with the serosa (García-Pascual et al. 2008).
Kit-positive ICC in the rabbit urethra. ICC in rabbit urethra labeled with anti-c-Kit (green). Smooth muscle is labeled with antismooth muscle myosin (red). Urethral ICC are located on the both the edge of and between the smooth muscle bundles, running in parallel with the bundle orientation. Figure from Lyons et al. (2007)
Investigation of the relationships between intramural nerves and ICC in the urethra in double-labeling experiments with anti-c-Kit and antineurofilament (or anti-PGP 9.5) showed a close association between ICC and nerves within the muscularis layer. This suggested that the activity of ICC could be controlled by neurotransmitters released by adjacent urethral nerves perhaps providing a means of pacemaker regulation. More specifically, close relationships have been reported between urethral ICC and nitergic nerves labeled with antinitric oxide synthase (Lyons et al. 2007; García-Pascual et al. 2008).
4.2 Physiological Properties of Urethral ICC
Investigation of urethral ICC with patch-clamp electrophysiology and fluorescent Ca2+-imaging has established that these cells possess properties expected of pacemaker cells. The initial study of Sergeant et al. (2000) demonstrated that noncontractile cells morphologically resembling ICC fired STDs, larger slow waves and STICs. The ionic basis of the pacemaking conductance was a depolarizing Ca2+-activated Cl− current which had previously been found in sheep urethral SMC (Cotton et al. 1997; Sergeant et al. 2001) and which depolarized the RMP until l-type Ca2+-currents were activated, carrying the “upstroke” of the slow wave. The finding that in rabbit urethra, the pacemaking current was exclusively found in ICC and notably absent in SMC attributed a pacemaking function to rabbit urethral ICC. This work defined the earlier findings of Hashitani et al. (1996) at the cellular level who had demonstrated Cl−-dependent STDs in rabbit urethral tissue preparations.
The pacemaker current was found to be regulated by exogenously applied noradrenaline via α1 adrenergic receptors or ATP mediated by purinergic P2Y receptors, as demonstrated by an increase in the frequency of firing (Sergeant et al. 2002, 2009), consistent with the morphological studies which showed structural relationships between ICC and nerves. The source of Ca2+ to activate the current was shown to be largely via release from the IP3 sensitive intracellular Ca2+-stores (Sergeant et al. 2001). Rhythmic Ca2+-waves were initiated by ryanodine-mediated release of Ca2+ and wave propagation was controlled by the IP3-sensitive stores and also found to be highly sensitive to the external Ca2+ concentration (Johnston et al. 2005). These Ca2+ events are the primary signal which activates Ca2+-activated Cl− channels, leading to depolarization of the cell membrane and subsequent opening of l-type Ca2+-channels and slow wave firing. Interestingly, the frequency of spontaneous Ca2+-waves was increased by noradrenaline or ATP (Sergeant et al. 2009), as suggested by the finding of adrenergic and purinergic modulation of the pacemaker conductance. Further characterization of the pacemaking mechanism has revealed the role of the membrane Na+/Ca2+ exchanger in regulating the frequency of the pacemaker conductance (Bradley et al. 2006); moreover, mitochondrial buffering of [Ca2+]i has also been demonstrated to regulate urethral ICC Ca2+-signaling (Sergeant et al. 2008).
4.3 Functional Role of Urethral ICC
The work described above from isolated cells has been furthered by Ca2+-imaging experiments from whole-sheet preparations of rabbit urethra Hashitani and Suzuki (2007). Spontaneous Ca2+-signals were recorded from both ICC and SMC, which like the bladder were different in frequency and duration, with the ICC firing events at a mean rate of three per minute of longer duration than SMC events. While signals from neighboring ICC were often synchronized, there was little evidence of correlation with the SMC events. The hypothesis that urethral ICC act as pacemakers, regulating the activity of the smooth muscle was not necessarily supported; however, the authors speculated that the ICC could act as a “loose” pacemaker, providing multiple random depolarizing inputs to the smooth muscle to maintain activity at the optimum level to generate appropriate urethral tone.
The physiological characteristics of urethral ICC have been convincingly established by the McHale laboratory and clearly, these novel cells are among the most promising areas in the study of urethral function. However, much work needs to be done in order to consolidate any clinical implications of ICC in the urethra, not least the need to develop robust animal models in which the functional roles of urethral ICC can be deduced and the necessary translation of work from animal cells to human urethral ICC. The limited availability of “normal” human urethral tissue has undoubtedly been a major limiting factor; however, van der Aa et al. (2004) demonstrated kit-positive cells resembling ICC in unfixed, frozen sections of human urethra. Urethral smooth muscle tone is known to contribute to urinary continence and if this tone is indeed regulated by ICC activity, it remains to be shown whether aspects of LUTS are attributable to defects in either the quantities or function of urethral ICC.
5 Upper Urinary Tract: Renal Pelvis and Ureter
The upper urinary tract has long been known to display spontaneous peristaltic activity which propels urine from the kidneys to the bladder via the ureters. This activity is predominant in the renal pelvis and lessens along the tract to the distal ureter which is comparatively quiescent in the majority of mammalian species, with the exception of human and pig (Constantinou et al. 1978; Constantinou 1977). For several decades, it has been known that the pacemaker region, driving the spontaneous electrical and mechanical activity is located in a group of specialized cells, termed atypical smooth muscle cells (ASMC; Gosling and Dixon 1971, 1974). These cells are dominantly present in the proximal renal pelvis but absent in the ureter and are morphologically and ultrastructurally distinct from the SMC (Klemm et al. 1999). A defining feature of ASMC was the fact that 40% of their cellular sectional area contained contractile filaments, compared with the SMC which had more than 60%. ASMC possessed branched processes, formed a cellular network and were not immunopositive for c-Kit. The high-frequency pacemaking properties of the ASMC were elegantly demonstrated in intracellular microelectrode recordings in which the cell of interest was filled with neurobiotin so that its morphology could be visualized (Klemm et al. 1999).
5.1 Location and Morphology of ICC in the Upper Urinary Tract
Klemm et al. (1999) first described a new cell type in guinea-pig renal pelvis which was absent from ureter, morphologically resembling ICC. These cells differed from fibroblasts by the presence of numerous membrane-associated caveolae and an incomplete basal lamina. Like the ASMC, the ICC also had many processes and formed junctional contacts with similar cells or SMC, but differed from both ASMC and SMC by their distinct lack of contractile filaments. While the initial study in guinea-pig did not find the ICC to be immunopositive for c-kit, others have since reported c-Kit-positive ICC in upper urinary tract tissues, indicating that as in bladder and urethra, detection of c-Kit positivity depends on the use of a panel of c-Kit antibodies and perhaps interspecies differences. Kit-positive ICC have been reported to be located between the smooth muscle bundles in uteropelvic junction samples (Solari et al. 2003; Lang and Klemm 2005) and in lamina propria and muscle layers in ureter from mouse (Pezzone et al. 2003; David et al. 2005), human (Metzger et al. 2004) and other mammalian species (Metzger et al. 2005, 2008). Typically, the presence of ICC is reported to decrease from proximal to distal segments.
5.2 Physiological Properties of Upper Urinary Tract ICC
Neurobiotin experiments, similar to those described above, revealed that the ICC cell population fired intermediate-type action potentials (Klemm et al. 1999) characterized by a single spike, quiescent plateau phase, and abrupt repolarization. Interestingly, ICC were found in distal regions of renal pelvis where pacemaker type activity was absent, leading the authors to speculate that ICC in the upper urinary tract were not primary pacemakers but could form a conduit for transmission of signals from the ASMC pacemakers to the SMC. Further study of the three cell types in the mouse renal pelvis examined the electrical and Ca2+-signaling basis of their spontaneous activity. Again, the ASMC were found to be the main pacemakers, whereas the ICC had more of a supportive role, firing less frequent Ca2+-transients, and long duration action potentials (Lang et al. 2007a and b). Spontaneous Ca2+ signals in ICC were sensitive to blockade of Ca2+ release from IP3 or ryanodine-dependent intracellular stores (Lang et al. 2010). Study of W/Wv transgenic mice unfortunately showed no change in ICC Ca2+-signaling (Lang et al. 2009), suggesting that the renal pelvis, like the urinary bladder, is not significantly affected by the c-Kit mutation (McCloskey et al. 2009).
Lang et al. (2007b) studied isolated ICC from mouse UPJ under voltage-clamp and found high-frequency STICs and long-lasting large inward currents (LICs). These currents were relatively insensitive to Cl− channel blockers and were considered to represent cationic-selective currents. This direct evidence of depolarizing spontaneous electrical activity in the ICC suggested that these cells could perhaps provide a type of pacemaking or modulatory input to adjacent smooth muscle bundles, especially if activity from ASMC was not present.
5.3 Functional Implications of ICC in the Upper Urinary Tract
As seems to be the case for bladder, the putative role of upper urinary tract ICC has been revealed in studies outside of the normal adult physiological situation. Solari et al. (2003) noted a decrease in the density of Kit-positive ICC in obstructed human UPJ specimens. Incubation of murine ureter tissues with neutralizing Kit antibodies under tissue culture conditions not only altered ureter morphology but also disrupted peristalsis leading the authors to suggest that Kit was required for the spontaneous activity (David et al. 2005). Consistent with this, the same study investigated the embryonic development of ureter ICC and contractility and reported a correlation between the ability of isolated ureter preparations to exhibit unidirectional contractions and an upregulation of c-Kit expression. To date, the literature supports pacemaking in the upper urinary tract coming primarily from ASMC but also with a convincing, although less dominant input from ICC.
6 Future Perspectives for Urinary Tract ICC
The last decade has seen significant advances in our knowledge of the complexity of cells present in the tissues of the urinary tract and their morphological and physiological characteristics. Cells with properties typical of ICC are expressed in renal pelvis, ureter, urinary bladder, and the outlet urethra in many species, including human. What has become clear is that these cells have a unique arrangement in urinary tract tissues compared with the gastrointestinal tract. The extensive and dense networks of ICC typical of the pacemaking myenteric plexus regions of the GI tract have not yet been shown in any of the urinary tract tissues. Whether this is a true reflection of the actual arrangement of ICC or represents limitations in currently available detection methods remains to be seen. However, the comparatively less-dense networks of ICC in the lamina propria regions of the tissues are clearly distinct from the ICC present in the muscularis regions, which do not appear to be widely networked but are placed along the boundary of smooth muscle bundles. Another common finding in the urinary tract is that the ICC do not appear to be the main pacemaker or “driver” for the adjacent smooth muscle. This is perhaps most evident in the renal pelvis and ureter where the ASMC population have already been directly demonstrated to be the primary pacemakers. In addition, in the bladder and urethra, the smooth muscle activity appears to be myogenic in origin, with the ICC perhaps providing positive or negative signals to regulate the activity.
For those who have perhaps lost faith in the urinary tract ICC having clearly defined physiological roles, the answer may lie in the study of pathological conditions. There is good agreement, for example, that numbers of ICC and spontaneous activity are increased in obstructed bladder, and the Kit-blocking drug Glivec appears to have a more convincing effect in those tissues compared with normal controls. Similar findings in spinal cord injury bladders add support to these findings. The studies of patient samples with megacystic-microcolon intestinal hypoperistalsis are especially relevant as the dysfunctional, distended, and unobstructed bladders were correlated with a clear lack of ICC. Future work should be directed to the study of ICC in patient samples with clinically defined lower urinary tract symptoms with the specific aim of assessing the presence of ICC and their activity in each condition. Furthermore, work on animal models will perhaps enable the field to progress more rapidly providing the opportunity to correlate urinary tract activity with the absence, presence, or increased numbers of ICC.
While there are many unknowns relating to urinary tract ICC and the field lags behind knowledge of gut ICC, the fact that urinary tract ICC persist under normal adult physiological conditions strongly suggests that they are important players in the concert type of activity from all the cells present within these complex tissues. Their roles may not be dominant in primary pacemaking as initially anticipated, yet they are unlikely to be maintained as uninvolved bystanders. To use an orchestral analogy, which is the most important instrument (or cell)? ICC may not be the applauded classical solo instruments such as violin, clarinet or flute, but even the percussionist’s humble triangle has its own part to play in the most wonderful of concertos. I have no doubt that the roles of ICC in the normal and pathophysiological urinary tract will ultimately be discovered and may even surprise us.
References
Anderson UA, Carson C, McCloskey KD (2009) KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol 182:330–336
Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB (1999) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162:1768–1778
Biers SM, Reynard JM, Doore T, Brading AF (2006) The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97(3):612–616
Brading AF (1999) The physiology of the mammalian urinary outflow tract. Exp Physiol 84(1):215–221
Brading AF, McCloskey KD (2005) Mechanisms of disease: specialized interstitial cells of the urinary tract-an assessment of current knowledge. Nat Clin Pract Urol 2:546–554
Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, McHale NG, Thornbury KD, Sergeant GP (2006) Contribution of reverse Na+-Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol 574:651–661
Cajal SR (1911) Histologie du système nerveux de l'homme et des vertébrés, vol 2. Maloine, Paris, pp 891–942
Callahan SM, Creed KE (1981) Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol 74:353–358
Constantinou CE, Silvert MA, Gosling J (1977) Pacemaker system in the control of ureteral peristaltic rate in the multicalyceal kidney of the pig. Invest Urol 14:440–441
Constantinou CE, Neubarth JL, Mensah-Dwumah M (1978) Frequency gradient in the autorhythmicity of the pyeloureteral pacemaker system. Experientia 34:614–615
Cotton KD, Hollywood MA, McHale NG, Thornbury KD (1997) Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. J Physiol 505:121–131
Cunningham RMJ, Larkin P, McCloskey KD (2011) Ultrastructural Properties of Interstitial Cells of Cajal in the Guinea Pig Bladder. J Urol (in press)
David SG, Cebrian C, Vaughan ED Jr, Herzlinger D (2005) c-kit and ureteral peristalsis. J Urol 173:292–295
Davidson RA, McCloskey KD (2005) Morphology and localization of interstitial cells in the guinea-pig bladder: structural relationships with smooth muscle and neurons. J Urol 173:1385–1390
Everaerts W, Sepúlveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D (2009) Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379:421–425
García-Pascual A, Sancho M, Costa G, Triguero D (2008) Interstitial cells of Cajal in the urethra are cGMP-mediated targets of nitrergic neurotransmission. Am J Physiol Renal Physiol 295:F971–F983
Gillespie JI, Markerink-van Ittersum M, de Vente J (2004) cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int 94(7):1114–1124
Gosling JA, Dixon JS (1971) Morphologic evidence that rhe renal calyx and pelvis control ureteric activity in the rabbit. Am J Anat 130:393–408
Gosling JA, Dixon JS (1974) Species variation in the location of upper urinary tract pacemaker cells. Invest Urol 11:418–423
Grol S, Essers PB, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI (2009) M(3) muscarinic receptor expression on suburothelial interstitial cells. BJU Int 104:398–405
Hashitani H, Suzuki H (2007) Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J Physiol 583:505–519
Hashitani H, Van Helden DF, Suzuki H (1996) Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol 118:1627–1632
Hashitani H, Yanai Y, Suzuki H (2004) Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol 559:567–581
Horowitz B, Ward SM, Sanders KM (1999) Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61:19–43
Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A (2007) Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293:F1018–F1025
Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG (2005) Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol 565:449–461
Johnston L, Carson C, Lyons AD, Davidson RA, McCloskey KD (2008) Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am J Physiol Renal Physiol 294:F645–F655
Johnston L, Woolsey S, O’Kane H, Duggan B, Keane P, McCloskey KD (2010) Expression of kit-positive interstitial cells of cajal in human bladder. J Urol 184:370–377
de Jongh R, van Koeveringe GA, van Kerrebroeck PE, Markerink-van Ittersum M, de Vente J, Gillespie JI (2007) The effects of exogenous prostaglandins and the identification of constitutive cyclooxygenase I and II immunoreactivity in the normal guinea pig bladder. BJU Int 100:419–429
de Jongh R, Grol S, van Koeveringe G, van Kerrebroeck P, de Vente J, Gillespie J (2009) The localisation of cyclo-oxygenase immuno-reactivity (COX I-IR) to the urothelium and to interstitial cells in the bladder wall. J Cell Mol Med 13:3069–3081
Klemm MF, Exintaris B, Lang RJ (1999) Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. J Physiol 519:867–884
Komuro T (1999) Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech 47(4):267–285
Komuro T, Seki K, Horiguchi K (1999) Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62:295–316
Kubota Y, Kajioka S, Biers SM, Yokota E, Kohri K, Brading AF (2004) Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci 30(115):64–73
Kubota Y, Biers SM, Kohri K, Brading AF (2006) Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn 25:205–210
Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K (2008) Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn 27:330–340
Lang RJ, Exintaris B, Teele ME, Harvey J, Klemm MF (1998) Electrical basis of peristalsis in the mammalian upper urinary tract. Clin Exp Pharmacol Physiol 25(5):310–321
Lang RJ, Klemm MF (2005) Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med 9:543–556
Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H (2007a) Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol 583:1049–1068
Lang RJ, Zoltkowski BZ, Hammer JM, Meeker WF, Wendt I (2007b) Electrical characterization of interstitial cells of Cajal-like cells and smooth muscle cells isolated from the mouse ureteropelvic junction. J Urol 177:1573–1580
Lang RJ, Hashitani H, Tonta MA, Bourke JL, Parkington HC, Suzuki H (2010) Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol 37:509–515
Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM (2007) Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol 579:487–501
Lyons AD, Gardiner TA, McCloskey KD (2007) Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int 99:687–694
Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S (1992) Requirement of c-kit for development of intestinal pacemaker system. Development 116:369–375
McCloskey KD (2005) Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol 173:296–301
McCloskey KD (2006) Calcium currents in interstitial cells from the guinea-pig bladder. BJU Int 97:1338–1343
McCloskey KD, Gurney AM (2002) Kit-positive cells in the guinea pig bladder. J Urol 168:832–836
McCloskey KD, Anderson UA, Davidson RA, Bayguinov YR, Sanders KM, Ward SM (2009) Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol 156(2):273–283
Metzger R, Schuster T, Till H, Stehr M, Franke FE, Dietz HG (2004) Cajal-like cells in the human upper urinary tract. J Urol 172:769–772
Metzger R, Schuster T, Till H, Franke FE, Dietz HG (2005) Cajal-like cells in the upper urinary tract: comparative study in various species. Pediatr Surg Int 21:169–174
Metzger R, Neugebauer A, Rolle U, Böhlig L, Till H (2008) c-Kit receptor (CD117) in the porcine urinary tract. Pediatr Surg Int 24:67–76
Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P (2006) Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol 176:367–373
Ost D, Roskams T, Van Der Aa F, De Ridder D (2002) Topography of the vanilloid receptor in the human bladder: more than just the nerve fibers. J Urol 168:293–297
Pezzone MA, Watkins SC, Alber SM, King WE, de Groat WC, Chancellor MB, Fraser MO (2003) Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol 284(5):F925–F929
Piaseczna Piotrowska A, Rolle U, Solari V, Puri P (2004) Interstitial cells of Cajal in the human normal urinary bladder and in the bladder of patients with megacystis-microcolon intestinal hypoperistalsis syndrome. BJU Int 94:143–146
Rasmussen H, Rumessen JJ, Hansen A, Smedts F, Horn T (2009) Ultrastructure of Cajal-like interstitial cells in the human detrusor. Cell Tissue Res 335:517–527
Roosen A, Datta SN, Chowdhury RA, Patel PM, Kalsi V, Elneil S, Dasgupta P, Kessler TM, Khan S, Panicker J, Fry CH, Brandner S, Fowler CJ, Apostolidis A (2009) Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin Type A treatment. Eur Urol 55:1440–1448
Sanders KM (1996) A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111:492–515
Sanders KM, Koh SD, Ward SM (2006) Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol 68:307–343
Sanders KM, Ordög T, Ward SM (2002) Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 282(5):G747–G756
Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM (1999) Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil 5:311–338
Sanders KM, Ward SM (2007) Kit mutants and gastrointestinal physiology. J Physiol 578:33–42
Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG (2000) Specialized pacemaking cells in the rabbit urethra. J Physiol 526:359–366
Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD (2001) Role of IP(3) in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol 280:C1349–C1356
Sergeant GP, Thornbury KD, McHale NG, Hollywood MA (2002) Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol 283:C885–C894
Sergeant GP, Bradley E, Thornbury KD, McHale NG, Hollywood MA (2008) Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol 586:4631–4642
Sergeant GP, Bradley E, Drumm B, Hollywood MA, McHale NG, Thornbury KD (2009) Regulation of spontaneous activity in interstitial cells of Cajal of the rabbit urethra by ATP. Proc Physiol Soc 15:C139
Shafik A, El-Sibai O, Shafik AA, Shafik I (2004) Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker. Urology 64:809–813
Smet PJ, Jonavicius J, Marshall VR, de Vente J (1996) Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71:337–348
Solari V, Piotrowska AP, Puri P (2003) Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol 170:2420–2422
Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ (2002) Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90:118–129
Sui GP, Wu C, Fry CH (2004) Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol 171:938–943
Sui GP, Wu C, Fry CH (2006) Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97:1327–1331
Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH (2008) Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol 295:F688–F697
Turner WH, Brading AF (1997) Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther 75:77–110
Wu C, Sui GP, Fry CH (2004) Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559:231–243
Wiseman OJ, Fowler CJ, Landon DN (2003) The role of the human bladder lamina propria myofibroblast. BJU Int 91:89–93
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
McCloskey, K.D. (2011). Interstitial Cells of Cajal in the Urinary Tract. In: Andersson, KE., Michel, M. (eds) Urinary Tract. Handbook of Experimental Pharmacology, vol 2011. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-16499-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-16499-6_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-16498-9
Online ISBN: 978-3-642-16499-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)