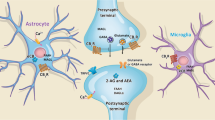
Abstract
Cannabinoid receptors and their endogenous ligands are located throughout the limbic, or “emotional,” brain, where they modulate synaptic neurotransmission. Converging preclinical and clinical data suggest a role for endogenous cannabinoid signaling in the modulation of anxiety and depression. Augmentation of endocannabinoid signaling (ECS) has anxiolytic effects, whereas blockade or genetic deletion of CB1 receptors has anxiogenic properties. Augmentation of ECS also appears to have anti-depressant actions, and in some assays blockade and genetic deletion of CB1 receptors produces depressive phenotypes. These data provide evidence that ECS serves in an anxiolytic, and possibly anti-depressant, role. These data suggest novel approaches to treatment of affective disorders which could include enhancement of endogenous cannabinoid signaling, and warrant cautious use of CB1 receptor antagonists in patients with pre-existing affective disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Human Studies Suggesting a Role for Endocannabinoid Signaling in Anxiety
Cannabis has been used for centuries for a variety of recreational and medicinal purposes. The primary psychoactive chemical in cannabis, Δ9-tetrahydrocannabinol (THC), is a partial agonist of the CB1 cannabinoid receptor (Breivogel et al. 1998). The most commonly cited reasons for continued recreational cannabis use are relaxation and reduction in tension (Reilly et al. 1998; Schofield et al. 2006; Thomas 1993). Paradoxically, the most commonly cited reasons for discontinuation of cannabis use are increased anxiety and panic reactions (Reilly et al. 1998; Szuster et al. 1988). Modulation of anxiety reactions by cannabis appears to be complex in that both dose and environmental context can modulate these effects. Subjects under “experimenter harassment” were more likely to experience anxiety reactions under the influence of cannabis than those in neutral environments (Gregg et al. 1976). Since the subjective effects of cannabis are mediated via the CB1 receptor (Huestis et al. 2001), these data suggest a role for endocannabinoid signaling (ECS) in the regulation of anxiety.
A CB1 receptor antagonist, rimonabant (also named Acomplia, SR141716 and SR141716A) has been developed and used in humans for the treatment of obesity, diabetes and dyslipidemia (Van Gaal et al. 2008). Psychiatric adverse effects, including anxiety, were cited as reasons for discontinuation by patients taking rimonabant significantly more than those taking placebo (Van Gaal et al. 2008), although objective measures of anxiety were not significantly increased in patients taking rimonabant (Scheen et al. 2006). A recent meta-analysis pooling data from four large clinical trials indicated that subjects taking rimonabant had a significantly greater increase in anxiety symptoms while taking the drug than patients taking placebo (Christensen et al. 2007). Therefore, human experience with a cannabinoid receptor agonist (THC) and antagonist (rimonabant) support the hypothesis that ECS regulates anxiety in humans and suggest that activation of the CB1 receptor by endocannabinoids could produce anxiolytic effects.
Support for an inverse relationship between ECS and anxiety in humans also comes from a recent study of serum endocannabinoids in women with depression (Hill et al. 2008). In this study, the severity of anxiety experienced by women with major depression was inversely correlated with serum content of N-arachidonylethanolamine (AEA). Although very little is known about the source or potential target of circulating endocannabinoids, these data suggest that some of the somatic manifestations of anxiety could be related to reduced ECS.
2 Animal Studies Indicating a Role for ECS in Anxiety
2.1 Effects of CB1 Receptor Blockade and Genetic Deletion on Unconditioned Anxiety Behaviors
A commonly used and well-validated test of unconditioned anxiety in rodents is the elevated plus-maze. This is an exploration-based test that utilizes the innate fear of open spaces exhibited by rodents. The maze measures the proportion of time rodents spend in well-lit “open” arms, compared to darker “closed” arms. A drug-induced increase in the proportion of time spent in the open arms is suggestive of an anxiolytic effect, whereas an increase in time spent in closed arms is suggestive of an anxiogenic effect. An anxiogenic effect of rimonabant has been demonstrated using an elevated plus-maze test in rats (Navarro et al. 1997) and mice (Arevalo et al. 2001; Patel and Hillard 2006). A second CB1 receptor antagonist, AM251, a structural analog of rimonabant, also shows anxiogenic effects in rodents in the elevated plus-maze (Haller et al. 2004b; Patel and Hillard 2006). Rimonabant exhibits an anxiogenic profile in the defensive-withdrawal (Navarro et al. 1997) and ultrasonic vocalization tests (McGregor et al. 1996) as well.
In contrast to these findings, other studies have demonstrated either no effect (Bortolato et al. 2006; Kathuria et al. 2003) or an anxiolytic effect of rimonabant (Degroot and Nomikos 2004; Griebel et al. 2005; Rodgers et al. 2003). In the studies in which no effect was seen, relatively low doses of the antagonists were used (Bortolato et al. 2006; Kathuria et al. 2003). Dose-dependent anxiolytic effects of rimonabant were seen in the elevated plus-maze and Vogel conflict test in mice (Griebel et al. 2005). Furthermore, using a design in which rodents were tested twice, rimonabant had no effect in the elevated plus-maze during the first trial, but produced an anxiolytic effect during the second exposure (Rodgers et al. 2003). Interestingly, rimonabant produced anxiolytic effects in CB1 receptor knockout (KO) mice, leading Haller et al. to suggest its anxiolytic actions are mediated via non-CB1-dependent mechanisms (Haller et al. 2002). These authors did not observe anxiogenic effects of AM251 in CB1 receptor KO mice, and concluded that AM251 does not share the non-receptor effect of rimonabant (Haller et al. 2004a). Anxiolytic effects of rimonabant have also been demonstrated in the shock-probe burying test, although this effect could be due to the effect of the drug to enhanced memory function, rather than direct effects of unconditioned anxiety per se (Degroot and Nomikos 2004).
Administration of rimonabant results in activation of brain regions involved in the generation of fear and anxiety. Systemic administration of rimonabant increased Fos expression, a marker of neuronal activity, within the central amygdala, bed nucleus of the stria terminalis, hypothalamus and brainstem (Alonso et al. 1999; Patel et al. 2005b; Rodriguez de Fonseca et al. 1997). These studies further support the hypothesis that ECS is an endogenous anxiolytic system that dampens neuronal activity within brain regions critical for the generation of fear and anxiety responses.
CB1 receptor KO mice exhibit increased anxiety-like behaviors in the elevated plus-maze (Haller et al. 2002, 2004a, b), and in the light–dark exploration model in young mice only (Maccarrone et al. 2002). Interestingly, these effects appear to be more prominent under environmentally stressful conditions (Haller et al. 2004a; Maccarrone et al. 2002). In particular, in a high light condition, which is considered stressful since rodents are nocturnal and have impaired vision under this condition, CB1 receptor KO mice exhibit an anxiogenic phenotype; while under low light conditions, this phenotypic difference is absent (Haller et al. 2004a). This finding may explain why some studies have failed to detect an anxiogenic phenotype in CB1 receptor KO mice (Marsicano et al. 2002). In addition to direct anxiogenic behaviors, CB1 receptor KO mice display impaired behavioral responses to non-cannabinoid anxiolytics including benzodiazepines and buspirone (Uriguen et al. 2004).
2.2 Effects of Pharmacological and Genetic Augmentation of ECS on Unconditioned Anxiety Behaviors
ECS occurs when synaptic concentrations of the endocannabinoids AEA and/or 2-arachidonoylglycerol (2AG) are increased through either increased synthesis or decreased catabolism. In particular, fatty acid amide hydrolase (FAAH) is a well-characterized enzyme that hydrolyzes and inactivates AEA and other N-acylethanolamines (Ho and Hillard 2005). Pharmacologic inhibition or genetic deletion results in significant increases in brain AEA but not 2AG content (Cravatt et al. 1996; Kathuria et al. 2003; Patel et al. 2005a). Systemic administration of a highly efficacious inhibitor of FAAH, URB597, produced anxiolytic effects in the elevated zero-maze (a slight modification of the elevated plus-maze described above) and in the ultrasonic vocalization test in rats (Kathuria et al. 2003). This effect was accompanied by an increase in brain AEA concentrations and blocked by the CB1 receptor antagonist rimonabant (Kathuria et al. 2003). These data suggest that increased CB1 receptor signaling by AEA produces anxiolytic behavioral effects that can be enhanced by pharmacological blockade of FAAH. This effect of URB597 has been replicated in mice using the elevated plus-maze (Moreira et al. 2008; Patel and Hillard 2006) and in rats using the light–dark box test (Scherma et al. 2008). FAAH KO mice also exhibit an anxiolytic phenotype in the elevated plus-maze and light–dark box test (Moreira et al. 2008; Naidu et al. 2007); effects that are blocked by pretreatment with rimonabant (Moreira et al. 2008). Taken together, these data support the hypothesis that the ECS in rodents provides an anxiolytic tone that can be enhanced if AEA-mediated signaling is increased. The role of 2AG in this system is not known.
These findings are consistent with data showing that exogenous administration of low doses of direct-acting CB1 receptor agonists also produce anxiolytic effects in rodents (Patel and Hillard 2006; Scherma et al. 2008). However, unlike direct CB1 receptor agonists that display anxiogenic effects at higher doses, FAAH inhibitors exhibit only dose-dependent anxiolytic effects without anxiogenic effects at high doses (Kathuria et al. 2003; Patel and Hillard 2006). These data suggest that the spatio-temporal properties of ECS are maintained by FAAH inhibition, in contrast to global CB1 activation by direct agonists, and that this property of FAAH inhibitors subserves their uniphasic, anxiolytic properties. In other words, global CB1 receptor activation can result in both decreased and increased anxiety, but the evidence using both inhibition of FAAH and CB1 receptor antagonism indicate that the anxiogenic “pool” of CB1 receptors is not endogenously active. We suggested earlier that the functional pools are anatomically distinct (Patel and Hillard 2006), a suggestion that is supported by a recent study using region-selective, virally mediated up-regulation of FAAH. Parolaro and co-workers showed that increasing FAAH expression within the prefrontal cortex (PFC) caused a reduction in AEA concentrations and an increase in anxiety behaviors in the elevated plus-maze (Rubino et al. 2008b). These data confirm a role for ECS in the regulation of anxiety behaviors and suggest that the anatomical site of this ECS function includes the PFC.
However, another explanation for the difference in the effects on anxiety between FAAH inhibition and direct CB1 receptor agonists is that the inhibition of FAAH increases levels of non-cannabinoid, fatty acid ethanolamides (NAEs) as well as AEA (Cravatt et al. 2001). Since the anxiolytic effects of FAAH inhibitors can be blocked by CB1 receptor antagonists (Kathuria et al. 2003; Moreira et al. 2008), it can be concluded that CB1 receptor activation is required for the anti-anxiety efficacy of FAAH inhibition. However, these data do not address the question of whether other NAEs contribute to the efficacy as well. In other words, it is not known whether CB1 receptor activation is sufficient for the anxiolytic efficacy of FAAH inhibition.
In addition to inhibition of FAAH, inhibitors of endocannabinoid transport have also demonstrated anxiolytic properties. AM404 is an arachidonic acid analog that inhibits uptake of both AEA (Beltramo et al. 1997) and 2AG (Beltramo and Piomelli 2000), inhibits FAAH activity (Jarrahian et al. 2000), and increases brain AEA concentrations (Bortolato et al. 2006). Several studies have demonstrated that systemic administration of AM404 produces anxiolytic effects in the elevated plus-maze, defensive withdrawal test, and social isolation test (Bortolato et al. 2006; Patel and Hillard 2006). These effects are blocked by the CB1 receptor antagonist rimonabant, consistent with the hypothesis that indirect activation of the ECS can produce anxiolytic effects (Bortolato et al. 2006). However, in another study in which drugs were administered into the periaqueductal gray of rats, AEA produced anxiolytic effects that were enhanced by AM404, but alone AM404 was not anxiolytic (Moreira et al. 2007).
2.3 Effects of CB1 Receptor Deletion and Pharmacological Blockade on Conditioned Anxiety Behaviors
Conditioned, or “learned,” fear is a model for certain types of anxiety disorders including post-traumatic stress disorder (PTSD). In this paradigm, a temporal contingency is established between environmental cues such as an auditory tone or a specific environmental context, i.e., “cage type,” and an aversive stimulus such as an electric shock. After single or repeated “paired” presentations of these two stimuli, the environmental cues presented alone can elicit an innate, conditioned fear response such as freezing, and signs of sympathetic nervous system activation. After “conditioned” fear responses to cue presentation are established, presentation of environmental cues in the absence of the aversive stimulus causes a gradual extinction of conditioned fear responses.
Two different conditioning paradigms, context and tone, have been used to examine the role of ECS in the acquisition of conditioned fear responses. Several studies have shown no effect of either CB1 receptor genetic deletion or pharmacological blockade on the acquisition of contextual or tonal fear conditioning (Marsicano et al. 2002; Suzuki et al. 2004). However, a recent study utilizing a multiple-trial acquisition model found enhanced acquisition of conditioned fear responses in trace and delayed fear conditioning paradigms, which are hippocampus- and amygdala-dependent, respectively (Reich et al. 2008). These data suggest that ECS could impair acquisition of conditioned anxiety responses under specific conditions.
It has been conclusively demonstrated that both pharmacological and genetic inhibition of CB1 receptors impair the extinction of both contextual and tonal conditioned anxiety responses (Kamprath et al. 2006; Marsicano et al. 2002; Reich et al. 2008; Suzuki et al. 2004). Impaired extinction of aversive associative learning has also been demonstrated using fear-potentiated startle and passive avoidance protocols (Chhatwal et al. 2005), but not an appetitively motivated instrumental responding paradigm (Niyuhire et al. 2007).
Data from a novel paradigm that attempts to separate the associative and non-associative components of conditioned fear responses suggest that impairments in extinction observed in CB1 receptor KO mice are due to deficits in habituation, the non-associative component of extinction (Kamprath et al. 2006). In this paradigm, presentation of the tone stimulus used in fear conditioning paradigms (preceded by a sensitizing shock) results in freezing behavior that habituates over repeated presentations; this represents a non-associative component of extinction of conditioned fear behavior. Mice lacking CB1 receptors do not show habituation of these innate fear responses after repeated tone presentation. These authors suggest that the impairments in extinction of conditioned fear behavior observed in CB1 receptor KO mice and after CB1 receptor blockade are a result of an impaired “habituation component” of the extinction process (Kamprath et al. 2006). This suggestion is consistent with a growing body of literature supporting a role of the ECS in habituation of the behavioral and endocrine responses to stress (Patel and Hillard 2008).
2.4 Effects of ECS Augmentation on Conditioned Anxiety Behaviors
Similarly to unconditioned anxiety, insight into the role of ECS in conditioned anxiety comes from studies in which CB1 receptor signaling is activated using low doses of agonists. For example, the CB1 receptor agonist WIN55212-2 impairs acquisition of context-, but not tone-, conditioned anxiety responses (Pamplona and Takahashi 2006) and low doses of WIN55212-2 facilitate extinction of conditioned anxiety responses in a contextual fear-conditioning paradigm (Pamplona et al. 2006). Similarly, the indirect agonist, AM404, impairs extinction of fear-potentiated startle responses (Chhatwal et al. 2005), and FAAH KO mice exhibit enhanced extinction of an aversively motivated, spatial memory task (Varvel et al. 2007). This appears selective for aversively motivated over appetitively motivated learning (Holter et al. 2005).
Taken together, data in animal models of unconditioned and conditioned anxiety support the hypothesis that activation or enhancement of ECS can produce a reduction in anxiety in rodents. This function of the ECS appears to be tonically “on” or easily activated since treatment of rodents in mildly aversive environments with CB1 receptor antagonists enhances anxiety behaviors. It is likely that changes in CB1 receptor activation can regulate anxiety in multiple brain regions and through multiple mechanisms (discussed further below). High doses of direct CB1 receptor agonists can be anxiogenic, which parallels the human experience in which cannabis use can be both anxiolytic and anxiogenic. However, the lack of anxiogenic effects by FAAH inhibitors and the nearly consistent finding that CB1 receptor blockade is monophasically anxiogenic support the hypothesis that the predominant effect of endogenous CB1 receptor activation is a reduction in anxiety.
3 Neural Mechanisms Underlying Endocannabinoid Modulation of Anxiety
The neural mechanisms by which ECS affects anxiety are not well understood, yet several mechanisms at the systems, synaptic, and molecular level can be posited based on available data. The majority of available data indicate that ECS has anxiolytic properties in both conditioned and unconditioned anxiety models, and that these effects are more active during states of stress or high arousal (Haller et al. 2004a). The anxiolytic effects of ECS are mimicked by low doses of direct CB1 receptor agonists (Patel and Hillard 2006); thus data exploiting this phenomenon can be used to increased our understanding of the neural mechanisms subserving the anxiolytic actions of the ECS system.
At the systems level, microinjections of low doses of the direct CB1 agonist THC into the PFC (Rubino et al. 2008a), ventral hippocampus (Rubino et al. 2008a), and dorsal periaqueductal gray area (Moreira et al. 2007) exert anxiolytic effects in the elevated plus-maze. These effects are blocked by the CB1 receptor antagonist AM251 (Moreira et al. 2007; Rubino et al. 2008b). Pharmacological inhibition of FAAH within the PFC produces CB1-receptor-dependent anxiolytic effects, and over-expression of FAAH (which reduces local AEA levels) causes an anxiogenic effect in the elevated plus-maze (Rubino et al. 2008b). In contrast to the PFC and hippocampus, very low doses of THC produce only anxiogenic effects when administered into the basolateral amygdala (BLA); this was also dependent upon CB1 receptor activation (Rubino et al. 2008a). These data suggest that the PFC and hippocampus are likely anatomical sites of action that subserve the anxiolytic effects of ECS. More specifically, the balance of ECS in favor of an increase in the PFC and/or hippocampus and reduced signaling in the amygdala could be required for maximal anxiolytic effects.
With regard to endocannabinoid facilitation of extinction of conditioned fear responses, direct administration of CB1 agonists into the lateral amygdala impairs fear memories by blocking reconsolidation in a fear-potentiated startle model (Lin et al. 2006). These data suggest that ECS in the amygdala during presentation of conditioned cues impairs reconsolidation of fear memories, and thus facilitates extinction of conditioned fear responses. Thus, in contrast to unconditioned anxiety responses (which are enhanced by CB1 receptor activation in the amygdala), impairments in conditioned anxiety responses are observed after amygdalar CB1 receptor activation. These data suggest a complex and potentially divergent role for amygdalar ECS in the modulation of conditioned vs. unconditioned anxiety behaviors.
At the synaptic level, activation of CB1 receptors inhibits glutamatergic inputs to principal neurons in the cortex, hippocampus and BLA (Hashimotodani et al. 2007). In addition, CB1 receptor activation inhibits GABA release from a subpopulation of cholecystokinin (CCK)-expressing interneurons that form perisomatic (and some dendritic) contacts with hippocampal principal neurons; however, this effect is only operative when the firing rates of these interneurons is low (Foldy et al. 2007). Haller and co-workers suggest that the anxiolytic effects of ECS are mediated via inhibition of GABAergic transmission within the hippocampus (Haller et al. 2007). This suggestion is based on data demonstrating an anxiolytic effect of WIN55212-2 in CD-1 mice, in which this compound was significantly more efficacious at inhibiting hippocampal GABAergic than glutamatergic transmission. By contrast, WIN55212-2 produced an anxiogenic effect and affected GABAergic and glutamatergic transmission equally in rats. In addition, AM251 blocked the anxiogenic effect of WIN55212-2 in mice, and blocked the effect of this compound on GABAergic transmission, but not glutamatergic transmission. These data led the authors to conclude that WIN55212-2 produced anxiolytic effects via inhibition of GABAergic transmission within the hippocampus. These pharmacologic studies led to the further suggestion that the anxiogenic effect of WIN55212-2 in rats is mediated by inhibition of glutamatergic transmission. These data provide an interesting hypothesis that requires further experimental evidence; particularly important will be studies using mouse models in which CB1 receptors on either glutamatergic or GABAergic terminals have been selectively abolished (Monory et al. 2006).
A synaptic mechanism subserving endocannabinoid facilitation of extinction of conditioned fear responses has also been proposed (Lafenetre et al. 2007). These authors incorporate the ability of endocannabinoids to modulate both GABAergic and glutamatergic transmission within the amygdala in their model. They suggest that under basal conditions ECS is not active in the amygdala; a conclusion that is supported by c-Fos studies from our laboratory (Patel et al. 2005b). After tonal fear conditioning, presentation of the tone alone increases ECS in the BLA, which has been demonstrated experimentally (Marsicano et al. 2002). This increase in ECS inhibits GABAergic transmission, which results in dis-inhibition of BLA projection neurons and facilitation of a “no fear” pathway mediated by activation of inhibitory neurons within intercalated cell masses. These neurons provide feed-forward inhibition onto central amygdala neurons, which are output neurons of the amygdala and activate conditioned behavioral and physiological responses. These authors also suggest that ECS signaling could decrease glutamatergic transmission in a “fear” pathway that transmits directly from the BLA to the central amygdala. Such depotentiation of the conditioned “fear” pathway could represent a synaptic mechanism for the habituation component of extinction of conditioned fear. The mechanisms that would segregate ECS into GABAergic and glutamatergic signaling in the “no fear” and “fear” pathways, respectively, remain to be determined.
Although the above data provide anatomical and synaptic insights into the mechanisms subserving the anxiolytic effects of ECS, they do not alone explain the context-dependent effects. Specifically, the anxiogenic effects of CB1 receptor deletions or blockade are more robust under stress or high arousal (Haller et al. 2004a), suggesting increased ECS counteracts the anxiety produced by environmental stress. These observations suggest that exposure to the fear-evoking or stressful context results in an increase in endocannabinoid release. A potential explanation could involve the neuropeptide CCK, which is expressed by CB1-receptor-positive, GABAergic interneurons. CCK is released under times of stress and high arousal (Nevo et al. 1996), and activation of CCK2 receptors appears to result in endocannabinoid release from hippocampal principal neurons, based on the effects of AM251 (Foldy et al. 2007). These endocannabinoids can then activate receptors on GABAergic interneurons to produce anxiolytic effects as suggested above. This hypothesis remains to be experimentally tested.
At the molecular level, anxiolytic effects of low doses of CB1 receptor agonists are associated with increased CREB expression within the PFC and hippocampus (Rubino et al. 2007). This increase was associated with an increase in ERK activation in the PFC, and a decrease in CAMKII (a kinase that inhibits CREB activation) within the hippocampus. In addition, anxiolytic doses of THC inhibited plus-maze exposure-induced Fos expression with the PFC and amygdala (Rubino et al. 2007). Behaviorally, the anxiolytic effects of low doses of THC are blocked by a mu-opioid receptor antagonist (Berrendero and Maldonado 2002), and a 5HT1A serotonin receptor antagonist (Marco et al. 2004); the anxiolytic effects of AM404 are also blocked by a 5-HT1A antagonist (Marco et al. 2004). These data suggest a role for opioid and serotonin receptors in the anxiolytic effects of ECS.
In the case of conditioned fear modulation, roles for ERK and calcineurin have been demonstrated. In response to conditioned tone presentation, CB1 receptor KO mice exhibit relatively increased freezing behavior as a consequence of impaired extinction (Marsicano et al. 2002). These mice also exhibited decreased tone-induced phosphorylation of ERK and calcineurin expression in the BLA and PFC, while showing increased expression of these two proteins in the central amygdala (Cannich et al. 2004). CB1 receptor KO mice also showed increased p-AKT in the BLA and dorsal hippocampus in response to conditioned tone presentation compared to wild-type mice (Cannich et al. 2004). It has been shown that ERK signaling in the BLA is required for the acquisition of extinction (Herry et al. 2006), suggesting that impaired ERK signaling in CB1 receptor KO mice could contribute to the impaired extinction observed in these mice. In addition, mice lacking forebrain calcineurin exhibit impaired extinction of conditioned fear behaviors (Havekes et al. 2008), supporting a role for this protein in the impaired extinction observed in CB1 receptor KO mice. These data suggest that ECS could facilitate extinction of conditioned fear via activation of ERK and calcineurin signaling (Davis et al. 2003; Galve-Roperh et al. 2002).
4 Human Studies Suggesting a Role for ECS in Depression
4.1 Cannabis Use and Depression
The thousands of years of human use of the CB1 receptor agonist, THC, in preparations of Cannabis sativa support the hypothesis that there is a relationship between cannabis use and depression. Elevation of mood is one of the commonly cited motivations for the use of cannabis. In a study of young, poly-substance users, 69% of the respondents reported that they used cannabis to “make themselves feel better when down or depressed” (Boys et al. 2001). While this is far less than the 97% who responded that they used cannabis to help relax, it argues that cannabis could exert anti-depressant effects in humans. Several clinical trials in the 1970s designed to determine the anti-depressant efficacy of THC found that it failed to improve symptoms of depression and produced unacceptable adverse effects (Ablon and Goodwin 1974; Kotin et al. 1973). Although it can be argued that these studies were small and did not take into consideration the heterogeneity in depressive illnesses, it is not likely that THC would be broadly useful as an anti-depressant in humans.
A similar hypothesis, that depressed individuals self-administer cannabis because it elevates mood, is not supported by available data (Kandel et al. 1986; Miller-Johnson et al. 1998; Patton et al. 2002). This hypothesis predicts that depressed people use cannabis to elevate mood more frequently than non-depressed users. This prediction was not upheld in a recent study (Arendt et al. 2007); in fact, depressed subjects experienced more depression, aggression and sadness when intoxicated with cannabis than when they were not intoxicated.
There are data to support an alternative hypothesis that cannabis use precipitates depression. For example, cannabis dependence and depression are co-morbid diagnoses more than would be expected by chance (Degenhardt et al. 2003). Furthermore, several prospective studies have found that cannabis use precedes the diagnosis of depression (Bovasso 2001; Patton et al. 2002; Rey and Tennant 2002). Cannabis use was identified in high-school students as a significant, independent predictor of suicidal behaviors after adjustment for depressive symptoms (Chabrol et al. 2008). However, a large (greater than 12,000 participants) longitudinal study did not find that past cannabis use was a significant predictor of depression in adults when baseline differences between users and non-users were carefully controlled (Harder et al. 2006). The authors of this study concluded that the available evidence does not support a causal relationship between cannabis use and depression, but does suggest that a common factor or factors predisposes individuals to both depression and cannabis dependence. In this regard, the hypothesis of a shared genetic predisposition for both cannabis use and depression has received support in the literature. Both cannabis use and dependence (Fu et al. 2002a; Kendler et al. 2000; Lynskey et al. 2002) and depressive/suicidal behaviors (Fu et al. 2002a, b; Statham et al. 1998; Sullivan et al. 2000) are moderately heritable. More importantly, several recent studies have demonstrated that the genetic factors for cannabis dependence and depression/suicidality are moderately correlated (Fu et al. 2002a; Lynskey et al. 2004). Twin studies suggest that shared environmental factors also contribute significantly to the co-morbidity of cannabis dependence and depression (Lynskey et al. 2004).
4.2 Depression and the ECS
The data described above lead to the hypothesis that dysregulation of ECS results in depression. Support for this hypothesis comes from the adverse events profile in humans of the CB1 receptor antagonist, rimonabant, which demonstrates a small, yet significant, increased likelihood for the development or exacerbation of depression (Van Gaal et al. 2008). The likelihood of depression or mood changes with depressive symptoms increases when patients with pre-existing depressive illness were not excluded from rimonabant treatment (Nissen et al. 2008). These data suggest that endogenous activation of CB1 receptors serves as a buffer against depression and its elimination or reduction in susceptible individuals can result in depressive symptoms. In another study, the incidence of depression in patients with Parkinson’s disease was found to be significantly correlated with polymorphisms in the CB1 receptor gene (Barrero et al. 2005). There was a trend for the same observation in non-Parkinson patients, but the study was not sufficiently powerful to determine whether CB1 receptor polymorphisms contribute to the likelihood of developing major depression in the general population.
There have also been some very interesting studies that have investigated the hypothesis that depression changes ECS. Patients with depression who died by suicide had significantly greater CB1 receptor agonist binding site density and agonist signaling in the dorsolateral PFC than matched controls (Hungund et al. 2004; Vinod et al. 2005). Tissue contents of both AEA and 2AG in the dorsolateral PFC were also increased in alcoholic patients who were depressed compared to alcoholics without depression (Vinod et al. 2005). In a study using immunohistochemical approaches, neuronal CB1 receptor density in the anterior cingulate cortex (ACC) was not found to be different between patients with major depression and controls (Koethe et al. 2007). However, CB1 receptor density was significantly decreased in subjects with major depression taking selective serotonin re-uptake inhibitors (SSRIs) compared to patients with major depression who were not being treated with SSRIs, suggesting that the drug therapy reduced CB1 receptor expression (Koethe et al. 2007). CB1 receptor density was also decreased in glial cells in the ACC of brains from patients who died with major depression compared to controls (Koethe et al. 2007). This finding is particularly interesting in light of other data suggesting that glial cell function and/or numbers are dysregulated in major depression (Cotter et al. 2001).
Our group has recently published a study in which circulating endocannabinoid concentrations were compared in non-medicated women with major depression and controls (Hill et al. 2008). 2AG contents in the serum were significantly lower in women with major depression than matched controls and were negatively correlated with the length of the current depressive episode. These data, while preliminary, support the possibility that some of the peripheral consequences of depression, such as cardiovascular and metabolic changes, could be related to ECS modulation.
To summarize, the available human data support the general hypothesis that CB1 receptor activity is involved in the regulation of mood and that pharmacological dysregulation of ECS can alter mood in some individuals. Data suggest that depressed individuals have altered ECS; however, whether changes in ECS precede or follow the development of depression is unknown.
5 Animal Studies Suggesting a Role for ECS in Depression
5.1 Evidence That Alteration of CB1 Receptor Signaling Results in Anti-Depressant-Like Effects
Immobility assays in rodents have been used extensively as preclinical models of anti-depressant efficacy of various pharmacologic agents. The Porsolt forced swim test is commonly employed; the time that rodents spend in an immobile, floating state is argued to represent a state of behavioral despair and is reduced by monoamine elevating anti-depressants (Porsolt et al. 1978). The highly efficacious CB1 receptor agonists, HU210 (Hill and Gorzalka 2005b) and WIN55212-2 (Bambico et al. 2007) reduce immobility duration in the forced swim test in male rats at very low doses, consistent with anti-depressant efficacy. These agonist effects are blocked by co-treatment with CB1 receptor antagonist. Indirect CB1 receptor agonists, including AM404 (Hill and Gorzalka 2005b) and the FAAH inhibitor, URB597 (Gobbi et al. 2005; Hill et al. 2007b), also exhibit anti-depressant efficacy in the forced swim test. URB597 also has anti-depressant efficacy in a second immobility assay, the mouse tail suspension (Gobbi et al. 2005).
While the direct and indirect agonist data are fairly consistent and support a role for the ECS in the coping response of mice in the forced swim, antagonist data have been inconsistent. In both male and female C57Bl/6N mice, rimonabant had no effect on the duration of immobility and increased struggling during the first exposure to the test (Steiner et al. 2008b). However, these investigators found that chronic treatment with high dose rimonabant significantly decreased immobility (Steiner et al. 2008a). Other studies using acute treatment with antagonists have also reported no effect (Bambico et al. 2007; Gobbi et al. 2005; Gobshtis et al. 2007; Hill and Gorzalka 2005b). On the other hand, several studies have demonstrated that acute treatment with antagonists, usually at high doses, reduces immobility (Shearman et al. 2003). The reasons for the discrepancies in these studies are not clear, but strain/species differences, differences in the parameters examined and differences in the environmental context of the assay (i.e., light vs. dark phase) are all plausible explanations.
Immobility tests comparing KO and wild-type mice have also been used to infer pro-depressant or anti-depressant roles for various proteins or signaling systems (Cryan and Holmes 2005). The duration of immobility of CB1 receptor KO mice on a CD-1 background is not different from wild-type (Jardinaud et al. 2005). In one study, Steiner and colleagues reported that immobility (floating) was significantly increased in CB1 receptor KO mice on a C57Bl/6N background compared to wild-type (Steiner et al. 2008b), while a second study from the same laboratory reported no difference in response when KO and wild-type mice were pretreated with a vehicle injection (Steiner et al. 2008a).
Taken together, these data suggest that activation of the CB1 receptor exogenously can produce an anti-depressant behavioral phenotype in immobility assays; and they provide some support for ECS tone. On the other hand, they suggest that CB1 receptor activation also contributes to behavioral despair since antagonist treatment can be anti-depressant as well. As for the effects of cannabinoid receptor ligands in anxiety discussed above, it is likely that there are “functional” pools of CB1 receptors that subserve pro- and anti-depressant behavioral effects.
Most depressive disorders in humans include decreased incentive to seek positive reinforcers or anhedonia as a core symptom (Rush and Weissenburger 1994). This aspect of depression can be modeled using several rodent assays; the most common is the sucrose consumption test. Activation of CB1 receptors results in a selective increase in the consumption of highly palatable foods, including increased sucrose drinking relative to the drinking of water (Sofia and Knobloch 1976). Inhibition of ECS by antagonists inhibits sucrose consumption in two bottle-choice paradigms (Arnone et al. 1997) and decreases responding reinforced by normal food and sucrose in operant procedures models (Freedland et al. 2001; Perio et al. 2001). CB1 receptor KO mice also display reduced sucrose intake (Poncelet et al. 2003; Sanchis-Segura et al. 2004). Therefore, there are consistent data that inhibition or removal of the CB1 receptor in otherwise normal rodents results in a decrease in their motivation to consume sucrose. These data lead to the hypothesis that reduced ECS could contribute to the anhedonia that occurs in depression. In support of this hypothesis, exposure of mice to stress results in a decrease in sucrose consumption that is reversed by direct and indirect CB1 receptor agonists (Rademacher and Hillard 2007). Interestingly, in this study, rimonabant reduced sucrose consumption in the stressed mice at doses that did not affect sucrose consumption in unstressed mice, consistent with a possible recruitment of ECS in the stressed condition (Rademacher et al. 2008).
5.2 Evidence That Environmental Contexts That Produce Depression-Like Symptoms Alter ECS
Repeated stress has been used to model depressive symptoms in rodents with a reasonable degree of biological and behavioral similarities to humans (Nestler et al. 2002). In particular, chronic exposure to an unpredictable and variable set of stressors (CUS) produces changes in rodents that parallel many aspects of human depression (Willner 2005). Several studies have demonstrated alterations in ECS in rodents exposed to CUS. Hippocampal CB1 receptor density is reduced in rats exposed to CUS; and perseveration in the water maze induced by CUS is reversed by cannabinoid agonist treatment (Hill et al. 2005a). In another study, CUS was found to reduce body weight and sucrose intake in rats, both of which were reversed by treatment with a FAAH inhibitor (Bortolato et al. 2007). These studies suggest that down-regulation of ECS contributes to the detrimental effects of CUS. This conclusion is supported by the finding that CB1 receptor KO mice exhibit increased sensitivity to the anhedonic effects of CUS (Martin et al. 2002).
Repeated exposure to the same stressor also recapitulates some of the behavioral effects of depression, including anhedonia. Repeated restraint results in changes in endocannabinoid content in several limbic regions, including a progressive increase in 2AG content within the PFC, amygdala and hypothalamus, as the number of restraint episodes increases (Rademacher et al. 2008). On the other hand, restraint decreases AEA contents in the PFC and amygdala regardless of the number of restraint episodes. These and other data support the hypothesis that repeated exposure to stress alters ECS and that these changes underlie the behavioral alterations induced by stress (Patel and Hillard 2008). Early life stress, which is known to promote the appearance of depression in adulthood, can be mimicked in mice using a 24-h maternal deprivation (Marco et al. 2009). Evidence from Macri and Laviola suggests that early life stress also down-regulates CB1-receptor-mediated signaling (Macri and Laviola 2004).
In a recent study, Rubino and colleagues demonstrated that chronic THC exposure during adolescence resulted in significantly increased immobility in the forced swim test in females but not males, and significant anhedonia in both males and females (Rubino et al. 2008c). These studies are very interesting, particularly since they bear on the hypothesis that cannabis consumption predisposes humans to depression.
Therefore, an evolving body of evidence supports the hypothesis that altered ECS accompanies the development of depressive-like behaviors in rodents. The specifics of the alteration are not completely clear, but hypofunctional ECS in subcortical regions, particularly the hippocampus and hypothalamus, have been seen in several models.
5.3 Evidence That Anti-Depressant Therapies Alter ECS
While THC itself is not a good anti-depressant in humans, the role of ECS in mood regulation prompts the question of whether altered ECS contributes to the efficacy of other anti-depressant drugs or manipulations. Chronic exposure of rats to desipramine results in a significant increase in CB1 receptor binding site density in the hippocampus and hypothalamus in non-stressed rats (Hill et al. 2006). Furthermore, the ability of chronic desipramine treatment to inhibit activation of Fos in the paraventricular nucleus (PVN) in response to stress was reversed by CB1 receptor antagonist treatment. In addition, rimonabant inhibited the weight gain in response to desipramine, but did not affect the ability of desipramine to reduce immobility in the forced swim assay (Gobshtis et al. 2007). These data suggest that the ability of chronic desipramine to inhibit the activation of the hypothalamic–pituitary–adrenal (HPA) axis and increase weight in normal rats is mediated by an increase in ECS, perhaps in the hypothalamus. In contrast to these results, the effect of an acute injection of desipramine to induce immobility was absent in CB1 receptor KO mice but the dampening effects of desipramine on HPA axis activation were intact (Steiner et al. 2008b). These results also suggest a difference in the mechanisms by which anti-depressants and ECS affect immobility and HPA axis activation, an observation that is discussed further below. The role of ECS in the effects of desipramine is not identical for other anti-depressants. For example, the SSRI citalopram significantly decreases CB1 -receptor-mediated signaling in the PVN (Hesketh et al. 2008). Electroconvulsive shock treatment (ECT) is the most effective therapeutic option for depression in humans in that it benefits a higher proportion of patients than chemical anti-depressant therapy and requires substantially less time to see benefit (Silverstone and Silverstone 2004). ECT also produces alterations in ECS that can be summarized as an increase in subcortical ECS and a decrease in cortical ECS (Hill et al. 2007a).
Therefore, the treatments for human depression modulate ECS in a regionally specific manner. However, the changes are not consistent with respect to brain region or directionality and more studies are needed to determine which, if any, of these changes are relevant to ECS in depression.
6 Neural Mechanisms Underlying Endocannabinoid Modulation of Depression
The neurobiology of depression is complex; however, a large body of evidence supports the hypothesis that dysregulation of the HPA axis plays a critical role (Hill and Gorzalka 2005a). In particular, HPA axis hyperactivation and reduced feedback inhibition are seen in humans with depression and in animal models of depression. The ability of anti-depressants to suppress HPA axis hyperactivity is coupled to their clinical efficacy (Appelhof et al. 2006). Recent studies strongly suggest that a primary role for ECS is to dampen HPA axis activation by stress and to allow for appropriate stress recovery (Barna et al. 2004; Di et al. 2003; Patel et al. 2004). These findings are consistent with the data obtained in rodents described above that inhibition of ECS is generally pro-depressive while its activation results in an anti-depressant phenotype, and lead to the hypothesis that dampening of the HPA axis is the mechanism by which ECS interacts with depression. However, HPA axis inhibition does not completely explain the effects of ECS to alter coping behaviors in the forced swim assay. For example, desipramine-induced behavioral effects are CB1 receptor-dependent while its effects on HPA axis activation are not (Steiner et al. 2008a). Recent studies in our laboratory demonstrate that female CB1 receptor KO mice exhibit normal HPA axis activation by stress but have increased immobility in the forced swim assay compared to wild-type (Roberts and Hillard, unpublished data).
The monoamine hypothesis of depression posits that dysregulation of serotonergic and noradrenergic signaling in the brain contributes to depressive symptoms (Belmaker and Agam 2008). ECS interactions with serotonergic signaling have been demonstrated in many studies. For example, serotonergic neurons have been shown to be involved in many cannabinoid effects, including hypothermia (Malone and Taylor 1998) and sleep (Mendelson and Basile 2001). The effect of WIN55212-2 to reduce immobility in the forced swim test is abolished by the serotonin (5-HT) depleting agent, para-chlorphenylalanine, indicating that this behavior is also 5-HT-mediated (Bambico et al. 2007). Low doses of WIN55212-2 enhance dorsal raphe serotonergic neuronal activity, an effect that is mimicked by the FAAH inhibitor URB597 (Gobbi et al. 2005). This effect appears to be due to ECS activation in the medial PFC since lesions there abolish the WIN55212-2 on raphe firing. Therefore, these studies suggest that activation of 5-HT-mediated signaling in the PFC is involved in the anti-depressant efficacy of activation of ECS. Recent studies have found that both CB1 receptor blockade (Tzavara et al. 2003) and chronic administration of THC result in increased serotonin levels in the PFC (Sagredo et al. 2006). Chronic administration of another agonist, HU210, results in an enhancement of 5-HT2A behavioral effects and a decrease in 5-HT1A effects (Hill et al. 2005b). On the other hand, the FAAH inhibitor, URB597, increases firing of serotonergic neurons in the dorsal raphe and noradrenergic neurons in the locus coeruleus and ECS has been shown to subserve the regulation of glutamate-induced activation of serotonergic neurons in the raphe (Haj-Dahmane and Shen 2005).
CB1 receptors are present throughout the limbic system (Herkenham et al. 1990) and can modulate both GABA and glutamate release (Freund et al. 2003). Therefore, it is not surprising that global activation or inhibition of ECS has confusing effects on behavior. A few studies have begun to dissect regional differences in the role of ECS in depression. HU210 injected into the rat hippocampus elicits reduced immobility in the forced swim test while URB597 is not active via this route (McLaughlin et al. 2007). These data, that activation of ECS in the hippocampus exerts anti-depressant effects, are consistent with findings that CUS, which produces depressive-like symptoms, down-regulates hippocampal ECS (Hill et al. 2005a). WIN55212-2 is also an effective anti-depressant when injected into the ventromedial PFC; the effects of indirect agonists and antagonists were not determined (Bambico et al. 2007). The possible role of 5-HT signaling in the PFC effects is discussed above. Interestingly, CUS has been shown to increase CB1 receptor mRNA expression in the PFC (Bortolato et al. 2007) and human suicides have increased CB1 receptor density and signaling (Hungund et al. 2004). It will be very interesting to determine the neuronal site of these up-regulated receptors.
7 Clinical Implications for Endocannabinoid-Based Therapeutics for Anxiety and Depressive Disorders
The data reviewed above indicate that ECS has an anxiolytic function. Data from studies of unconditioned anxiety measures suggest that pharmacological augmentation of ECS could represent a novel approach to the treatment of generalized anxiety disorder, and anxiety symptoms associated with depressive disorders. Endocannabinoid augmentation could also be useful in the treatment of PTSD based on the role of ECS in stress response habituation (Patel and Hillard 2008) and enhancement of extinction of conditioned fear and anxiety.
Initial augmentation strategies have focused on inhibition of AEA catabolism by FAAH and endocannabinoid uptake inhibitors. Both of these approaches have been successful in preclinical models. Future drug discovery should be aimed at development of selective inhibitors of 2AG degradation, which could also have anxiolytic properties. It is likely that pharmacological augmentation of ECS will have several advantages over direct CB1 receptor agonists including less likelihood of precipitating anxiety or panic reactions and less socio-political resistance to widespread clinical use. Lastly, these data suggest that the use of CB1 receptor antagonists should be minimized in patients with anxiety disorders, due to an increased risk of exacerbating symptoms (Christensen et al. 2007).
The issue of treating depression with ECS-based therapies is far more murky. Human depression is a heterogeneous disease and only a fraction of those treated with conventional therapies have long-term disease remission. There are strong indications (discussed at length above) that ECS dysregulation could contribute to depression in some humans. The challenge to research at this stage is to further our understanding of both depression and ECS in order to elucidate which depressed patients will benefit from ECS-based therapy.
Abbreviations
- 2AG:
-
2-Arachidonoylglycerol
- 5-HT:
-
5-Hydroxytryptamine, serotonin
- ACC:
-
Anterior cingulate cortex
- AEA:
-
Anandamide
- BLA:
-
Basolateral amygdala
- CCK:
-
Cholecystokinin
- CUS:
-
Chronic exposure to an unpredictable and variable set of stressors
- ECS:
-
Endocannabinoid signaling
- ECT:
-
Electroconvulsive therapy
- FAAH:
-
Fatty acid amide hydrolase
- HPA:
-
Hypothalamus–pituitary–adrenal
- KO:
-
Knockout
- PFC:
-
Prefrontal cortex
- PTSD:
-
Post-traumatic stress disorder
- PVN:
-
Paraventricular nucleus
- SSRI:
-
Selective serotonin re-uptake inhibitors
References
Ablon SL, Goodwin FK (1974) High frequency of dysphoric reactions to tetrahydrocannabinol among depressed patients. Am J Psychiatry 131:448–453
Alonso R, Voutsinos B, Fournier M et al. (1999) Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience 91:607–620
Appelhof BC, Huyser J, Verweij M et al. (2006) Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression). Biol Psychiatry 59:696–701
Arendt M, Rosenberg R, Fjordback L et al. (2007) Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychol Med 37:935–945
Arevalo C, de Miguel R, Hernandez-Tristan R (2001) Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav 70:123–131
Arnone M, Maruani J, Chaperon F et al. (1997) Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106
Bambico FR, Katz N, Debonnel G, et al. (2007) Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci 27:11700–11711
Barna I, Zelena D, Arszovszki AC et al. (2004) The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci 75:2959–2970
Barrero FJ, Ampuero I, Morales B et al. (2005) Depression in Parkinson's disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J 5:135–141
Belmaker RH, Agam G (2008) Major depressive disorder. N Engl J Med 358:55–68
Beltramo M, Piomelli D (2000) Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport 11:1231–1235
Beltramo M, Stella N, Calignano A et al. (1997) Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277:1094–1097
Berrendero F, Maldonado R (2002) Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 163:111–117
Bortolato M, Campolongo P, Mangieri RA et al. (2006) Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 31:2652–2659
Bortolato M, Mangieri RA, Fu J et al. (2007) Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry 62:1103–1110
Bovasso GB (2001) Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry 158:2033–2037
Boys A, Marsden J, Strang J (2001) Understanding reasons for drug use amongst young people: a functional perspective. Health Educ Res 16:457–469
Breivogel CS, Selley DE, Childers SR (1998) Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem 273:16865–16873
Cannich A, Wotjak CT, Kamprath K et al. (2004) CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Memory 11:625–632
Chabrol H, Chauchard E, Girabet J (2008) Cannabis use and suicidal behaviours in high-school students. Addict Behav 33:152–155
Chhatwal JP, Davis M, Maguschak KA et al. (2005) Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30:516–524
Christensen R, Kristensen PK, Bartels EM et al.(2007) Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370:1706–1713
Cotter D, Mackay D, Landau S et al. (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553
Cravatt BF, Giang DK, Mayfield SP et al. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Cravatt BF, Demarest K, Patricelli MP et al. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 98:9371–9376
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Davis MI, Ronesi J, Lovinger DM (2003) A predominant role for inhibition of the adenylate cyclase/protein kinase a pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J Biol Chem 278:48973–48980
Degenhardt L, Hall W, Lynskey M (2003) Exploring the association between cannabis use and depression. Addiction 98:1493–1504
Degroot A, Nomikos GG (2004) Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur J NeuroSci 20:1059–1064
Di S, Malcher-Lopes R, Halmos KC et al. (2003) Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857
Foldy C, Lee SY, Szabadics J et al. (2007) Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci 10:1128–1130
Freedland CS, Sharpe AL, Samson HH et al. (2001) Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res 25:277–282
Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signalling. Physiol Rev 83:1017–1066
Fu Q, Heath AC, Bucholz KK et al. (2002a) Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry 59:1125–1132
Fu Q, Heath AC, Bucholz KK et al. (2002b) A twin study of genetic and environmental influences on suicidality in men. Psychol Med 32:11–24
Galve-Roperh I, Rueda D, Gomez del Pulgar T et al. (2002) Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol 62:1385–1392
Gobbi G, Bambico FR, Mangieri R et al. (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102:18620–18625
Gobshtis N, Ben-Shabat S, Fride E (2007) Antidepressant-induced undesirable weight gain: prevention with rimonabant without interference with behavioral effectiveness. Eur J Pharmacol 554:155–163
Gregg JM, Small EW, Moore R et al. (1976) Emotional response to intravenous delta9tetrahydrocannabinol during oral surgery. J Oral Surg 34:301–313
Griebel G, Stemmelin J, Scatton B (2005) Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry 57:261–267
Haj-Dahmane S, Shen RY (2005) The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signalling. J Neurosci 25:896–905
Haller J, Bakos N, Szirmay M et al. (2002) The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J NeuroSci 16:1395–1398
Haller J, Varga B, Ledent C et al. (2004a) Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J NeuroSci 19:1906–1912
Haller J, Varga B, Ledent C et al. (2004b) CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol 15:299–304
Haller J, Matyas F, Soproni K et al. (2007) Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J NeuroSci 25:2445–2456
Harder VS, Morral AR, Arkes J (2006) Marijuana use and depression among adults: Testing for causal associations. Addiction 101:1463–1472
Hashimotodani Y, Ohno-Shosaku T, Kano M (2007) Endocannabinoids and synaptic function in the CNS. Neuroscientist 13:127–137
Havekes R, Nijholt IM, Visser AK et al. (2008) Transgenic inhibition of neuronal calcineurin activity in the forebrain facilitates fear conditioning, but inhibits the extinction of contextual fear memories. Neurobiol Learn Memory 89:595–598
Herkenham M, Lynn AB, Little MD et al. (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936
Herry C, Trifilieff P, Micheau J et al. (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J NeuroSci 24:261–269
Hesketh SA, Brennan AK, Jessop DS et al. (2008) Effects of chronic treatment with citalopram on cannabinoid and opioid receptor-mediated G-protein coupling in discrete rat brain regions. Psychopharmacology (Berl) 198:29–36
Hill MN, Gorzalka BB (2005a) Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol 16:333–352
Hill MN, Gorzalka BB (2005b) Pharmacological enhancement of cannabinoid CB(1) receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol 15(6):593–599
Hill MN, Patel S, Carrier EJ et al. (2005a) Downregulation of endocannabinoid signalling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 30:508–515
Hill MN, Sun JC, Tse MT et al. (2005b) Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol 9:277–286
Hill MN, Ho WS, Sinopoli KJ et al. (2006) Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology 31:2591–2599
Hill MN, Barr AM, Ho WS et al. (2007a) Electroconvulsive shock treatment differentially modulates cortical and subcortical endocannabinoid activity. J Neurochem 103:47–56
Hill MN, Karacabeyli ES, Gorzalka BB (2007b) Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 32:350–357
Hill MN, Miller GE, Ho WS et al. (2008) Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 41:48–53
Ho W-SV, Hillard CJ (2005) Modulators of endocannabinoid enzymic hydrolysis and membrane transport. In: Pertwee R (ed) Cannabinoids (handbook of experimental pharmacology). Springer, Freiburg, pp 187–207
Holter SM, Kallnik M, Wurst W et al. (2005) Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol 510:69–74
Huestis MA, Gorelick DA, Heishman SJ et al. (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328
Hungund BL, Vinod KY, Kassir SA et al. (2004) Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry 9:184–190
Jardinaud F, Crete D, Canestrelli C et al. (2005) CB1 receptor knockout mice show similar behavioral modifications to wild-type mice when enkephalin catabolism is inhibited. Brain Res 1063:77–83
Jarrahian A, Manna S, Edgemond WS et al. (2000) Structure–activity relationships among N-arachidonylethanolamine (Anandamide) head group analogues for the anandamide transporter. J Neurochem 74:2597–2606
Kamprath K, Marsicano G, Tang J et al. (2006) Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26:6677–6686
Kandel DB, Davies M, Karus D et al. (1986) The consequences in young adulthood of adolescent drug involvement. An overview. Arch Gen Psychiatry 43:746–754
Kathuria S, Gaetani S, Fegley D et al. (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81
Kendler KS, Karkowski LM, Neale MC et al. (2000) Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry 57:261–269
Koethe D, Llenos IC, Dulay JR et al. (2007) Expression of CB(1) cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm 114:1055–1063
Kotin J, Post RM, Goodwin FK (1973) Delta9-tetrahydrocannabinol in depressed patients. Arch Gen Psychiatry 28:345–348
Lafenetre P, Chaouloff F, Marsicano G (2007) The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res 56:367–381
Lin HC, Mao SC, Gean PW (2006) Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Memory 13:316–321
Lynskey MT, Heath AC, Nelson EC et al. (2002) Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med 32:195–207
Lynskey MT, Glowinski AL, Todorov AA et al. (2004) Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry 61:1026–1032
Maccarrone M, Valverde O, Barbaccia ML et al. (2002) Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J NeuroSci 15:1178–1186
Macri S, Laviola G (2004) Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposure during adolescence. Behav Brain Res 154:231–238
Malone DT, Taylor DA (1998) Modulation of delta9-tetrahydrocannabinol-induced hypothermia by fluoxetine in the rat. Br J Pharmacol 124:1419–1424
Marco EM, Perez-Alvarez L, Borcel E et al. (2004) Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55, 940 in male rats. Behav Pharmacol 15:21–27
Marco EM, Adriani W, Llorente R et al. (2009) Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: Altered responses to cannabinoid exposure. Neurosci Biobehav Rev 33(4):498–507
Marsicano G, Wotjak CT, Azad SC et al. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Martin M, Ledent C, Parmentier M et al. (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 159:379–387
McGregor IS, Dastur FN, McLellan RA et al. (1996) Cannabinoid modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol 313:43–49
McLaughlin RJ, Hill MN, Morrish AC et al. (2007) Local enhancement of cannabinoid CB1 receptor signalling in the dorsal hippocampus elicits an antidepressant-like effect. Behav Pharmacol 18:431–438
Mendelson WB, Basile AS (2001) The hypnotic actions of the fatty acid amide, oleamide. Neuropsychopharmacology 25:S36–S39
Miller-Johnson S, Lochman JE, Coie JD et al. (1998) Comorbidity of conduct and depressive problems at sixth grade: substance use outcomes across adolescence. J Abnorm Child Psychol 26:221–232
Monory K, Massa F, Egertova M et al. (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51:455–466
Moreira FA, Aguiar DC, Guimaraes FS (2007) Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology 52:958–965
Moreira FA, Kaiser N, Monory K et al. (2008) Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 54:141–150
Naidu PS, Varvel SA, Ahn K et al. (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 192:61–70
Navarro M, Hernandez E, Munoz RM et al. (1997) Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. NeuroReport 8:491–496
Nestler EJ, Gould E, Manji H et al. (2002) Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528
Nevo I, Becker C, Hamon M et al. (1996) Stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of the freely moving rat: prevention by diazepam but not ondansetron. J Neurochem 66:2041–2049
Nissen SE, Nicholls SJ, Wolski K et al. (2008) Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 299:1547–1560
Niyuhire F, Varvel SA, Thorpe AJ et al. (2007) The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 191:223–231
Pamplona FA, Takahashi RN (2006) WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci Lett 397:88–92
Pamplona FA, Prediger RD, Pandolfo P et al. (2006) The cannabinoid receptor agonist WIN 55, 212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 188:641–649
Patel S, Hillard CJ (2006) Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signalling. J Pharmacol Exp Ther 318:304–311
Patel S, Hillard CJ (2008) Adaptations in endocannabinoid signalling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci 27(11):2921–2929
Patel S, Roelke CT, Rademacher DJ et al. (2004) Endocannabinoid signalling negatively modulates stress-induced activation of the hypothalamic–pituitary–adrenal axis. Endocrinology 145:5431–5438
Patel S, Carrier EJ, Ho WS et al. (2005a) The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res 46:342–349
Patel S, Cravatt BF, Hillard CJ (2005b) Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology 30:497–507
Patton GC, Coffey C, Carlin JB et al. (2002) Cannabis use and mental health in young people: cohort study. Br Med J 325:1195–1198
Perio A, Barnouin MC, Poncelet M et al. (2001) Activity of SH141716 on post-reinforcement pauses in operant responding for sucrose reward in rats. Behav Pharmacol 12:641–645
Poncelet M, Maruani J, Calassi R et al. (2003) Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett 343:216–218
Porsolt RD, Anton G, Blavet N et al. (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Rademacher DJ, Hillard CJ (2007) Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog Neuropsychopharmacol Biol Psychiatry 31:633–641
Rademacher DJ, Meier SE, Shi L et al. (2008) Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 54:108–116
Reich CG, Mohammadi MH, Alger BE (2008) Endocannabinoid modulation of fear responses: learning and statedependent performance effects. J Psychopharmacol 22(7):769–777
Reilly D, Didcott P, Swift W et al. (1998) Long-term cannabis use: characteristics of users in an Australian rural area. Addiction 93:837–846
Rey JM, Tennant CC (2002) Cannabis and mental health. Br Med J 325:1183–1184
Rodgers RJ, Haller J, Halasz J et al. (2003) 'One-trial sensitization' to the anxiolytic-like effects of cannabinoid receptor antagonist SR141716A in the mouse elevated plus-maze. Eur J NeuroSci 17:1279–1286
Rodriguez de Fonseca F, Carrera MR, Navarro M et al. (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2054
Rubino T, Sala M, Vigano D et al. (2007) Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral delta(9)-tetrahydrocannabinol in rats. Neuropsychopharmacology 32:2036–2045
Rubino T, Guidali C, Vigano D et al. (2008a) CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 54:151–160
Rubino T, Realini N, Castiglioni C et al. (2008b) Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex 18:1292–1301
Rubino T, Vigano D, Realini N et al. (2008c) Chronic delta(9)-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33:2760–2771
Rush AJ, Weissenburger JE (1994) Melancholic symptom features and DSM-IV. Am J Psychiatry 151:489–498
Sagredo O, Ramos JA, Fernandez-Ruiz J et al. (2006) Chronic delta(9)-tetrahydrocannabinol administration affects serotonin levels in the rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol 372:313–317
Sanchis-Segura C, Cline BH, Marsicano G et al. (2004) Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 176:223–232
Scheen AJ, Finer N, Hollander P et al. (2006) Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368:1660–1672
Scherma M, Medalie J, Fratta W et al. (2008) The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54:129–140
Schofield D, Tennant C, Nash L et al. (2006) Reasons for cannabis use in psychosis. Aust NZ J Psychiatry 40:570–574
Shearman LP, Rosko KM, Fleischer R et al. (2003) Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol 14:573–582
Silverstone PH, Silverstone T (2004) A review of acute treatments for bipolar depression. Int Clin Psychopharmacol 19:113–124
Sofia RD, Knobloch LC (1976) Comparative effects of various naturally occurring cannabinoids on food, sucrose and water consumption by rats. Pharmacol Biochem Behav 4:591–599
Statham DJ, Heath AC, Madden PA et al. (1998) Suicidal behaviour: an epidemiological and genetic study. Psychol Med 28:839–855
Steiner MA, Marsicano G, Nestler EJ et al. (2008a) Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signalling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology 33:54–67
Steiner MA, Wanisch K, Monory K et al. (2008b) Impaired cannabinoid receptor type 1 signalling interferes with stress-coping behavior in mice. Pharmacogenomics J 8:196–208
Sullivan PF, Neale MC, Kendler KS (2000) Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 157:1552–1562
Suzuki A, Josselyn SA, Frankland PW et al. (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24:4787–4795
Szuster RR, Pontius EB, Campos PE (1988) Marijuana sensitivity and panic anxiety. J Clin Psychiatry 49:427–429
Thomas H (1993) Psychiatric symptoms in cannabis users. Br J Psychiatry 163:141–149
Tzavara ET, Davis RJ, Perry KW et al. (2003) The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol 138:544–553
Uriguen L, Perez-Rial S, Ledent C et al. (2004) Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology 46:966–973
Van Gaal L, Pi-Sunyer X, Despres JP et al. (2008) Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the rimonabant in obesity (RIO) program. Diabetes Care 31(Suppl 2):S229–S240
Varvel SA, Wise LE, Niyuhire F et al. (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32:1032–1041
Vinod KY, Arango V, Xie S et al. (2005) Elevated levels of endocannabinoids and CB1 receptor-mediated G-protein signalling in the prefrontal cortex of alcoholic suicide victims. Biol Psychiatry 57:480–486
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110
Acknowledgements
CJH was supported during the writing of this review by Research for a Healthier Tomorrow, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and NIH grant R21 DA022439.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Patel, S., Hillard, C.J. (2009). Role of Endocannabinoid Signaling in Anxiety and Depression. In: Kendall, D., Alexander, S. (eds) Behavioral Neurobiology of the Endocannabinoid System. Current Topics in Behavioral Neurosciences, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-88955-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-540-88955-7_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-88954-0
Online ISBN: 978-3-540-88955-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)