Abstract
Train yourself adequately in laryngotracheal surgery and upper airway endoscopy before addressing the challenging surgery of laryngotracheal stenosis (LTS). Remember that inappropriate initial management of LTS may lead to permanent intractable sequelae and that the best chance for the patient lies in the first operation. Perform a thorough preoperative assessment of the patient’s medical condition and of the stenosis to choose the best surgical option and timing. Address only mature cicatricial stenosis for a definitive endoscopic or open surgical repair. Perform a bacteriologic aspirate of the trachea prior to any treatment. Treat gastro-oesophageal reflux. Master all types of surgeries starting from appropriate use of CO2 laser for minor stenosis to laryngotracheal reconstruction with cartilage expansion and partial cricotracheal resection for the most severe grades of stenosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Core Messages
-
Train yourself adequately in laryngotracheal surgery and upper airway endoscopy before addressing the challenging surgery of laryngotracheal stenosis (LTS).
-
Remember that inappropriate initial management of LTS may lead to permanent intractable sequelae and that the best chance for the patient lies in the first operation.
-
Perform a thorough preoperative assessment of the patient’s medical condition and of the stenosis to choose the best surgical option and timing.
-
Address only mature cicatricial stenosis for a definitive endoscopic or open surgical repair.
-
Perform a bacteriologic aspirate of the trachea prior to any treatment.
-
Treat gastro-oesophageal reflux.
-
Master all types of surgeries starting from appropriate use of CO2 laser for minor stenosis to laryngotracheal reconstruction with cartilage expansion and partial cricotracheal resection for the most severe grades of stenosis.
10.2 Introduction
The management of laryngotracheal stenosis (LTS) remains a challenging problem for the otolaryngologist, especially in the pediatric age group. The complexity of the various preoperative situations implies that no single treatment modality can solve the problem. One has to take into consideration the type of the stenosis (congenital or acquired), its location (supraglottic, glottic, subglottic, combined), its degree of obstruction and length in the craniocaudal axis, and finally its association with vocal cord ankylosis or neurogenic paralysis. Furthermore, the presence of tracheal damage (stenosis or localized malacia) related to the tracheostoma or to the tracheotomy cannula can further complicate the surgical management. According to the nature and severity of the condition, a variety of treatments exists. They range from endoscopic laser sessions with or without dilatation or stenting [13, 14, 36, 51] to laryngotracheal reconstruction (LTR) with anterior, posterior, or combined costal cartilage grafts [8, 43, 44], to partial cricotracheal resection (PCTR) for the most severe grades of stenosis, and to extended PCTR for combined glotto-subglottic stenosis (SGS) [37, 55].
Needless to say, thorough preoperative endoscopic assessment is prerequisite to selecting the best surgical option for a given condition.
10.3 Etiology
10.3.1 Infants and Children
10.3.1.1 Subglottis
In the pediatric age group, the most common reason for SGS is prolonged intubation. In newborns, however, congenital SGS represents the third most common laryngeal anomaly after laryngomalacia and bilateral vocal fold paralysis [24]. According to Holinger, congenital SGS is classified into cartilaginous and soft tissue stenoses [25]. It is present when the lumen of the cricoid region measures less than 4 mm in diameter in a full-term infant or 3 mm in a premature infant. The cartilaginous type results from failure of complete recanalization of the laryngeal lumen after the eighth week of gestation. The cricoid may have a normal shape but is too small for the infant’s size, or it may show different abnormalities, such as general thickening, a large anterior or posterior lamina, or an elliptical shape. Sometimes a trapped first tracheal ring is responsible for the small size of the subglottis.
In approximately 50% of cases, congenital SGS is associated with mediastinal malformations including cardiovascular, tracheobronchial, or esophageal anomalies [41]. For the otolaryngologist, thoracic surgeon, and anesthetist, this implies that any mediastinal malformation warrants bronchoesophagoscopy before treatment to rule out minor asymptomatic congenital SGS.
Injuries leading to acquired SGS in infants and children are more likely to occur after traumatic intubation for resuscitation, after intubation for severe cranial injuries, when laryngoscopy is difficult because of anatomical problems, or when mild congenital subglottic stenosis has been overlooked. Any systemic condition that diminishes capillary perfusion (i.e., shock, anemia) or that increases the susceptibility to infection (i.e., diabetes, immunosuppression) aggravates the subglottic damage caused by the indwelling endotracheal (ET) tube, as does gastroesophageal reflux [29]. The evolution of acute lesions of intubation into cicatricial sequelae of the glottis and subglottis were clearly described by Benjamin in 1993 [3]. They are similar in adults and children but with more prominent involvement of the glottis in children.
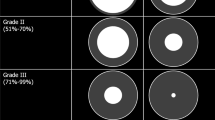
10.3.1.1.1 Grading System
In the pediatric community, the Myer-Cotton grading system is routinely used. This system classifies SGS into four grades (Table 10.1) [42].
10.3.1.2 Trachea
In infants, tracheal stenosis is most commonly related to congenital anomalies of the trachea itself (tracheomalacia, web, long-segment stenosis with circular rings) or to extrinsic compressions from cardiovascular malformations. Even after correcting the vascular anomaly, localized tracheomalacia may necessitate further specific tracheal surgery. These entities have been clearly described in textbooks [26]. Their detailed description is beyond the scope of this chapter.
In older children, the etiology of benign tracheal stenoses is similar to that seen in the adult population (see Sect. 3.2.2).
10.3.2 Adults
10.3.2.1 Subglottis
Although several conditions—e.g., blunt trauma, inhalation injuries, high tracheotomy or cricothyroidotomy, Wegener’s granulomatosis—and idiopathic causes can lead to benign SGS, postintubation injury remains by far the most common cause of SGS that is amenable to resection and primary reconstruction [35].
In the supraglottic region, sequelae of endotracheal intubation are usually absent or minimal. At the level of the glottis, they predominantly appear as bands of scar tissue tethering the vocal cords posteriorly, with or without cricoarytenoid ankylosis. This is the so-called posterior glottic stenosis (PGS). More seldomly, fusion of the vocal cords is also encountered. In the subglottis, circumferential ulcerations can induce granulation tissue formation which matures into contracting scars leading to SGS (Fig. 10.1).
10.3.2.1.1 Grading System
In adults where cricotracheal resection is now routinely used for the cure of laryngotracheal stenosis, Mc Caffrey’s grading system of SGS helps predict the rate of success of the operation (Table 10.2) [32].
10.3.2.2 Trachea
Benign tracheal stenoses result from cuff lesions induced by endotracheal or tracheotomy tubes or from the sequelae of tracheotomy (i.e., triangular deformation at the site of the former stoma, tip of cannula anterior stenosis, or suprastomal granuloma and collapse) (Fig. 10.2) [35].
Tracheal stenosis resulting of sequelae of tracheotomy. (a) Potential complications (i.e., suprastomal collapse, ostial or cuff-induced stenosis, tip of cannula stenosis). (b) Suprastomal collapse with granulation tissue. (c) Severe triangular narrowing of the trachea at the site of the former stoma. (d) Tip of cannula stenosis with granulation tissue
10.4 Indications
10.4.1 Primary Endoscopic Treatment
Cautious carbon dioxide (CO2) laser incision combined with dilatation may be effective in thin, web-like cicatricial stenoses of the subglottis, but extensive laser resection is liable to make an acquired stenosis worse [36].
The indications set down by Simpson et al. (Table 10.3) [52] are still valuable today as a basis for the endoscopic treatment of LTS. CO2 laser should be set to superpulse or ultrapulse mode and the laser beam directed to the target with a microspot manipulator (250 μm spot size at 400 mm focal distance) to minimize heat diffusion into the surrounding tissue. Radial incisions in the stenosis are made using Shapsay’s technique [51], and gentle dilatation is done with tapered bougies or angioplasty balloons. Then, a cotton swab soaked in a solution of 2 mg/ml mitomycin C may be topically applied to the subglottis for 2 minutes. Repeated mitomycin C application should probably be avoided, however, owing to uncertainty regarding possible late adverse effects [16, 48].
Finally, if the primary endoscopic treatment (CO2 laser/dilatation/stenting) leads to a recurrence of the stenosis to its initial grade, any further endoscopic treatment is strictly contraindicated. Open surgery should be considered instead. The expected result is superior if the stenosis does not involve the posterior wall of the airway, especially at the level of the membranous trachea.
10.4.2 Laryngotracheal Reconstruction with Cartilage Expansion
Laryngotracheal reconstruction with cartilage expansion is almost exclusively reserved for mild grades of pediatric SGS or for combined glotto-subglottic stenoses. Although in adults LTR has been replaced by cricotracheal resection it is still used when further resection of the subglottis and trachea is impossible because of previous failed surgeries.
In children, LTR with an anterior graft alone is used as a single-stage operation to cure grade II stenosis [9, [33]. Mild grade III stenosis is likely to need an anterior graft with posterior cricoid split supported by an endoluminal stent, and severe grade III stenosis requires both anterior and posterior grafts with stenting [7, 43-[55]. However, over the last decade, PCTR has shown to be superior to LTR for curing grade III and IV SGS [27].
In case of congenital stenosis, the LTR may be combined with submucosal resection of cartilage to increase the size of a thickened anterior lamina of the cricoid ring. Posterior glottic stenosis presents particular difficulties in children. A posterior cartilage graft is needed, but overexpansion of the posterior commissure should be avoided as it impairs voice quality and induces potential aspiration. Stenting is essential until the glottis and subglottis are completely healed.
10.4.3 Partial Cricotracheal Resection
In infants and children, PCTR is the procedure of choice for the treatment of severe (> 70% luminal obstruction) SGS of congenital or acquired etiology, but it is generally advisable to wait until the child reaches 10 kg body weight before surgery is undertaken [2, 38, 53-[55]. The latter is performed as a single-stage operation (with concomitant resection of the tracheostoma during the surgery) when the stenosis is purely subglottic and the child is otherwise healthy. The only exception to this rule is a very distal location of the tracheostoma (fifth or sixth tracheal ring) with normal, steady tracheal rings available for the anastomosis between the subglottic stenosis and the upper margin of the tracheostoma. The latter is then closed in a second stage.
In children with multiple congenital anomalies or impaired neurological or cardiopulmonary function, a double-stage PCTR (with postoperative maintenance of the tracheostoma) is preferable. In adults, PCTR is almost exclusively performed as a single-stage operation for simple SGS or SGS combined with posterior glottic stenosis if cricoarytenoid joint mobility can be restored during the surgery and there is no bilateral neurogenic vocal cord paralysis [10, 23, 30, 31, 46].
10.4.4 Extended PCTR
In the pediatric age group, when SGS is combined with glottic involvement (e.g., posterior glottic stenosis), cicatricial fusion of the vocal cords, or distortion of the laryngeal framework resulting from failed LTRs, the PCTR is supplemented with posterior cricoid split and costal cartilage graft that needs stenting with an LT-Mold (see Sect. 4.5) for about 3 weeks until the subglottic area is completely healed. The tracheostoma is then closed in a second stage [37, 50]. The alternative to this treatment is an LTR with anterior and posterior costal cartilage grafts with stenting [8, 43, 44].
In adults, a posterior cricoid split and costal cartilage graft is rarely necessary. Resection of the scar tissue constituting the interarytenoid aspect of the stenosis suffices in most cases, although sometimes stenting with an LT-Mold is necessary with maintenance of the tracheostoma until complete healing has occurred.
10.4.5 Stenting
Laryngeal stents are mainly used to keep the airway expanded after surgical reconstruction (LTR with costal cartilage grafts or extended PCTR). They provide support for cartilage grafts, allow approximation and immobilization of mucosal grafts to the recipient site, and maintain the lumen in a reconstructed area that lacks adequate support. Unfortunately, laryngeal stents can also act as foreign bodies in a reconstructed airway and induce mucosal injuries, ulcerations, granulation tissue formation, and subsequent restenosis if their anatomical conformity to the inner laryngeal contours is not perfect or if their consistency is too hard.
Several laryngeal stents are currently available on the market, but none truly meets the requirements for safe use without potential damage to the reconstructed airway. The simplest ones—the finger cot and the rolled Silastic sheet—are custom-made [18]). They are quite primitive, however, and have now been largely replaced by the Aboulker stent [1], Montgomery T-tube [39], Healy pediatric T-tube, and Montgomery or Eliachar laryngotracheal stents [17].
Unfortunately, none of these stents is devoid of potential severe complications [6, 19, 57]. To overcome this problem, the LT-Mold has been designed for temporary stenting of the airway after surgical treatment of cicatricial stenoses of the larynx. Its design was created after molding cadaver larynges and increasing the interarytenoid distance to obtain the intralaryngeal contours of a fully abducted larynx (Fig. 10.3) [34]. Made of silicone at a strength of 50 Shore-A, it is soft and thus prevents pressure necrosis at the medial aspect of the arytenoids. To avoid possible granulation tissue formation at the distal extremity of the LT-Mold, a dedicated silicone cap is manufactured for each prosthesis. The prosthesis comes in 10 sizes, from 6 to 15 mm in outer diameter, and can be used in pediatric and adult populations. Moreover, the prosthesis can be used during open surgery (intraoperative use) or after endoscopic resection of a laryngotracheal stenosis (endoscopic use) with temporary maintenance of the tracheostoma.
LT-Mold stent. (a) Made of soft silicone at a strength of 50 Shore-A, the LT-mold was designed after molding adult and pediatric cadaver larynges and increasing the interarytenoid distance to obtain the intralaryngeal contours of a fully abducted larynx. (b) The LT-Mold comes in 10 sizes, from 6 to 15 mm in outer diameter. To avoid possible granulation tissue formation at its distal extremity, a dedicated silicone cap has been manufactured for each prosthesis. (c) Retrograde view of the stent through the tracheostoma : absence of granulation tissue at the stent-mucosal interface
The current experience in 28 patients shows excellent tolerance of the stent, without erosion or granulation tissue formation in the supraglottis or glottis or in the vicinity of the tracheostoma when the prosthesis is used with a distal cap.
10.4.6 Tracheal Resection
For simple tracheal stenosis amenable to segmental resection, end-to-end anastomosis with immediate postoperative extubation is the preferred method of treatment in adults and children. In the latter group. a short-time intubation may be necessary. For multilevel stenoses (SGS + cuff tracheal stenoses), a two-stage approach is sometimes necessary if the tracheal segment to be resected is too long (cf. specific recommendations to the technique).
10.5 Preoperative Workup
10.5.1 Basic Assessment
Thorough endoscopic evaluation usually provides all the information needed for carefully planned surgery.
If precise description and measurement of the stenosis are obtained via endoscopy, radiography adds little to the preoperative workup. However, lateral soft tissue and anteroposterior high-kilovoltage radiography or computed tomography scans with three-dimensional reconstructions are useful for documenting the length of the segment to be resected. When malformation of the mediastinum is suspected, CT or magnetic resonance imaging (MRI) are the examinations of choice.
Finally, pulmonary, cardiac, and neurological evaluations should be considered in children with congenital anomalies or previous long-term intubation for neonatal dyspnea of various etiologies. In adults, the same investigations should be undertaken on an individual basis depending on the patient’s medical condition.
Last but not least, gastroesophageal reflux should be systematically ruled out or actively treated, if present.
10.5.2 Endoscopic Evaluation
Considering the potential dramatic consequences of failed PCTR or LTR, careful attention should be given to the preoperative endoscopic workup. It should comprise transnasal flexible laryngoscopy (TNFL) with spontaneous respiration or direct laryngotracheoscopy under general anesthesia with suspension microlaryngoscopy (if needed) and bronchoesophagoscopy.
10.5.2.1 Transnasal Flexible Laryngoscopy
In adults, neonates, and cooperative children, TNFL is done in the awake patient without sedation. For noncooperative children, TNFL under deep sevoflurane anesthesia with mask ventilation and spontaneous respiration is the preferred method (Fig. 10.4). This examination should not only give information on the mobility of the vocal cords but also on the patency of the nose, choanae, nasopharynx, and oropharynx. However, it provides only adequate visualization of the supraglottis and is unhelpful in the assessment of SGS. If there is any doubt regarding the mobility of the vocal cords, additional investigation by suspension microlaryngoscopy is mandatory [38].
10.5.2.2 Direct Laryngotracheoscopy and Suspension Microlaryngoscopy
The location, extent, and degree of stenosis are assessed using a bare magnifying telescope and an intubation laryngoscope while the patient is under general anesthesia and fully relaxed (Fig. 10.5). The exact location of the stenosis with respect to the vocal folds, the tracheostoma, and the carina are measured in millimeters. The degree of the stenosis is measured by passing telescopes, endotracheal tubes, or bougies of different given sizes through the stricture. In the pediatric community, the Myer-Cotton airway grading system is routinely used [42]. This system classifies SGS into four grades and helps predict the rate of success after laryngotracheal reconstruction: The less severe grades (I and II) have a far better outcome than do severe grades (III and IV), which correspond to subtotal or total obstruction. This grading system is not useful as a predictor of success or failure for partial cricotracheal resection because the stenotic segment is fully resected.
Endoscopic assessment of laryngotracheal stenosis. (a) Direct laryngotracheoscopy with a 0° bare telescope. Examination down to the carina is possible without mucosal trauma in nontracheostomized patients. (b) Endoscopy report for subglottic stenosis. Precise measurements with reference to the vocal folds, tracheostoma, and carina should be given in all cases, including the number of residual normal tracheal rings. A precise surgical strategy is chosen at that time, taking all parameters into account: vocal fold mobility, extension and degree of stenosis, location of tracheostoma, length of residual normal trachea, and segments of tracheomalacia
Differentiating vocal fold immobility due to a neurogenic cause from an interarytenoid fibrous adhesion is done by carefully inspecting the posterior commissure of the larynx using a 30°, angled telescope and by direct palpation the arytenoid cartilages during suspension microlaryngoscopy [15, 36]. The systematic use of Lindholm’s self-retaining vocal cord retractor (Storz no. 8654B) helps differentiate bilateral vocal fold paralysis from posterior glottic stenosis. A fixed arytenoid raises the suspicion of fibrous ankylosis of the joint; but in the most difficult cases, this diagnosis is safely made only during open surgery (Fig. 10.6).
The endoscopy report should also mention the presence of any localized tracheomalacia as well as a possible infection of the airway. A bacteriological smear is routinely taken and submitted to the laboratory.
10.5.2.3 Bronchoesophagoscopy
In infants and children, this additional examination is mandatory in all cases of congenital SGS to rule out an associated mediastinal malformation (i.e., tracheoesophageal fistula, tracheobronchial anomalies, extrinsic vascular compression of the airway), gastroesophageal reflux, and eosinophilic esophagitis [28, 41, 56].
For adult acquired SGS, a bronchoesophagoscopic examination is undertaken based on individual criteria. A systematic workup for gastroesophageal reflux is routinely done in patients with a positive medical history.
10.6 Operative Technique
10.6.1 Primary Endoscopic Treatment
Strict adherence to proper indications as described in Sect. 4.1 is a prerequisite if one wishes to avoid the dramatic consequences of overuse of laser [36]. In many cases, cold instruments, such as the sickle knife, can be used to incise a web-like cicatricial SGS. However, preference must be given to the use of CO2 laser, which offers more precision and a bloodless field. Proper selection of CO2 laser parameters is essential (Table 10.4) to obtain a char-free resection bed. Radial incisions of the cicatricial SGS according to Shapsay’s technique [51] preserve mucosal bridges for better, faster reepithelialization of the subglottis. In addition to laser incisions, gentle dilatation with tapered Savary-Gilliard bougies or angioplasty balloons blown up to a pressure of 8 atm optimally enlarges the subglottic lumen. Then, topical application with a cotton swab soaked in a solution of mitomycin at a concentration of 1-2 mg/ml for 2 minutes gives satisfactory clinical results, although no experimental study has yet set up the optimal dosage or duration of topical application of the drug (Fig. 10.7) [16, 48]. However, if the SGS recurs at its initial grade after the first endoscopic treatment, open surgery should be considered without delay. [36].
The management of posterior glottic stenosis (PGS) requires expertise in selecting the appropriate candidate for the right type of treatment. Interarytenoid adhesion with a residual posterior opening is usually not associated with cricoarytenoid (CA) joint fixation. Dividing the scar with CO2 laser is thus the first appropriate choice of treatment with a potentially high success rate [13, 14]. True PGS without residual posterior opening deserves first-try endoscopic treatment with CO2 laser and adjuvant topical application of mitomycin C. Postoperative intubation with a soft blue-line Portex tube for 5-7 days helps obtain a satisfactory result. If CA joint mobility is restored, the abductive force of both posterior cricoarytenoid muscles prevents recurrence of the PGS at least to some degree. In tracheostomized patients, 2-3 weeks of stenting with an Easy LT-Mold [34] ensures reepithelialization of the posterior commissure in abduction, thus re-creating an adequate airway for breathing. In the case of true fixation of the CA joints, laser arytenoidectomy, posterior cordotomy, or posterior costal cartilage grafting should be envisaged. When PGS is combined with a subglottic stenosis, open surgery is mandatory in most cases.
10.6.2 Laryngotracheal Reconstruction with Cartilage Expansion
The LTR with cartilage expansion is performed through a small collar incision placed at the superior edge of the tracheostoma (Fig. 10.8). The strap muscles are separated from the midline to expose the anterior portion of the larynx and upper trachea. For simple LTR with an anterior cartilage graft only, the incision typically extends through the lower third of the thyroid cartilage, the thyrocricoid membrane, the cricoid, and the first two tracheal rings. The costal cartilage harvested from the fifth, sixth, or seventh rib is boat-shaped and placed with the perichondrium intraluminally, serving as a lattice for reepithelialization. Lateral flanges of cartilage to the inset portion of the graft are secured to the thyroid, cricoid, and tracheal rings with 4.0 Vicryl sutures, thus preventing the graft from prolapsing into the airway.
Laryngotracheal reconstruction with cartilage expansion. (a) Costal cartilage harvested from the seventh rib. (b) Anterior costal cartilage graft. (c) Laryngofissure and posterior cricoid split for the treatment of posterior glottic stenosis (PGS) combined with subglottic stenosis (SGS). (d) Posterior costal cartilage graft. Note that the flanges of cartilage are wedged between the divided cricoid laminae. (e) Combined anterior and posterior cartilage grafts for grade III SGS
The treatment of an associated PGS or a grade III SGS requires both anterior and posterior grafts with stenting. Placement of the posterior cartilage graft requires that the anterior thyrotracheal incision be extended into a full laryngofissure. The cricoid plate is then divided in the midline, and interarytenoid scarring is accurately resected. A rectangular costal cartilage graft is then inserted between the two parts of the posterior cricoid plate with the perichondrium facing the lumen. The graft must fit flush between the divided posterior cricoid laminae. It is sutured into place with 4.0 Vicryl sutures. An LT-Mold of appropriate diameter is placed at that stage and securely fixed with two 3.0 Prolene sutures, one placed exactly at the anterior commissure of the larynx and the other craniocaudally on one lateral side of the trachea. Depending on the individual situation, the vertical incision of the trachea is closed over the stent with or without additional anterior costal cartilage grafting (Fig. 10.9).
Peroperative use of the LT-Mold. (a) The LT-Mold is cut at the appropriate length, and the distal cap is glued to the prosthesis with silicone glue. (b) Diagram showing proper LT-Mold placement in the larynx. (c) Endoscopic view of the LT-Mold in the larynx. (d) View of the larynx immediately after stent removal
10.6.2.1 Single-Stage Versus Double-Stage LTR
The decision of whether to perform single-stage versus double-state LTR is based on the severity of the initial stenosis, the type of LTR that was performed (anterior graft only versus anterior and posterior costal cartilage grafts), and the patient’s medical condition. Poor cardiopulmonary function and neurological impairment are contraindications to a single-stage LTR, even for a grade II or mild grade III SGS. Depending on the stability of the reconstructed airway, an LT-Mold stent is secured in place and left in the airway for a period of 3 weeks to 6 months [34].
10.6.3 Partial Cricotracheal Resection for Subglottic Stenosis
PCTR for SGS is performed with the neck fully extended. A collar incision is usually made at the level that will best expose the stenotic segment of the airway (Fig. 10.10). In tracheotomized patients, a horizontal crescent-shaped excision of the skin is made around the stoma. This is necessary for upward mobilization of the trachea even if the tracheostoma is kept at the end of the operation. The subplatysmal skin flaps are elevated, and the strap muscles are separated from the midline to provide exposure from the hyoid bone to the suprasternal notch. At that stage, the use of a Denys-Brown retractor (Lone Star Medical Products, Stafford TX, USA) is extremely useful. As the dissection progresses, the surgeon advances the position of stays into deeper tissue (i.e., strap muscles and thyroid lobes reflected aside). As a consequence, the larynx and trachea are almost at the level of the skin, which markedly facilitates the dissection and resection-anastomosis. With benign stenosis, the trachea is dissected anteriorly and laterally without identifying the recurrent laryngeal nerves by staying in close contact with the underlying cartilaginous rings. With neoplastic stenosis, one or the other recurrent laryngeal nerve is usually identified to determine the extent of the disease. The vascular supply coming laterally from the tracheoesophageal grooves should always be carefully preserved, especially during extensive mobilization of the distal trachea. Prior to any incision of the trachea or subglottis, this mobilization gives an idea on how much of the trachea can safely be resected without a laryngeal release procedure.
Partial cricotracheal resection. (a) After careful preparation and mobilization of the trachea and larynx, the superior resection line is made at the inferior margin of the thyroid cartilage. The inferior resection line is carried out one ring below the first normal tracheal ring to harvest an anterior pedicled wedge of cartilage that will be used to enlarge the subglottic lumen. The lateral resection line is made just anterior to the cricothyroid joint on both sides. The recurrent laryngeal nerve is shown here for anatomical purposes only; it is deliberately not identified during the surgery. (b) After resection of the anterior arch of the cricoid, the fibrous tissue constituting the posterior aspect of the stenosis is fully resected. The uppermost posterior section of the mucosa passes immediately below the cricoarytenoid joints. The denuded cricoid plate is then flattened with a diamond burr. This allows better adaptation of the tracheal stump to the subglottis. The cartilaginous wedge of the anterior trachea will be used to enlarge the subglottic lumen. (c) Except for the most posterolateral suture, which is placed between the trachea and the cricoid plate, all lateral and anterior stitches are passed between the tracheal ring and the thyroid cartilage. The adaptation of the large tracheal ring to the narrower subglottic space is facilitated by enlargement of the subglottic lumen with the partial inferior midline thyrotomy. The triangular defect is then filled in with the cartilaginous wedge pedicled to the tracheal ring used for the anastomosis
At the level of the cricoid arch, the cricothyroid muscles are sharply dissected off the underlying cartilage and reflected laterally over the cricothyroid joint. This maneuver protects the recurrent laryngeal nerves, which run posteriorly to the cricothyroid joint. After having placed stay sutures to the distal normal tracheal wall, the inferior resection line is made first at the lower end of the stenosis or at the level of the tracheostoma if the latter is to be resected during the same surgical procedure. This allows a view of the stenosis from below and affords a good distal airway for ventilation. The membranous trachea is then dissected and separated from the anterior wall of the esophagus over a distance that corresponds to the height of the cricoid plate. Unnecessary extensive separation of the trachea from the esophagus should be avoided to preserve an optimal vascular supply. Advancement of the distal tracheal stump upward is achieved by freeing the cartilaginous rings from the mediastinal structures anteriorly and laterally only.
Owing to its elasticity, the esophagus shortens spontaneously without anterior bulging. The superior incision is started at the inferior margin of the thyroid cartilage in front and is passed laterally just anterior to the cricothyroid joints. This results in complete resection of the anterior cricoid arch while avoiding injury to the recurrent laryngeal nerves that run posterior to the joint. In the subglottis, the uppermost incision of the posterior mucosa is made just below the cricoarytenoid joints; and the submucosal fibrosis, constituting the posterior aspect of the subglottic stenosis, is fully resected, thereby exposing the cricoid plate completely. As the luminal diameter of the distal airway is always larger than that of the subglottis, the first normal tracheal ring used for the anastomosis must be adapted to the size of the subglottic lumen. In adults, this is best achieved by plicating the membranous trachea to accommodate the differences in diameter. In children, the discrepancy in luminal diameter between the subglottic space and the tracheal stump is even more pronounced than it is in adults. Any attempt to reduce the caliber of the trachea should be avoided. Instead, one should enlarge the subglottic lumen as much as possible without compromising voice quality. This is best achieved by widening the cricoid plate posteriorly and laterally with a diamond burr and by performing an inferior midline thyrotomy up to the level of the anterior commissure of the larynx without transecting it. Because the thyroid cartilage is usually soft and pliable in infants and children, the inferior margins of both thyroid alae are easily spread apart. In this way, the subglottic lumen is enlarged considerably while the anterior commissure is kept intact, thus preserving a good voice. The triangular defect is filled in with a mucosa-lined cartilaginous wedge that is obtained from the first normal tracheal ring below the resected stricture. This requires additional resection of the lateral portion of the first normal tracheal ring used for the anastomosis.
Unfortunately, this technique is not feasible in adults, whose thyroid cartilage is often calcified or even ossified. The denuded cricoid plate is covered with the membranous trachea after its upward mobilization. Interrupted Vicryl sutures (4.0 or 3.0 in adults, 6.0 or 5.0 in children) are used for the posterior anastomosis, with the knots tied inside the lumen. The disadvantage of having a few sutures tied inside the lumen posteriorly is largely compensated for by the optimal approximation of the mucosa, which is difficult to obtain with the knots tied outside the lumen. At this stage, tension-releasing sutures are also placed bilaterally between the third or fourth tracheal ring laterally and the inferior border of the cricoid plate. Fibrin glue is used to secure the membranous trachea to the cricoid plate. Vicryl sutures (2.0 in adults, 3.0 or 4.0 in children) are used for the anterior and lateral anastomosis. The first stitch is passed through the posterolateral aspect of the first normal tracheal ring and through the cricoid plate laterally. It should emerge in a subperichondrial plane from the outer surface of the cricoid plate to avoid any lesion to the recurrent laryngeal nerves. This stitch is extremely important and should be placed as meticulously as possible to bring the mucosa of the subglottis in close contact with the mucosa of the trachea. The thyrotracheal anastomosis is then completed by placing the Vicryl sutures between the tracheal ring and the thyroid cartilage anteriorly, with the knots tied on the outside.
At the end of the procedure, the neck is maintained in a flexed position. It is not necessary to place sutures from the chin to the chest to limit extension of the neck during the postoperative period. Motivated patients receiving clear explanations maintain their neck flexed for a period of 10 days without any difficulty.
10.6.3.1 Single-Stage Versus Double-Stage PCTR
If the patient is fit for single-stage surgery, two options usually exist, depending on the location of the tracheostoma. Single-stage PCTR with preoperative resection of the tracheostoma is chosen if no more than five tracheal rings must be resected with the subglottic stenosis. The absence of a postoperative tracheostoma is highly favorable for healing of the anastomosis, but longer tracheal resections carry greater risk of anastomotic dehiscence.
If the location of the tracheostoma requires resection of six or more tracheal rings, or if for anatomical reasons mobilization of the tracheal stump is difficult, it is preferable to use a steady, normal ring (situated between the SGS and the upper margin of the tracheostoma) for the anastomosis and to close the tracheostoma separately or keep it into the postoperative period. In some cases, owing to proximal damage of the trachea, insufficient steadiness of the upper tracheal rings prevents their use for the anastomosis. A longer tracheal resection must then be envisaged with a laryngeal and/or hilar release procedure.
10.6.4 Extended PCTR for Subglottic Stenosis Combined with Glottic Pathologies
Initially used for purely subglottic stenosis, partial cricotracheal resection with primary thyrotracheal anastomosis has proved to be efficient also for the cure of combined subglottic and glottic pathologies: posterior glottic stenosis, cicatricial fusion of the vocal cords, anterior glottic web extending into the subglottis, combined supraglottic, glottic, and suglottic scarring, and distortion of the larynx after failed LTR (Fig. 10.11). The surgical procedure (extended PCTR) is then modified as follows. A complete laryngofissure is created. In adults, the scar tissue constituting the posterior glottic stenosis is removed; and, if necessary, the transverse interarytenoid muscle is sectioned in the midline. Most often, a posterior cricoid split with costal cartilage grafting is not necessary because the interarytenoid distance is large enough in adults. On the contrary, the latter procedure is almost always necessary in children. The two parts of the posterior cricoid plate are then sufficiently distracted to allow correct positioning of the costal cartilage harvested from the sixth or seventh rib. The graft must fit flush between the divided posterior cricoid laminae with the perichondrium facing the lumen. Lateral flanges of perichondrium on the luminal side and small cartilaginous extensions of the graft under the cricoid plate stabilize the graft, which is fixed in place with 4.0 Vicryl sutures. In both adults and children, a pedicled flap of membranous trachea is created by resecting one or two additional rings of the tracheal stump distally.
Extended PCTR. (a) PCTR is performed according to the conventional technique. A temporary midline thyrotomy gives access to the cricoid plate. A posterior midline incision of the cricoid plate is made, and a costal cartilage graft is interposed between the divided cricoid laminae (blue arrow). (b) A pedicled flap of membranous trachea is obtained by removing one or two more rings of the tracheal stump distally. This allows delineation of the anterior cartilaginous wedge that will be used to fill in the triangular defect resulting from the inferior midline thyrotomy. (c) The trachea is advanced upward, and its membranous portion is sutured to the mucosa of the posterior commissure of the larynx. The lateral and anterior anastomosis is completed as in conventional PCTR with a pedicled wedge of tracheal cartilage filling in the triangular defect of the inferior midline thyrotomy. Precise repositioning of the anterior commissure is essential to preserve a good voice when suturing the alae of the thyroid cartilage together
The trachea is then advanced upward, and its membranous portion is sutured with 5.0 Vicryl stitches to the mucosa of the posterior commissure of the larynx. The lateral and anterior anastomosis is completed as for conventional PCTR using 3.0 or 4.0 Vicryl sutures. A fully mucosalized anastomosis is thus obtained. Closure of the laryngofissure over a nasotracheal tube or a stent is performed meticulously by placing a Vicryl suture exactly at the level of the vocal cords to restore a sharp anterior commissure. In adults, stenting is not usually necessary. In children, we currently use an LT-Mold that conforms to the inner laryngeal contours to restore it as close to a normal laryngotracheal airway as possible.
The cartilaginous wedge pedicled to the tracheal ring used for the anastomosis is inserted between the alae of the thyroid cartilage inferiorly to enlarge the subglottic lumen without compromising voice quality in children. Next, the isthmus of the thyroid gland is resutured in the midline, over the anastomosis, to optimize the vascular supply.
10.6.5 Tracheal Resection with End-to-End Anastomosis
Tracheal resection with an end-to-end anastomosis is basically identical to that described for PCTR, but the initial dissection is carried out only up to the level of the cricoid cartilage unless a laryngeal release procedure is envisaged during the course of the surgery [22].
When a tracheostoma is present, hemostats are placed for traction on either side of the ellipse of skin that has been left around the stoma. This helps mobilize the mediastinal portion of the trachea cranially while its dissection is carried out anteriorly and laterally only and in close contact with the cartilaginous rings. At that stage, all the vascular supply coming bilaterally from the tracheal esophageal grooves should be preserved. Before resecting the stenotic segment, pulling on the mediastinal trachea gives a good idea on how much cranial mobilization can be achieved without a laryngeal release procedure. If there is any doubt about the exact location of the upper and lower edges of the intrinsic portion of the stenosis, it is best to incise the trachea transversally through the narrowest portion of the airway and to progress cranially and caudally by slicing the trachea until normal rings are found. The steadiness and quality of the tracheal rings are essential to the success of the surgery. No compromise should be made at this point. If necessary, a laryngeal and/or hilar and pericardial release procedure should be performed with the help of a thoracic surgeon, instead of using unstable tracheal rings for the anastomosis.
Once appropriate tension-free approximation of the trachea has been achieved, only minimal separation of the membranous trachea from the anterior esophageal wall should be performed in order to preserve an optimal vascular supply. The esophagus shortens spontaneously without anterior bulging.
Because the proximal and distal stumps of the trachea are usually well matched, performing the anastomosis is much easier than with PCTR. It is done with 3.0 interrupted Vicryl sutures for the membranous trachea and 2.0 Vicryl sutures for the lateral and anterior anastomosis. All inverted stitches should emerge in a submucosal plane with the knots tied on the outer surface of the trachea. At that stage, lifting the head of the patient helps obtain a tension-free anastomosis. A Penrose drain is placed distal to the site of the anastomosis, after which the thyroid isthmus and strap muscles are resutured on the midline and the skin is closed in two layers.
10.7 Specific Recommendations
This section essentially focuses on resections and anastomoses for subglottic or tracheal stenosis.
10.7.1 Primary Location of the Tracheostoma
Although the primary location of the tracheostoma at the time of acute lesions of intubation may seem straightforward, it is of significant importance for delayed reconstructive surgery.
In case of incipient SGS, the tracheostoma should be placed either between the cricoid and the first tracheal ring or as low as possible in the neck. In the former situation, this allows single-stage PCTR with limited resection of the trachea or single-stage LTR and easy closure of the tracheostoma with the same cartilage graft. In the latter situation, the tracheostoma located at the fifth or sixth tracheal ring always preserves a sufficient number of normal tracheal rings between the inferior border of the SGS and the upper rim of the tracheostoma, thus allowing double-stage PCTR with a short tracheal resection. In case of LTR, the SGS can be treated without too much proximity to the tracheostoma, thus allowing better healing and closure of the tracheostoma during a second stage procedure.
Knowing that the risk of anastomotic dehiscence is proportional to the length of the tracheal resection, correct placement of the tracheostoma is of utmost importance for the future success of reconstructive surgery. In case of incipient tracheal stenosis, the tracheostoma should always be placed through the stenosis, thus avoiding further damage to the normal trachea.
If these basic principles were respected by all surgeons, the outcome of surgery for subglottic and tracheal stenoses would certainly be much better. Improved medical education is of key importance in this field.
10.7.2 Extensive Airway Resection
The usual question that arises is how much trachea can be resected and the anastomosis still safely accomplished. There is no straightforward answer. The permissible length varies with patient’s age, body build, and height and any prior surgery performed. A young adult with a long, supple neck and cranial location of the larynx differs completely from an old, kyphotic patient with the cricoid ring situated at the level of the sternal notch. In the former, 50% of the trachea can certainly be resected and reanastomosed safely, whereas in the latter only a short (less than one-fourth) segment of trachea is amenable to safe reconstruction. Furthermore, cranial mobilization of the mediastinal tracheal stump is unpredictable and varies significantly from one patient to another, even without previous surgery.
Although experimental studies on dogs and human cadavers have been conducted, no clear answer has yet emerged. For the time being, surgical experience shows that approximately one-half of the pediatric or adult trachea may be removed and reconstruction safely made. However, this is where the surgeon’s experience and judgment contribute to the final decision of what is likely to be safely possible in each patient, with or without laryngeal and/or hilar and pericardial release.
10.7.3 Recapture of Tracheal Length
Various techniques of tracheal and supralaryngeal release may be used to diminish the tension on the suture line for PCTR and segmental resection of the trachea [4, 12, 40, 58] (Fig. 10.12). It depends on the length of the tracheal segment to be resected and the individual anatomy. Usually, advancement of the distal tracheal stump upward is much easier in children than it is in adults. If a laryngeal release procedure is necessary, however, it is best performed by sectioning the thyrohyoid muscle at the level of its insertion line on the thyroid cartilage. Then, by pulling the cartilage caudally with a laryngeal hook placed at the thyroid notch, the thyrohyoid membrane can be cut along the upper border of the thyroid cartilage. This leads laterally to its upper cornu, which is then sectioned with a pair of straight Mayo scissors. A laryngeal drop of 1.5-2.0 cm can thus be obtained. A hilar and pericardial release procedure, performed through a sternotomy and a T incision into the left fourth interspace is sometimes necessary in adults but not in children. However, it hugely increases the extent of surgery otherwise planned as a cervicomediastinal procedure. It does provide another 2 cm of length for a tension-free thyrotracheal anastomosis though. It is best to plan this additional surgery with the thoracic surgeon prior to the day of cricotracheal resection. It can be anticipated by a thorough preoperative endoscopic and radiological workup.
Laryngeal release procedure. (a) At the level of the second layer of the strap muscles. The sternohyoid muscle is preserved. The thyrohyoid muscle is cut bilaterally just above its insertion on the thyroid cartilage. Then, the thyrohyoid membrane is cut in the midline and along the upper edge of the thyroid cartilage, leading laterally to the upper cornu, which is cut bilaterally with a pair of straight Mayo scissors. (b) Result of the laryngeal drop: a distance of 1.5-2.0 cm has been obtained
10.8 Postoperative Care and Follow-Up
10.8.1 Laryngotracheal Reconstruction
Patients with long-standing tracheotomies may be colonized with Pseudomonas aeruginosa or Staphylococcus aureus— hence the importance of the preoperative bacteriological smear.
Appropriate antibiotics are given until the subglottic airway is fully healed. If reflux is present, proton pump inhibitors are continued for up to 6 months postoperatively.
Patients with single-stage LTRs are kept intubated without paralysis for 7-14 days, depending on the type of reconstruction (anterior graft versus posterior graft). Control endoscopy is mandatory on the day of extubation to ensure proper incipient healing of the reconstructed airway and again at 3 months if clinically the patient shows no sign of upper airway obstruction.
With double-stage LTRs, tracheotomized patients may return to the ward on the first operative day as the parents are already familiar with care of the tracheotomy cannula. The first control endoscopy is done at the time of stent removal and then 3 weeks later. Plugging the cannula early after stent removal gives information on the patency of the reconstructed airway above the tracheostoma. If for some reason (e.g., suprastomal collapse) this is not possible, a control endoscopy should be scheduled after 10 days to ensure that no incipient subglottic stenosis is redeveloping. When the subglottis is fully healed and stable, downsizing the cannula over a period of several days facilitates eventual closure of the tracheostoma.
10.8.2 Partial Cricotracheal Resection and Segmental Tracheal Resection
Postoperative management after single-stage PCTR or segmental tracheal resection is more challenging in children, whose airways are much smaller than those of adults.
Nontracheotomized children stay under close supervision in the intensive care unit until extubation is achieved. Broad-spectrum antibiotics and antireflux medication are given to all patients until a mucosalized anastomosis is obtained. Proton pump inhibitors are continued postoperatively over a period of up to 6 months. Corticosteroids are started on the day prior to extubation and are continued for the following days if necessary. Depending on the child’s age, a first control endoscopy is performed at 5, 7, or 10 days postoperatively. If there is only slight to moderate edema of the vocal folds and subglottis, the child is tentatively extubated. Because of its very low viscosity, heliox (a mixture of helium and oxygen) is sometimes used to diminish the inspiratory stridor resulting from postoperative laryngeal edema. In the case of significant edema, the child is reintubated with a one-size-smaller tube, and a plug of corticosteroid-gentamicin ointment is applied to the endolarynx. The next tentative extubation is planned for 2 days later. Additional endoscopic controls are routinely performed at 3 weeks and 3 months. The final result may then be optimized at 3 months by gentle bougienage with Savary-Gilliard dilators.
If a double-stage PCTR is performed without stenting, no clinical information on subglottic airway patency is available as the child breathes through the tracheostoma. A control endoscopy at the third postoperative week is then mandatory to assess the quality of healing at the site of the anastomosis. To salvage a suboptimal result (i.e., incipient restenosis), a laryngeal stent (LT-Mold) should be placed endoscopically.
With extended PCTRs and double-stage PCTRs with stenting, the treacheostoma is left in place until the subglottic anastomosis is completely healed. Stenting is usually necessary for about 3 weeks. However, depending on the complexity of the reconstruction after LTR or extended PCTR, stenting is sometimes maintained for up to 6 months or longer, especially after reconstruction of distorted larynges resulting from previous failed LTRs.
10.9 Results
10.9.1 Pediatric LTR and PCTR
Results from the most experienced centers [8, 43, 44] show a decannulation rate of just under 90% for grades I and II SGS following LTR. However, it drops to around 80% for grade III and to 50% or less for grade IV SGS, implying the need for revision surgery in a significant number of cases [8, 43, 44]. From the six international centers that published their results on pediatric PCTR for severe (grades III and IV) SGS, a global decannulation rate of 96% (258/269 cases) was achieved [2, 37, 49, 53-[55]. Looking at these results in a more detailed manner, the two centers with the largest experience in PCTR [37, 55] showed a decannulation rate of 98% (56/57 cases) for primary surgery and 93% (70/75 cases) for salvage surgery after failed previous airway reconstruction (unpublished results on 86 cases from Lausanne).
Extended PCTRs for SGS with glottic involvement showed an interesting decannulation rate of 91% (42/46 cases), but 11 of 25 (44%) patients in the Cincinnati group and 6 of 21 (29%) patients in the Lausanne group required more than one open procedure to achieve these results (Table 10.5). This reflects the challenge of treating some of these extremely complex cases that have undergone several prior open surgeries.
In a recent publication on the long-term outcome of 57 PCTRs with a median follow-up of 5.1 year (minimum follow-up of 1 year), 55 of the 57 (96%) patients were decannulated [27]. Only one patient had moderate exertional dyspnea, and all other patients could participate in sports without restriction. Voice quality was found to improve after PCTR of 1.0 ± 1.34 grade, according to the GRBAS grading system.
10.9.2 Adult PCTR
The overall results in the international literature published from 1972 to 2000 [10, 20, 21, 31, 45] on 249 patients in the adult age group were similar to those of the pediatric population, with a 95% (237/249 cases) success rate and a 1% (2/249 cases) mortality rate.
10.9.3 Segmental Tracheal Resection
Grillo’s large series of 503 patients who underwent segmental tracheal resection for postintubation stenosis shows a 94% (471/503 cases) good to satisfactory postoperative result [22]. The latter was classified as good if the patient could play sports without exertional dyspnea, and satisfactory if the patient could perform normal activities but was stressed on exercise. These results are in accordance with smaller reported series of tracheal resections with end-to-end anastomosis in adults [5, 11, 47]. Failure occurred in 20 (3.9%) and death in 12 (2.4%) of the cases.
10.10 Tips and Pearls to Avoid Complications
-
Perform a thorough preoperative assessment of the patient’s medical condition.
-
Establish a precise endoscopy report on extralaryngeal sites of obstruction, vocal fold mobility and site, extent, and degree of airway stenosis.
-
Address only mature cicatricial stenosis for a definitive endoscopic or open surgical treatment.
-
Do workup studies for gastroesophageal reflux.
-
Carefully respect the indications for the management of each type of stenosis and for single-stage or double-stage procedures.
-
Use meticulous technique for LTR, PCTR, and segmental tracheal resection.
-
Follow up your patient carefully during the postoperative period.
-
Remember that inappropriate initial management of LTS may lead to permanent intractable sequelae and that the best chance for the patient lies in the first operation.
References
Aboulker P, Sterkers JM, Demaldent JE, Sauton P (1966) Modifications apportées à l’intervention de Rethi. Intérêt dans les sténoses laryngo-trachéales et trachéales. Ann Otolaryngol Paris 83:98-106
Alvarez-Neri H, Penchyna-Grub J, Porras-Hernandez JD, Blanco-Rodriguez G, Gonzalez R, Rutter MJ (2005) Primary cricotracheal resection with thyrotracheal anastomosis for the treatment of severe subglottic stenosis in children and adolescents. Ann Otol Rhinol Laryngol 114:2-6
Benjamin B (1993) Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification and treatment. Ann Otol Rhinol Laryngol 160:S1-15
Biller HF, Munier MA (1992) Combined infrahyoid and inferior constrictor muscle release for tension-free anastomosis during primary tracheal repair. Otolaryngol Head Neck Surg 107:430-433
Bisson A, Bonnette P, El Kadi B (1992) Tracheal sleeve resection for iatrogenic stenoses. J Thorac Cardiovasc Surg 104: 882-887
Calhoun KH, Deskin RW, Bailey BJ (1988) Near-fatal complication of tracheal T-tube use. Ann Otol Rhinol Laryngol 97: 542-544
Cotton RT (2000) Management of subglottic stenosis. Otolaryngol Clin North Am 33:111-130
Cotton RT, Gray SD, Miller RP (1989) Update of the Cincinnati experience in laryngotracheal reconstruction. Laryngoscope 99:1111-1116
Cotton RT, Myer CM 3rd, O’Connor DM, Smith ME (1995) Pediatric laryngotracheal reconstruction with cartilage grafts and endotracheal tube stenting: the single stage approach. Laryngoscope 105:818-821
Couraud L, Brichon PY, Velly JF (1988) The surgical management of inflammatory and fibrous laryngotracheal stenosis. Eur J Cardiothorac Surg 2:410-415
Couraud L, Jongon JB, Velly JF (1995) Surgical treatment of non tumoral stenosis of the upper airway. Ann Thorac Surg 60:250-260
Dedo HH, Fishman NH (1969) Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 78:285-296
Dedo HH, Sooy CD (1984) Endoscopic laser repair of posterior glottic, subglottic and tracheal stenosis by division or micro-trapdoor flap. Laryngoscope 94:445-450
Duncavage JA, Ossoff RH, Toohill RJ (1985) Carbon dioxide laser management of laryngeal stenosis. Ann Otol Rhinol Laryngol 94:565-569
Eckel HE, Wittekindt C, Klussmann JP, Schroeder U, Sittel C (2003) Management of bilateral cricoarytenoid cartilage fixation versus recurrent laryngeal nerve paralysis. Ann Otol Rhinol Laryngol 112:103-108
Eliachar R, Eliachar I, Esclamado R, Gramlich T, Strome M (1999) Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope 109:1594-1600
Eliachar I, Nguyen D (1990) Laryngotracheal stent for internal support and control of aspiration without loss of phonation. Otolaryngol Head Neck Surg 103:837-840
Evans JNG, Todd GB (1974) Laryngotracheoplasty. J Laryngol Otol 87:589-597
Froehlich P, Truy E, Stamm D (1993) Role of long-term stenting in treatment of pediatric subglottic stenosis. Int J Pediatr Otorhinolaryngol 27(3):273-280
George M, Lang F, Pasche Ph, Monnier P (2005) Surgical management of laryngotracheal stenosis in adults. Eur Arch Otorhinolaryngol 262:609-615
Gerwat J, Bryce DP (1974) The management of subglottic stenosis by resection and direct anastomosis. Laryngoscope 84:940-947
Grillo HC, Donahue DM, Mathisen DJ (1995) Postintubation tracheal stenosis. Treatment and result. J Thorac Cardiovasc Surg 109:486-493
Grillo HC, Mathisen Dj, Wain JC (1992) Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 53:54-63
Holinger LD (1980) Etiology of stridor in the neonate, infant, and child. Ann Otol Rhinol Laryngol 89:397
Holinger LD. Congenital laryngeal anomalies. In: Pediatric laryngology and bronchoesophagology,. Philadelphia, PA/New York: Lippincott-Raven; 1997
Holinger LD, Green CG, Benjamin B, Shorp JK (1997) Tracheobronchial tree. In: Holinger LD, Lusk RP, Green CG (eds) Pediatric laryngology and bronchoesophagology. Lippincott-Raven, Philadelphia, PA/New York
Jaquet Y, Lang F, Pilloud R, Savary M, Monnier P (2005) Partial cricotracheal resection for pediatric subglottic stenosis: long-term outcome in 57 patients. J Thorac Cardiovasc Surg 130(3):726-732
Johnson L, Cotton RT, Rutter MJ (2003) Airway stenosis and eosinophilic esophagitis. Abstract presented at Annual Meeting of the American Academy, May 3, Nashville, TN
Lang F, Pasche P, Monnier P (2001) Sténoses laryngotrachéales. Encycl Med Chir Pneumologie 6-035-A-20:1-20. Elsevier, Paris
Macchiarini P, Verhoye JP, Chapelier A, Fadel E, Dartevelle P (2001) Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation subglottic stenosis. J Thorac Cardiovasc Surg 121:68-76
Maddaus MA, Toth JL, Gullane PJ, Pearson FG (1992) Subglottic tracheal resection and synchronous laryngeal reconstruction. J Thorac Cardiovasc Surg 104:1443-1450
McCaffrey TV (1992) Classification of laryngotracheal stenosis. Laryngoscope 102:1335-1340
McQueen CT, Shapiro NL, Leighton S, Guo XG, Albert DM (1999) Single-stage laryngotracheal reconstruction: the Great Ormond Street experience and guidelines for patient selection. Arch Otolaryngol Head Neck Surg 125:320-322
Monnier Ph (2003) A new stent for the management of adult and pediatric laryngotracheal stenosis. Laryngoscope 113: 1418-1422
Monnier Ph (1999) Complications laryngées et trachéales après intubation et trachéotomie. In: Cros AM, Bourgain JL, Ravussin P (eds) Les voies aériennes: leur contrôle en anesthésie - réanimation. Pradel Revil-Malmaison, France
Monnier Ph, George M, Monod ML, Lang F (2005) The role of the CO2 laser in the management of laryngotracheal stenosis: a survey of 100 cases. Eur Arch Otorhinolaryngol 262: 602-608
Monnier P, Lang F, Savary M (2003) Partial cricotracheal resection for pediatric subglottic stenosis: a single institution’s experience in 60 cases. Eur Arch Otorhinolaryngol 260:295-297
Monnier P, Savary M, Chappuis G (1993) Partial cricoid resection with primary tracheal anastomosis for subglottic stenosis in infants and children. Laryngoscope 103:1273-1283
Montgomery W (1965) T-tube tracheal stent. Arch Otolaryngol 82:320-321
Montgomery WW (1974) Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 99:255-260
Morimitsu T, Matsumoto I, Okada S, Takahashi M, Kosugi T (1981) Congenital cricoid stenosis. Laryngoscope 91:1356-1364
Myer CM, O’Connor DM, Cotton RT (1994) Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 103:319-323
Ndiaye I, Van de Abbeele T, François M, Viala P, Tanon-Anoh MJ, Narcy P (1999) Traitement chirurgical des sténoses laryngées de l’enfant. Ann Otolaryngol Chir Cervicofac 116: 143-148
Ochi JW, Evans JNG, Bailey CM (1992) Pediatric airway reconstruction at Great Ormond Street: a 10-year review. Ann Otol Rhino Laryngol 101:465-468
Ogura JH, Roper CL (1972) Surgical correction of traumatic stenosis of the larynx and pharynx. Laryngoscope 72:468-470
Pearson FG, Gullane P (1996) Subglottic resection with primary tracheal anastomosis including synchronous laryngotracheal reconstruction. Semin Thorac Cardiovasc Surg 4: 381-391
Pearson FG, Andrews MJ (1971) Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg 12:359-374
Rahbar R, Shapsay SM, Healy GB (2001) Mitomycin: effects on laryngeal and tracheal stenosis, benefits and complications. Ann Otol Rhinol Laryngol 110:1-6
Ranne RD, Lindley S, Holder TM, Ashcraft KW, Sharp RJ, Amoury RA (1991) Relief of subglottic stenosis by anterior cricoid resection: an operation for the difficult case. J Pediatr Surg 26:255-259
Rutter MJ, Hartley BEJ, Cotton RT (2001) Cricotracheal resection in children. Arch Otolaryngol Head Neck Surg 127:289-292
Shapshay SM, Beamis JF, Hybels RL (1987) Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol 96: 661-664
Simpson GT, Strong MS, Healy GB, Shapshay SM, Vaughan CW (1982) Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol 91:384-388
Triglia JM, Nicollas R, Roman S (2001) Primary cricotracheal resection in children: indications, technique and outcome. Int J Pediatr Otorhinolaryngol 58:17-25
Vollrath M, Freihorst J, von der Hardt H (1999) Die Chirurgie der erworbenen laryngotrachealen Stenosen im Kindesalter. Erfahrungen und Ergebnisse von 1988-1998. Teil II: Die cricotracheale Resektion. HNO 47:611-622
White DR, Cotton RT, Bean JA, Rutter MJ (2005) Pediatric cricotracheal resection. Surgical outcomes and risk factor analysis. Arch Otolaryngol Head Neck Surg 131:896-899
Yellon RF, Szeremeta W, Grandis JR, Diguisseppe F, Dickman PS (1997) Role of subglottic injury, gastric juice and peptide growth factors in a porcine model. Int Anesthesiol Clin 35:115-125
Zalzal GH (1988) Use of stents in laryngotracheal reconstruction in children: indications, technical considerations, and complications. Laryngoscope 98:849-854
Zitch RP III, Mullins JB, Tampler J, Davis WE (1995) Suprahyoid and inferior constrictor release for laryngeal lowering. Arch Otolaryngol Head Neck Surg 121: 1310-1313
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Monnier, P. (2009). Subglottic and Tracheal Stenosis. In: Remacle, M., Eckel, H. (eds) Surgery of Larynx and Trachea. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-79136-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-540-79136-2_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-79135-5
Online ISBN: 978-3-540-79136-2
eBook Packages: MedicineMedicine (R0)