Abstract
Cells, including endothelial cells, continuously sense their surrounding environment and rapidly adapt to changes in order to assure tissues and organs homeostasis. The extracellular matrix (ECM) provides a physical scaffold for cell positioning and represents an instructive interface allowing cells to communicate over short distances. Cell surface receptors of the integrin family emerged through evolution as essential mediators and integrators of ECM-dependent communication. In preclinical studies, pharmacological inhibition of vascular integrins suppressed angiogenesis and inhibited tumor progression. αVβ3 and αVβ5 were the first integrins targeted to suppress tumor angiogenesis. Subsequently, additional integrins, in particular α1β1, α2β1, α5β1, and α6β4, emerged as potential therapeutic targets. Integrin inhibitors are currently tested in clinical trials for their safety and antiangiogenic/antitumor activity. In this chapter, we review the role of integrins in angiogenesis and present recent advances in the use of integrin antagonists as potential therapeutics in cancer and discuss future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumor Angiogenesis
- Isolation Limb Perfusion
- Antiangiogenic Activity
- Angiogenic Vessel
- Integrin Antagonist
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Integrin Structure
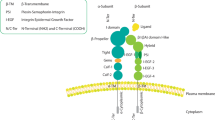
Integrins comprise a family of cell surface heterodimeric complexes formed by the noncovalent association of two subunits, α and β (Takada et al. 2007). There are 18 α and 8 β subunits capable of forming 24 different functional heterodimers. The αβ composition of the heterodimer largely determines the ligand specificity, although some ligands can bind directly to individual subunits, such as collagens, to the I-domain on the α subunit. Each individual subunit consists of a large extracellular domain (about 750 amino acids for the β subunits and around to 1000 amino acids for the α subunits), a single transmembrane domain (22–24 amino acids), and a short cytoplasmic tail (15–58 amino acids, except for the β4 subunit, which contains over 1000 intracellular residues). The cytoplasmic domain is essential for the regulation of integrin activity and function: on the one side it controls extracellular ligand-binding activity of the complex (“inside-out” signaling), while on the other, it initiates cellular responses upon ligand binding (“outside-in” signaling) (Ginsberg et al. 2005). In resting, nonligated integrins, the β subunit cytoplasmic domain interacts with the α subunit cytoplasmic domain, thereby maintaining the receptor in its inactive state (Luo et al. 2007). Binding of the cytoplasmic protein talin to the β subunit cytoplasmic domain disrupts this interaction, resulting in a conformational change of the extracellular domain leading to a high affinity ligand-binding state (affinity maturation). The “released” β cytoplasmic tail interacts with additional intracellular structural (e.g., paxillin, vinculin), adaptor (e.g., Shc, Cas), and signaling (e.g., FAK, ILK) proteins, thereby initiating cytoskeletal rearrangement and cell signaling events. Ligated integrins can cluster to form small focal contacts at the cell periphery, large focal adhesions retracted from the cell border, or fibrillar adhesions located underneath the cell body along actin stress fibers (Romer et al. 2006).
Integrin Functions
Cell Adhesion
Integrins are the main cell adhesion receptors for ECM proteins for virtually every cell, including endothelial cells (Hynes 2007). A particular feature of integrins is their ability to recognize short amino acid sequences on exposed loops of their cognate ligands, the tripeptide RGD being the best known and studied. In addition, integrins also bind matricellular proteins, such as thrombospondins, and cell surface molecules, such as ICAMs (for a comprehensive detailed list of ligands, see (Takada et al. 2007)). Ligand binding specificity is promiscuous and redundant: that is, one integrin can bind several different ligands, and many different integrins can bind to the same ligand. Redundancy may be an advantage when the cellular response needed in a particular context (e.g., survival or migration during matrix remodeling) is more important than the nature of the ECM protein eliciting. For example integrin αVβ3 binds to many ECM proteins present at sites of inflammation, coagulation, and tissue remodeling. Promiscuity may reflect the need to initiate different signaling events and cellular responses from the same ECM. For example, integrin α5β1 and αVβ6 bind to fibronectin, but α5β1 suppresses cell migration, while αVβ6 stimulates it (Coutifaris et al. 2005; Scott et al. 2004). More recently, integrins have been reported to bind to a multitude of noncanonical ligands, which themselves are known modulators of vascular functions, including VEGF (Vlahakis et al. 2007), FGF (Murakami et al. 2008), angiopoietins (Camenisch et al. 2002), or matrix-bound VEGFR-1 (Orecchia et al. 2003). These observations open the intriguing possibility that angiogenic growth factors, when associated to the ECM, may modulate endothelial cell functions by signaling through integrins in complement to their activities mediated by their canonical receptors.
Cell Signaling
Integrin ligation also initiates signaling cascades, modulating complex cell functions like spreading, migration, survival, proliferation, or differentiation (Alghisi and Ruegg 2006; Stupack 2007) (Fig. 6.1). As integrins do not have intrinsic enzymatic activity, they need to recruit cytoplasmic structural (e.g., α-actinin, talin, vinculin) and signaling (e.g., FAK, paxillin, and Src family kinases) proteins at adhesion complexes to initiate signal transduction (Luo et al. 2007; Romer et al. 2006). Many signaling pathways activated by integrins are also activated by growth factor receptors, and maximal signal transduction is achieved when integrins and growth factor receptors are concomitantly engaged. Signaling pathways activated by integrins, including in angiogenesis, comprise: MAPK, Akt/PKB, Rho family GTPases, and NF-κB (Mahabeleshwar et al. 2006).
Integrin functions and how to inhibit them. Integrins act as cell adhesion and motility mediators (green arrows) or as signal transducers (red arrows). These functions can be modulated by growth factors and their receptors (green and red dashed arrows). On one hand, the inhibition or integrin function can be achieved extracellularly by the action of peptidomimetics (a), peptides, most frequently RGD-based, but also as noncanonical peptides (b) or antibodies (c). These three classes of inhibitors could also be used as imaging tools if they are labeled with a detectable tracers. On the other hand, peptides disrupting or blocking the interaction between the β integrin cytoplasmic tail with cytoplasmic adaptor or signaling proteins inhibit integrin function and may be developed in the future as therapeutic tools (d)
A pathway activated by integrin, particularly relevant to vascular biology and angiogenesis, is the COX-2/prostaglandin pathway. Integrin-mediated adhesion and binding of soluble ligands induce COX-2 mRNA expression and stabilize COX-2 protein in endothelial cells resulting in enhanced prostaglandin production (Zaric and Ruegg 2005). In turn, prostaglandins activate the adenylcyclase via prostane receptor signaling, resulting in PKA activation, accelerated αVβ3-dependent cell adhesion, spreading, and migration in a Rac1-dependent manner (Dormond and Ruegg 2003). Consistent with these findings, COX-2 inhibitors inhibit αVβ3-dependent endothelial cell spreading and migration in vitro and angiogenesis in vivo.
Integrins in Tumor Angiogenesis
-
Integrin αVβ3 was the first integrin associated with angiogenesis (Brooks et al. 1994). αVβ3 is highly expressed in angiogenic endothelial cells in granulation tissue and in malignant tumors, but is virtually absent from quiescent endothelial cells (Hynes 2007). Inhibition of αVβ3 with a function-blocking monoclonal antibody, or RGD-based peptides, or peptidomimetics suppressed corneal neovascularization (Klotz et al. 2000), hypoxia-induced retinal neovascularization (Hammes et al. 1996), tumor angiogenesis, and tumor progression in various in vivo models (MacDonald et al. 2001; Reinmuth et al. 2003), and endothelial cell sprouting and angiogenesis in an in vitro 3D model of angiogenesis (Nisato et al. 2004). Importantly, quiescent and pre-existing vessels were not perturbed by these treatments. The results obtained with pharmacological antagonists of integrins αVβ3/αVβ5 contrast with results obtained through genetic approaches. Mice deficient in αV integrins and lacking αVβ1, αVβ3, αVβ5, αVβ6, and αVβ8 expression, were still able to undergo extensive developmental vasculogenesis and angiogenesis, although they died in utero or shortly after birth (Bader et al. 1998). Analysis of the phenotype of individual β integrin knock-out mice showed that the β8 knock-out was the only one to reproduce the αV knock-out phenotype (Zhu et al. 2002), thereby revealing a role for αVβ8 in the association between cerebral microvessels and brain parenchymal cells. Deletion of the β3 subunit did not significantly disrupt vascular development, although some embryos died in utero due to placenta defects, while others died postnatally due to bleeding and anemia (Hodivala-Dilke et al. 1999). β3-deficiency reproduce the inherited human bleeding disorder known as Glanzmann thrombasthenia due to the concomitant lack of αIIbβ3 (Tomiyama 2000). Paradoxically, mice lacking αVβ3 integrins had enhanced pathological angiogenesis, including tumor angiogenesis (Reynolds et al. 2002), associated with enhanced VEGFR-2 signaling (Reynolds et al. 2004). The reason for the divergence of the results obtained with pharmacological inhibition vs. genetic deletion of αVβ3 are not yet fully clear (Hynes 2002).
-
More recently, β1 integrins (i.e., α1β1, α2β1, α3β1, α4β1, α5β1, αVβ1), αVβ8, and α6β4 have also been shown to promote angiogenesis (Alghisi and Ruegg 2006; Serini et al. 2006). β1 integrin expression on vascular endothelial cells is dispensable for vasculogenesis, but crucial for embryonic angiogenesis (Tanjore et al. 2007). Deletion of the α5 gene is embryonically lethal and is associated with vascular and cardiac defects (Francis et al. 2002). α5β1 is up regulated in angiogenesis and blocking anti-α5 antibodies suppressed VEGF-induced tumor angiogenesis in both chick embryo and murine models (Collo and Pepper 1999; Kim et al. 2000). An α5β1 antagonist in combination with chemotherapy reduced metastasis and suppressed angiogenesis at metastatic lesions (Stoeltzing et al. 2003).
-
Integrins α1β1 and α2β1 are highly upregulated by VEGF in cultured endothelial cells, resulting in enhanced cell spreading on collagen I, while anti-α1β1 and anti-α2β1 antibodies inhibited VEGF-driven angiogenesis in vivo. Combined administration of anti-α1β1 and anti-α2β1 antibodies to mice bearing squamous cell carcinoma xenografts, resulted in reduced tumor angiogenesis and tumor growth (Hong et al. 2004; Perruzzi et al. 2003; Senger et al. 2002). The role of α2β1 for the regulation of murine wound angiogenesis was confirmed in a genetic approach (Zweers et al. 2007).
-
α6β4 promotes an invasive endothelial cell phenotype at the early phase of angiogenesis in response to growth factors (FGF-2, VEGF) (Nikolopoulos et al. 2004). Genetic studies have revealed that α6β4 signaling promotes both angiogenesis and tumorigenesis. Importantly, α6β4 combines with multiple receptor tyrosine kinases, including ErbB2, EGF-R and c-Met, and enhances their signaling function (Giancotti 2007).
Integrin Antagonists with Antiangiogenic Activities
Four different types of integrin antagonists have been developed: antibodies, endogenous inhibitors, peptides, and nonpeptidic antagonists. We describe here the main representative drugs within each class that have shown antiangiogenic activity in preclinical models, with particular emphasis on drugs that entered clinical testing. These and additional inhibitors are summarized in Table 6.1.
Antibodies
-
LM609/MEDI-522/Vitaxin. The anti-αVβ3 monoclonal antibody LM609 blocked endothelial cell adhesion, migration, and sprouting in vitro and angiogenesis in vivo in the CAM assay (Brooks et al. 1994). Subsequently, LM609 was humanized and affinity maturated allowing the isolation of an antibody with a 90-fold improved affinity (MEDI-522 or Vitaxin) (Wu et al. 1998). Phase I studies demonstrated that treatment was well tolerated with little or no toxicity. The most common side effect was infusion-related fevers. Doses of 1 mg/kg/week or more produced plasma concentrations sufficient to saturate αVβ3 in vitro. Vitaxin demonstrated a half-life longer than five days with no tendency to accumulation. One partial response and several stable diseases were observed (Posey et al. 2001). Combination of Vitaxin with chemotherapy was well tolerated. There was possible effect on tumor perfusion detected by dynamic computed tomography imaging, but no objective antitumor responses (McNeel et al. 2005). In treated patients, there was evidence of reduced FAK phosphorylation in skin wound vessels, consistent with inhibition of αVβ3 signaling (Zhang et al. 2007). Vitaxin has entered Phase II trials mostly on hormone-refractory prostate cancers or metastatic melanoma (www.clinicaltrials.gov).
-
CNTO 95. CNTO 95 is a pan anti-αV fully humanized antibody. In a human melanoma xenograft model, wherein CNTO 95 recognized αVβ3 and αVβ5 on human tumor cells but not mouse cells, CNTO 95 treatment inhibited tumor growth by 80%. In a nude rat, human skin xenograft tumor model where CNTO 95 blocks αVβ3 and αVβ5 on both human tumor cells and human skin endothelial cells, treatment reduced final tumor weight by >99% (Trikha et al. 2004). The antibody did not show any adverse effects in monkeys (Martin et al. 2005) and entered Phase I clinical trials in various solid tumors, including ovarian, colorectal, melanoma, and renal cell carcinoma. Results on these patients showed that CNTO 95 was well tolerated up to weekly doses of 10 mg/kg (www.asco.org). CNTO 95 is now in Phase I/II in combination with other chemotherapeutic drugs in Stage IV melanoma or metastatic HRPC.
-
M200/Volociximab. M200 is an affinity maturated humanized chimeric monoclonal antibody blocking α5β1 integrin. It inhibited tumor angiogenesis and tumor growth in a rabbit syngenic tumor model (M200 does not bind to rodent α5β1) despite a 20-fold lower affinity for rabbit integrin, relative to human (Bhaskar et al. 2007a). A function blocking rat-anti-mouse α5β1 antibody with features similar to M200 was shown to inhibit angiogenesis and suppress tumor growth and metastasis in mice (Bhaskar et al. 2007b; Ramakrishnan et al. 2006). Based on this activity profile, Volociximab was tested in Phase I trials in various refractory solid tumors including renal cell carcinoma and metastatic melanoma. The study data showed that adverse events were generally mild to moderate in intensity and there were no dose limiting toxicities. Volociximab is currently evaluated in Phase II trials as a single agent (Kuwada 2007). Combination trials with chemotherapy are planned.
-
c7E3/Abciximab/ReoPro. c7E3 is a humanized monoclonal antibody Fab fragment approved for use as adjunct therapy to prevent cardiac ischemic complications in patients undergoing coronary angioplasty (Cohen et al. 2000). c7E3 also interacts with integrins αVβ3 and Mac-1 (αMβ2). In animal models, c7E3 inhibited tumor growth and angiogenesis (Nakada et al. 2006).
Endogenous Antagonists
-
Endostatin is a carboxyl-terminal fragment of Collagen XVIII inhibiting endothelial cell proliferation in vitro and angiogenesis and tumor growth in vivo (O’Reilly et al. 1997). The generation of endostatin from Collagen XII is mediated by various proteases (e.g., cathepsin L and MMPs). The antiangiogenic activity of endostatin is due, at least in part, to binding to integrin α5β1 and caveolin-1 on endothelial cells, causing downregulation of RhoA activity and Src family kinase-dependent disassembly of focal adhesions and actin stress fibers, resulting in decreased matrix deposition and migration (Wickstrom et al. 2005). Recombinant human endostatin entered clinical testing and was found to be safe and well tolerated (Hansma et al. 2005; Herbst et al. 2002).
-
Tumstatin consists of the carboxyl-terminal noncollagenous 1 (NC1) domain of the α3 chain of Collagen IV. It inhibited in vivo neovascularization in Matrigel plug assays, suppressed tumor growth in xenograft models, and induced endothelial cell apoptosis (Hamano and Kalluri 2005). Tumstatin binds to αVβ3 integrin in endothelial cells, and selectively inhibits protein synthesis by suppressing mTOR (Maeshima et al. 2002).
Peptides
-
EMD121974/Cilengitide. The discovery that many integrins recognize their ligands through short amino acid sequences, most notably RGD, led to the development of small peptides that competitively blocked ligand–receptor interaction. Cyclized peptides were up to 100-fold more selective than linear counterparts, and cyclic pentapeptides that possessed two hydrophobic amino acids next to the recognition sequence proved to be highly active and selective for αVβ3/αVβ5. The cyclic pentapeptide cyclo(-Arg-Gly-Asp-D-Phe-Val-) (EMD66203 Merck KGaA) showed nanomolar inhibition of vitronectin binding to the αVβ3 integrin without interfering with αIIbβ3 integrin (Haubner et al. 1996). Modification of the amino acids flanking the RGD sequence led to the synthesis of EMD121974 (Cilengitide) inhibiting αVβ3 integrin binding to vitronectin with an IC50 of 0.6 nM versus 900 nM for the αIIbβ3 integrin (Smith 2003). Cilengitide showed antitumor effects in brain, melanoma, head and neck, and brain tumors (MacDonald et al. 2001; Mitjans et al. 2000; Raguse et al. 2004; Taga et al. 2002). In Phase I, studies of cilengitide were well tolerated with no dose-limiting toxicities, and showed evidence of activity in recurrent malignant gliomas (Nabors et al. 2007). Cilengitide is now in Phase II clinical trials, alone and in combination with radio and chemotherapies, in solid tumors, leukemia, and lymphoma (Stupp and Ruegg 2007).
-
ATN-161 is a peptide derived from the α5β1-binding sequence PHSRN present in fibronectin (Livant et al. 2000). Chemical modifications of this sequence led to the synthesis of ATN-161 (Ac-PHSCN-NH2) that, in contrast to other integrin antagonist peptides, is not a RGD-based peptide. ATN-161 possesses antitumorigenic antiangiogenic activities in mice in the absence of toxicities (Livant et al. 2000). ATN-161 was tested in a Phase I study in patients with advanced solid tumors for up to 14 cycles of 4 weeks and was well tolerated at all dose levels. Approximately, one-third of the treated patients manifested prolonged stable disease (Cianfrocca et al. 2006).
Non-peptidic Inhibitors
Peptidomimetics are compounds containing non-peptidic structural elements mimicking the action(s) of a natural parent peptide. Peptidomimetics can be administered orally, are insensitive to protease-mediated degradation, and have longer stability (Cacciari and Spalluto 2005).
-
SCH221153 was obtained by screening and further modifying an RGD-based peptidomimetic library. It targets αVβ3 and αVβ5 integrins with IC50 of 3.2 and 1.7 nM, respectively. SCH221153 inhibited endothelial cell adhesion to vitronectin and suppressed angiogenesis in a CAM assay (Kumar et al. 2001).
-
BCH-14661 and BCH-15046 are integrin antagonists that induce cell detachment and apoptosis of angiogenic endothelial grown on RGD-based matrices (i.e., vitronectin and fibronectin). BCH-14661 is specific for αVβ3, while BCH-15046 antagonizes αVβ3, αVβ5 and α5β1 (Meerovitch et al. 2003). BCH-15046 was also capable to induce endothelial cell apoptosis independently of cell detachment.
-
Thiolutin is a non-peptidic antagonist of cell adhesion interfering with integrin-post-receptor events (Minamiguchi et al. 2001). The antiadhesive effect of thiolutin is due to decreased paxillin protein expression, disruption of focal adhesions, and cell detachment.
-
SJ749, which structure mimics RGD-based sequences, is a potent inhibitor of α5β1 integrin (IC50 around 0.8 nM), and it inhibited angiogenesis in the CAM assay (Kim et al. 2000). The structure of this non-peptidic compound bound to the head of the α5β1 integrin has been resolved, thereby opening new perspectives in rational design to improve its specificity and binding constant (Marinelli et al. 2005).
-
JSM6427 is another α5β1-specific peptidomimetic inhibitor with antiangiogenic activities (Umeda et al. 2006). Interestingly, JSM6427 inhibited inflammatory lymphangiogenesis (Dietrich et al. 2007) suggesting the possibility of combined targeting angiogenesis and lymphangiogenesis by targeting one integrin.
Open Questions and Current Developments
Preclinical studies suggest that vascular integrins are valuable targets for antiangiogenic treatments. Results obtained in Phase I clinical trials have shown that the integrin antagonists tested so far are well tolerated and hint some antitumor activity. Phase II trials aimed at demonstrating antiangiogenic/antitumor activities are ongoing (Stupp and Ruegg 2007). Important basic questions on the role of integrins in angiogenesis and their therapeutic targeting, however, have remained unanswered and new ones have emerged. In this section we review some of the open outstanding questions.
Most Relevant Targets
Endothelial cells can express up to 12 integrins (αVβ3, αVβ5, α5β1, α1β1, α2β1, α6β4, α3β1, α4β1, α6β1, α8β1, αVβ1, and αVβ8) (Alghisi and Ruegg 2006). At this point, we do not know which one of these integrins is the best therapeutic target for antiangiogenic treatments, and if angiogenic vessels in different tumors or at different stages of development may use different integrins. More preclinical work is needed to test and compare the suitability of individual integrins as therapeutic target and to evaluate the possibility of combined targeting.
Combination Therapies
Combined treatment with integrin antagonists and chemotherapy or radiotherapy has shown enhanced therapeutic efficacy in preclinical models (Ruegg and Mutter 2007). Since combination therapy appears to be a general rule for antiangiogenic treatments in humans, the critical issue is to define the best combination in term of drug association, timing, and schedule.
-
Radiotherapy. Integrin antagonists enhance efficacy of radiotherapy. Radiation was found to upregulate αVβ3 expression in endothelial cells and to induce activation of Akt/PKB, possibly as a mechanism for the tumor vasculature to escape or recover from radiation-induced injury. Inhibitors of αVβ3 integrin suppressed radiation-induced Akt/PKB phosphorylation, increased cell death and enhanced antiangiogenic and antitumor effects in xenograft models (Abdollahi et al. 2005; Ning et al. 2007). Using a different model, cilengitide sensitized tumors to radioimmunotherapy (Burke et al. 2002). These results reinforce the rationale of combining vascular integrin antagonists with radiotherapy.
-
Chemotherapy. Combination of ATN-161 (α5β1 antagonist) with 5-fluoroacil synergized the reduction of the number of liver metastases and tumor burden of CT26 colon cancer cells in mice (Stoeltzing et al. 2003). Liver tumors in the ATN-161 and ATN-161/5-FU groups had significantly fewer microvessels than tumors in the control or 5-FU-treated groups.
-
Tumor Necrosis Factor (TNF). TNF is used in combination with high dose chemotherapy in an isolation limb perfusion setting to treat advanced cancers of the limbs (Lejeune et al. 2006). The mechanism by which TNF exerts its antitumor activity involves detachment and death of angiogenic endothelial cells expressing αVβ3 (Ruegg et al. 1998). Integrin-mediated adhesion is required for TNF-induced Akt/PKB activation, an event essential for the survival of TNF-stimulated endothelial cells (Bieler et al. 2007). Consistent with these results, cilengitide sensitizes endothelial cells to TNF-induced death in vitro. Thus, combined administration of cilengitide may open new perspectives to the therapeutic use of TNF as anticancer agent.
-
Tyrosine Kinase Inhibitors (TKI). Since integrins facilitate signaling from several receptor tyrosine kinases, including ErbB2, VEGFR-2, EGF-R, and Met, it is reasonable to hypothesize that integrin inhibition may sensitize endothelial cells to currently available TKI antiangiogenic drugs (e.g., bevacizumab, sorafenib, sunitinib, temsirolimus) or to other TKI with antiangiogenic activities, such as EGFR antagonists (e.g., cetuximab or gefitinib), or PDGFRs inhibitors (e.g., Imatinib). Indeed, combined administration of cilengitide and SU5416, a VEGR-2 TKI reduced tumoral vessel density and intratumoral blood flow compared to single drug treatments (Strieth et al. 2006). α6β4 might be an interesting integrin to target in combination with ErbB2, EGFR, and Met inhibitors, since, in addition to antiangiogenic effects, it may also have direct antitumor activity, as α6β4 and ErbB2, EGF-R and Met are expressed on many carcinoma cells (Giancotti 2007). The endogenous antiangiogenic peptide tumstatin was shown to exert direct antitumoral effects in αVβ3, expressing glioma cells in vitro and in vivo by suppressing αVβ3-dependent Akt and mTOR signaling (Kawaguchi et al. 2006), suggesting the possibility that a combination strategy may be chosen in a way to target angiogenic endothelial cells and tumor cells.
Drug Targeting
Vascular integrins expressed on tumoral vessels, such as αVβ3 have been used to target drugs to tumors. Cationic nanoparticles coupled with an integrin αVβ3-targeting ligand were used to deliver a dominant-negative mutant Raf gene to angiogenic blood vessels in tumor-bearing mice, resulting in apoptosis of the tumor vessels and regression of established primary and metastatic tumors (Hood et al. 2002). Paclitaxel (Taxol), an antitumor drug commonly used for the treatment of advanced metastatic breast cancer, conjugated with an RGD-based peptide had a better uptake kinetic in vivo compared to free paclitaxel (i.e., 4 h for free PTX vs. 2 h for the PTX-RGD conjugate), although it did not show enhanced potency at the cellular level(Chen et al. 2005). These experiments demonstrate the feasibility of integrin-based targeted drug delivery to tumors. More recently, several studies reported that conjugation of αVβ3-targeting RGD peptides or peptidomimetics to carrier proteins (e.g., antibody), synthetic scaffold structures, or micelles (e.g., PEG-polyLys-associated with plasmid DNA) resulted in improved pharmacokinetics, retention in the tumor tissue, and cellular uptake (Mitra et al. 2006; Oba et al. 2007; Shin et al. 2007). αVβ3-integrin-targeted nanoparticles rapidly taken up by αVβ3-positive angiogenic vessels and tumors were developed for delivery and imaging purposes (Xie et al. 2007).
Tumor Imaging
Vascular integrins upregulated in angiogenic vessels have also been explored for noninvasive tumor imaging purposes. Most approaches have targeted αVβ3 in combination with positron emission tomography (PET) and Magnetic Resonance Imaging (MRI) imaging techniques (Choe and Lee 2007). The proof of concept experiment was reported already in 1998, where gadolinium-labeled LM609 was used to detect angiogenesis in a rabbit tumor model.
Before this approach be successfully translated into the clinic, however, substantial gains in sensitivity brought about by improved coils, pulse sequences, and contrast agents were needed (Barrett et al. 2007). Thanks to its high sensitivity, PET technology has been preferred and used in animal models and in humans to detect αVβ3 using 18F-labeled monomeric or multimeric RGD peptides. The level of expression αVβ3 detected by PET, correlated with the level of αVβ3 determined by immunohistochemistry, suggesting that this approach may be used for the noninvasive measurement of αVβ3, and monitoring antiangiogenic therapy in patients (Beer et al. 2006). 64Cu-DOTA-labeled Vitaxin (Abegrin) were used in animal models and showed high levels of late tumor activity accumulation (i.e., 71 h) post injection (Cai et al. 2006). Similarly, 99mTc-labeled RGD peptides were used to image tumors and angiogenic vascular beds by gamma camera (Decristoforo et al. 2006) or single photon emission computed tomography (SPECT) in experimental models (Liu et al. 2007). Recently, near-infrared fluorescence imaging coupled with 3D optical imaging systems have been used to image αVβ3-positive tumor vessels and tumor cells in mice using Cy5.5-RGD peptides (Hsu et al. 2006). Taken together, these results illustrate the potential of employing integrin-targeted molecular probes to image tumor vasculature and monitoring response to therapy.
Future Directions
New Generation of Extracellular Antagonists
While most efforts have been focused on the generation of small molecular inhibitors based on the RGD sequence or the ligand-binding pocket, recent studies have reported inhibitors acting in a RGD-independent manner, such as tumstatin (Maeshima et al. 2000) or ATN-161 (Livant et al. 2000). The resolution of the 3D structure of cilengitide-αVβ3 complex (Xiong et al. 2002) and of SJ749-α5β1 complex (Marinelli et al. 2005), allows for the exploration of additional regions of the receptor for binding of novel inhibitory molecules, “in silico” design and virtual screenings of improved or fully novel antagonists (Zhou et al. 2006). Furthermore, “broad spectrum” inhibitors blocking several angiogenic integrins (e.g., β1 and β3) may be developed, as suggested by the recent report of BCH-15046, a αVβ3, αVβ5 and α5β1 antagonist (Meerovitch et al. 2003).
Targeting the Integrin Intracellular Domains
Interaction of the cytoplasmic tail of the β subunit is essential for integrin function (Travis et al. 2003). Expression of isolated β integrin subunit cytoplasmic and transmembrane domains in adherent endothelial cells caused cell detachment and death in vitro and in vivo (Hasmim et al. 2005; Oguey et al. 2000), consistent with the notion that overexpression of isolated β-cytoplasmic domain competes for binding of essential cytoplasmic adaptor proteins (e.g., talin), resulting in “mechanical uncoupling”of the integrins from focal adhesions and cytoskeletal structures. These results also suggest the possibility of targeting the cytoplasmic domain for therapeutic purposes. Two main problems need to be addressed: the first one concerns integrin specificity (current constructs that do not differentiate between β1 and β3 integrins). The second problem concerns intracellular delivery: to allow penetration into the cell, an inhibitory peptide has to be fused to cytoplasmic transduction peptides (Kim et al. 2006). Alternatively, non-peptidic drugs may be developed to disrupt β3-tail interaction with structural or signaling cytoplasmic proteins.
Targeting Angiogenic Precursor Cells and Inflammatory Cells
Bone marrow cells are mobilized during tumor growth and recruited at tumor sites to promote tumor angiogenesis. While some of the cells include true endothelial precursors giving rise to mature endothelial cells, most of them are of monocyte/macrophage lineage (De Palma and Naldini 2006). Monocyte/macrophage is very sensitive to hypoxia and produces angiogenic factors and chemokines that stimulate tumor angiogenesis, progression, and metastasis (Condeelis and Pollard 2006). Since leukocytes and inflammatory cells use integrins to extravasate and migrate through the stroma, such as αLβ2, α4β1, αMβ2, αVβ3, or α5β1, it may be reasonable to inhibit their recruitment to tumor sites by targeting their integrins (Ulbrich et al. 2003). For example, antagonists of integrin α4β1 blocked extravasation of monocytes into tumor tissue and prevented monocyte macrophage colonization of tumors and tumor angiogenesis (Jin et al. 2006). Since it is also possible that some of the antiangiogenic effects observed with αVβ3 or α5β1 antagonists may be due to the inhibition of leukocyte recruitment, in future experimental and clinical studies it will be important to monitor the effect of integrin inhibitors on the recruitment of inflammatory cells to tumor sites. Of note is the observation that Cilengitide inhibited proliferation and differentiation of human endothelial progenitor cells in vitro (Loges et al. 2007).
Conclusions
Preclinical evidence indicates that integrins expressed in angiogenic endothelial cells are potentially relevant targets for antiangiogenic therapies in cancer. Early clinical trials have provided initial evidence of activity in human cancers, and ongoing clinical trials tell us whether they may bring benefits to human cancer treatment. If this is the case, besides further clinical trials, there will be a race to generate novel, more potent, and orally bioavailable “second generation” antagonists as well as to define the best combination strategy. The possibility of coupling the use of integrin antagonists for therapeutic purposes with non-invasive imaging of vascular integrins would open the possibility to select patients expressing high levels of the target, and therefore those who are most likely to benefit from the treatment.
References
Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Grone H-J, Hallahan DE, Reisfeld RA, Debus J, Niethammer AG, Huber PE (2005) Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res 11: 6270–6279
Alghisi GC, Ruegg C (2006) Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium 13:113–135
Bader B, Rayburn H, Crowley D, Hynes R (1998) Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95:507–519
Barrett T, Brechbiel M, Bernardo M, Choyke PL (2007) MRI of tumor angiogenesis. J Magn Reson Imaging 26:235–249
Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, Schnell O, Niemeyer M, Kessler H, Wester HJ, Weber WA, Schwaiger M (2006) Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res 12:3942–3949
Bello L, Lucini V, Carrabba G, Giussani C, Machluf M, Pluderi M, Nikas D, Zhang J, Tomei G, Villani RM, Carroll RS, Bikfalvi A, Black PM (2001) Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res 61: 8730–8736
Belvisi L, Riccioni T, Marcellini M, Vesci L, Chiarucci I, Efrati D, Potenza D, Scolastico C, Manzoni L, Lombardo K, Stasi MA, Orlandi A, Ciucci A, Nico B, Ribatti D, Giannini G, Presta M, Carminati P, Pisano C (2005) Biological and molecular properties of a new alpha(v)beta3/alpha(v)beta5 integrin antagonist. Mol Cancer Ther 4:1670–1680
Bhaskar V, Fox M, Breinberg D, Wong MH, Wales PE, Rhodes S, Dubridge RB, Ramakrishnan V (2007a) Volociximab, a chimeric integrin alpha5beta1 antibody, inhibits the growth of VX2 tumors in rabbits. Invest New Drugs 26(1):7–12
Bhaskar V, Zhang D, Fox M, Seto P, Wong MH, Wales PE, Powers D, Chao DT, Dubridge RB, Ramakrishnan V (2007b) A function blocking anti-mouse integrin alpha5beta1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med 5:61
Bieler G, Hasmim M, Monnier Y, Imaizumi N, Ameyar M, Bamat J, Ponsonnet L, Chouaib S, Grell M, Goodman SL, Lejeune F, Ruegg C (2007) Distinctive role of integrin-mediated adhesion in TNF-induced PKB/Akt and NF-kappaB activation and endothelial cell survival. Oncogene 26: 5722–5732
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264:569–571
Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL (2002) Cilengitide targeting of alpha(v)beta3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res 62:4263–4272
Cacciari B, Spalluto G (2005) Non peptidic alphavbeta3 antagonists: recent developments. Curr Med Chem 12:51–70
Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X (2006) In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res 66:9673–9681
Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP (2002) ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem 277: 17281–17290
Chen X, Plasencia C, Hou Y, Neamati N (2005) Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem 48:1098–1106
Choe YS, Lee KH (2007) Targeted in vivo imaging of angiogenesis: present status and perspectives. Curr Pharm Des 13:17–31
Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, Lewis N, Weiner L, Lam GN, Brown SC, Shaw DE, Mazar AP, Cohen RB (2006) Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer 94:1621–1626
Cohen S, Trikha M, Mascelli M (2000) Potential future clinical applications for the GPIIb/IIIa antagonist, abciximab in thrombosis, vascular and oncological indications. Pathol Oncol Res 6: 163–174
Collo G, Pepper MS (1999) Endothelial cell integrin alpha5beta1 expression is modulated by cytokines and during migration in vitro. J Cell Sci 112: 569–578
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266
Coutifaris C, Omigbodun A, Coukos G (2005) The fibronectin receptor alpha5 integrin subunit is upregulated by cell-cell adhesion via a cyclic AMP-dependent mechanism: implications for human trophoblast migration. Am J Obstet Gynecol 192:1240–1253; discussion 1253–1255
De Palma M, Naldini L (2006) Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta 1766: 159–166
Decristoforo C, Faintuch-Linkowski B, Rey A, von Guggenberg E, Rupprich M, Hernandez-Gonzales I, Rodrigo T, Haubner R (2006) [99mTc]HYNIC-RGD for imaging integrin alphavbeta3 expression. Nucl Med Biol 33:945–952
Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C (2007) Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol 171: 361–372
Dormond O, Ruegg C (2003) Regulation of endothelial cell integrin function and angiogenesis by COX-2, cAMP and protein kinase A. Thromb Haemost 90:577–585
Eskens FALM, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT (2003) Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins [alpha]v[beta]3 and [alpha]v[beta]5 in patients with advanced solid tumours. Eur J Cancer 39: 917–926
Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO (2002) Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 22:927–933
Giancotti FG (2007) Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci 28:506–511
Ginsberg MH, Partridge A, Shattil SJ (2005) Integrin regulation. Curr Opin Cell Biol 17:509–516
Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA (2000) Targeted antiangiogenic therapy for cancer using vitaxin: a humanized monoclonal antibody to the integrin aVb3. Clin Cancer Res 6:3056–3061
Hamano Y, Kalluri R (2005) Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun 333:292–298
Hammes H, Brownlee M, Jonczyk A, Sutter A, Preissner K (1996) Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med 2:529–533
Hansma AHG, Broxterman HJ, van der Horst I, Yuana Y, Boven E, Giaccone G, Pinedo HM, Hoekman K (2005) Recombinant human endostatin administered as a 28-day continuous intravenous infusion, followed by daily subcutaneous injections: a phase I and pharmacokinetic study in patients with advanced cancer. Ann Oncol 16: 1695–1701
Hasmim M, Vassalli G, Alghisi G, Bamat J, Ponsonnet L, Bieler G, Bonnard C, Paroz C, Oguey D, Rüegg C (2005) Expressed isolated integrin b1 subunit cytodomain induces endothelial cell death secondary to detachment. Thromb Haemost 94: 1060–1070
Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H (1996) Structural and functional aspects of rgd-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J. Am. Chem. Soc. 118:7461–7472
Herbst RS, Hess KR, Tran HT, Tseng JE, Mullani NA, Charnsangavej C, Madden T, Davis DW, McConkey DJ, O’Reilly MS, Ellis LM, Pluda J, Hong WK, Abbruzzese JL (2002) Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol 20: 3792–3803
Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO (1999) β3-integrin–deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103:229–238
Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M (2004) VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J 18: 1111–1113
Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA (2002) Tumor regression by targeted gene delivery to the neovasculature. Science 296:2404–2407
Hsu AR, Hou LC, Veeravagu A, Greve JM, Vogel H, Tse V, Chen X (2006) In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol Imaging Biol 8:315–323
Hynes RO (2002) A reevaluation of integrins as regulators of angiogenesis. Nat Med 8:918–921
Hynes RO (2007) Cell-matrix adhesion in vascular development. J Thromb Haemost 5(Suppl 1): 32–40
Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J (2006) Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res 66:2146–2152
Kawaguchi T, Yamashita Y, Kanamori M, Endersby R, Bankiewicz KS, Baker SJ, Bergers G, Pieper RO (2006) The PTEN/Akt pathway dictates the direct alphaVbeta3-dependent growth-inhibitory action of an active fragment of tumstatin in glioma cells in vitro and in vivo. Cancer Res 66:11331–11340
Kim D, Jeon C, Kim JH, Kim MS, Yoon CH, Choi IS, Kim SH, Bae YS (2006) Cytoplasmic transduction peptide (CTP): new approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res 312:1277–1288
Kim S, Bell K, Mousa SA, Varner JA (2000) Regulation of angiogenesis in vivo by ligation of integrin a5b1 with the central cell-binding domain of fibronectin. Am J Pathol 156: 1345–1362
Klotz O, Park JK, Pleyer U, Hartmann C, Baatz H (2000) Inhibition of corneal neovascularization by alpha(v)-integrin antagonists in the rat. Graefes Arch Clin Exp Ophthalmol 238:88–93
Koivunen E, Wang B, Ruoslahti E (1994) Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J Cell Biol 124:373–380
Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L (2001) Inhibition of angiogenesis and tumor growth by SCH221153, a dual avb3 and avb5 integrin receptor antagonist. Cancer Res 61:2232–2238
Kuwada SK (2007) Drug evaluation: volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr Opin Mol Ther 9:92–98
Lejeune FJ, Lienard D, Matter M, Ruegg C (2006) Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun 6:6
Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F (2007) Evaluation of a (99m)Tc-labeled cyclic RGD tetramer for noninvasive imaging integrin alpha(v)beta3-positive breast cancer. Bioconjug Chem 18:438–446
Livant DL, Brabec RK, Pienta KJ, Allen DL, Kurachi K, Markwart S, Upadhyaya A (2000) Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res 60:309–320
Loges S, Butzal M, Otten J, Schweizer M, Fischer U, Bokemeyer C, Hossfeld DK, Schuch G, Fiedler W (2007) Cilengitide inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Biochem Biophys Res Commun 357:1016–1020
Luo BH, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25:619–647
MacDonald T, Taga T, Shimada H, Tabrizi P, Zlokovic B, Cheresh D, Laug W (2001) Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery 48:151–157
Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R (2000) Distinct antitumor properties of a Type IV collagen domain derived from basement membrane. J Biol Chem 275:21340–21348
Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, Sonenberg N, Hynes RO, Kalluri R (2002) Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 295:140–143
Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV (2006) Integrin signaling is critical for pathological angiogenesis. J Exp Med 203: 2495–2507
Marinelli L, Meyer A, Heckmann D, Lavecchia A, Novellino E, Kessler H (2005) Ligand binding analysis for human alpha5beta1 integrin: strategies for designing new alpha5beta1 integrin antagonists. J Med Chem 48:4204–4207
Martin PL, Jiao Q, Cornacoff J, Hall W, Saville B, Nemeth JA, Schantz A, Mata M, Jang H, Fasanmade AA, Anderson L, Graham MA, Davis HM, Treacy G (2005) Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-av integrin monoclonal antibody, despite widespread tissue binding. Clin Cancer Res 11:6959–6965
McNeel DG, Eickhoff J, Lee FT, King DM, Alberti D, Thomas JP, Friedl A, Kolesar J, Marnocha R, Volkman J, Zhang J, Hammershaimb L, Zwiebel JA, Wilding G (2005) Phase I trial of a monoclonal antibody specific for avb3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res 11:7851–7860
Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H, Bowlin T, Attardo G (2003) A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol 40:77–89
Minamiguchi K, Kumagai H, Masuda T, Kawada M, Ishizuka M, Takeuchi T (2001) Thiolutin, an inhibitor of HUVEC adhesion to vitronectin, reduces paxillin in HUVECs and suppresses tumor cell-induced angiogenesis. Int J Cancer 93:307–316
Mitjans F, Sander D, Adan J, Sutter A, Martinez JM, Jaggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL (1995) An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci 108:2825–2838
Mitjans F, Meyer T, Fittschen C, Goodman S, Jonczyk A, Marshall J, Reyes G, Piulats J (2000) In vivo therapy of malignant melanoma by means of antagonists of alphav integrins. Int J Cancer 87: 716–723
Mitra A, Coleman T, Borgman M, Nan A, Ghandehari H, Line BR (2006) Polymeric conjugates of mono- and bi-cyclic alphaVbeta3 binding peptides for tumor targeting. J Control Release 114:175–183
Mould AP, Burrows L, Humphries MJ (1998) Identification of amino acid residues that form part of the ligand-binding pocket of integrin alpha 5beta 1. J. Biol. Chem. 273:25664–25672
Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, Hope L, Valle JW, Radford JA, Lawrance J, Saunders MP, Munteanu MC, Nakada MT, Nemeth JA, Davis HM, Jiao Q, Prabhakar U, Lang Z, Corringham RE, Beckman RA, Jayson GC (2007) Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res 13:2128–2135
Murakami M, Elfenbein A, Simons M (2008) Non-canonical fibroblast growth factor signaling in angiogenesis. Cardiovasc Res 78(2):223–231
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA (2007) Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25:1651–1657
Nakada MT, Cao G, Sassoli PM, DeLisser HM (2006) c7E3 Fab inhibits human tumor angiogenesis in a SCID mouse human skin xenograft model. Angiogenesis 9:171–176
Nam JO, Jeong HW, Lee BH, Park RW, Kim IS (2005) Regulation of tumor angiogenesis by fastatin, the fourth FAS1 domain of betaig-h3, via alphavbeta3 integrin. Cancer Res 65:4153–4161
Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG (2004) Integrin [beta]4 signaling promotes tumor angiogenesis. Cancer Cell 6:471–483
Ning S, Chen Z, Dirks A, Husbeck B, Hsu M, Bedogni B, O’Neill M, Powell MB, Knox SJ (2007) Targeting integrins and PI3K/Akt-mediated signal transduction pathways enhances radiation-induced anti-angiogenesis. Radiat Res 168: 125–133
Nisato RE, Tille J-C, Jonczyk A, Goodman SL, Pepper MS (2004) alphav beta3 and alphav beta5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 6:105–119
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
Oba M, Fukushima S, Kanayama N, Aoyagi K, Nishiyama N, Koyama H, Kataoka K (2007) Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing alphavbeta3 and alphavbeta5 integrins. Bioconjug Chem 18:1415–1423
Oguey D, George P, Ruegg C (2000) Disruption of integrin-dependent adhesion and survival of endothelial cells by recombinant adenovirus expressing isolated beta integrin cytoplasmic domains. Gene Ther 7:1292–1303
Orecchia A, Lacal PM, Schietroma C, Morea V, Zambruno G, Failla CM (2003) Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the alpha 5 beta 1 integrin. J Cell Sci 116:3479–3489
Perruzzi CA, de Fougerolles AR, Koteliansky VE, Whelan MC, Westlin WF, Senger DR (2003) Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis. J Invest Dermatol 120: 1100–1109
Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM (2000) Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. PNAS 97:12227–12232
Posey J, Khazaeli M, DelGrosso A, Saleh M, Lin C, Huse W, LoBuglio A (2001) A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm 16:125–132
Raguse J-D, Gath HJ, Bier J, Riess H, Oettle H (2004) Cilengitide (EMD 121974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral Oncol 40:228–230
Ramakrishnan V, Bhaskar V, Law DA, Wong MH, DuBridge RB, Breinberg D, O’Hara C, Powers DB, Liu G, Grove J, Hevezi P, Cass KM, Watson S, Evangelista F, Powers RA, Finck B, Wills M, Caras I, Fang Y, McDonald D, Johnson D, Murray R, Jeffry U (2006) Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol 5: 273–286
Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM (2003) Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res 63:2079–2087
Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM (2004) Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in b3-integrin-deficient mice. Cancer Res 64:8643–8650
Reynolds L, Wyder L, Lively J, Taverna D, Robinson S, Huang X, Sheppard D, Hynes R, Hodivala-Dilke K (2002) Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 8:27–34
Romer LH, Birukov KG, Garcia JG (2006) Focal adhesions: paradigm for a signaling nexus. Circ Res 98:606–616
Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ (1998) Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med 4:408–414
Ruegg C, Mutter N (2007) Anti-angiogenic therapies in cancer: achievements and open questions. Bull Cancer 94:753–762
Sabherwal Y, Rothman VL, Dimitrov S, L’Heureux DZ, Marcinkiewicz C, Sharma M, Tuszynski GP (2006) Integrin alpha2beta1 mediates the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumor-associated protein. Exp Cell Res 312:2443–2453
Scott KA, Arnott CH, Robinson SC, Moore RJ, Thompson RG, Marshall JF, Balkwill FR (2004) TNF-alpha regulates epithelial expression of MMP-9 and integrin alphavbeta6 during tumour promotion. A role for TNF-alpha in keratinocyte migration? Oncogene 23:6954–6966
Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M (1997) Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha 1beta 1 and alpha 2beta 1 integrins. Proc Natl Acad Sci 94: 13612–13617
Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M (2002) The a1b1 and a2b1 integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol 160:195–204
Serini G, Valdembri D, Bussolino F (2006) Integrins and angiogenesis: a sticky business. Exp Cell Res 312(5):651–658
Shin IS, Jang BS, Danthi SN, Xie J, Yu S, Le N, Maeng JS, Hwang IS, Li KC, Carrasquillo JA, Paik CH (2007) Use of antibody as carrier of oligomers of peptidomimetic alphavbeta3 antagonist to target tumor-induced neovasculature. Bioconjug Chem 18:821–828
Smith JW (2003) Cilengitide Merck. Curr Opin Investig Drugs 4:741–745
Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry G, Parikh A, McCarty M, Bucana C, Mazar A, Ellis L (2003) Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer 104:496–503
Strieth S, Eichhorn ME, Sutter A, Jonczyk A, Berghaus A, Dellian M (2006) Antiangiogenic combination tumor therapy blocking alpha(v)-integrins and VEGF-receptor-2 increases therapeutic effects in vivo. Int J Cancer 119:423–431
Stupack DG (2007) The biology of integrins. Oncology (Williston Park) 21:6–12
Stupp R, Ruegg C (2007) Integrin inhibitors reaching the clinic. J Clin Oncol 25:1637–1638
Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, Barsky L, Weinberg KI, Laug WE (2002) Alphav-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer 98:690–697
Takada Y, Xiaojing X, Scott S (2007) The integrins. Genome Biol 8:215
Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R (2007) Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn 237:75–82
Tomiyama Y (2000) Glanzmann thrombasthenia: integrin alpha IIb beta 3 deficiency. Int J Hematol 72:448–454
Travis MA, Humphries JD, Humphries MJ (2003) An unraveling tale of how integrins are activated from within. Trends Pharmacol Sci 24:192–197
Trikha M, Zhou Z, Nemeth J, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada M (2004) CNTO 95, a fully human monoclonal antibody that inhibits av integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer 110:326–335
Tsopanoglou NE, Papaconstantinou ME, Flordellis CS, Maragoudakis ME (2004) On the mode of action of thrombin-induced angiogenesis: thrombin peptide, TP508, mediates effects in endothelial cells via alphavbeta3 integrin. Thromb Haemost 92: 846–857
Ulbrich H, Eriksson EE, Lindbom L (2003) Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci 24:640–647
Umeda N, Kachi S, Akiyama H, Zahn G, Vossmeyer D, Stragies R, Campochiaro PA (2006) Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol 69:1820–1828
Varner JA, Nakada MT, Jordan RE, Coller BS (1999) Inhibition of angiogenesis and tumor growth by murine 7E3, the parent antibodyof c7E3 Fab (abciximab; ReoProTM). Angiogenesis 3:53–60
Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D (2007) Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem 282:15187–15196
Wickstrom SA, Alitalo K, KeskiOja J (2005) Endostatin signaling and regulation of endothelial cell-matrix interactions. Adv Cancer Res 94:197–229
Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV (2008) Integrin alpha 2beta 1 is the required receptor for endorepellin angiostatic activity. J Biol Chem 283(4):2335–2343
Wu H, Beuerlein G, Nie Y, Smith H, Lee BA, Hensler M, Huse WD, Watkins JD (1998) Stepwise in vitro affinity maturation of Vitaxin, an alpha vbeta 3-specific humanized mAb. Proc Natl Acad Sci 95:6037–6042
Xie J, Shen Z, Li KC, Danthi N (2007) Tumor angiogenic endothelial cell targeting by a novel integrin-targeted nanoparticle. Int J Nanomed 2:479–485
Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA (2002) Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296:151–155
Zaric J, Ruegg C (2005) Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J Biol Chem 280:1077–1085
Zhang D, Pier T, McNeel DG, Wilding G, Friedl A (2007) Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels – a pharmacodynamic study. Invest New Drugs 25:49–55
Zhou Y, Peng H, Ji Q, Qi J, Zhu Z, Yang C (2006) Discovery of small molecule inhibitors of integrin alphavbeta3 through structure-based virtual screening. Bioorg Med Chem Lett 16: 5878–5882
Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF (2002) β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129:2891–2903
Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, Leutgeb B, Krieg T, Eckes B (2007) Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol 127:467–478
Acknowledgments
Work in our laboratory was supported by funds from the Molecular Oncology Program of the National Center for Competence in Research (NCCR), a research instrument of the Swiss National Science Foundation, the Swiss Cancer League/Oncosuisse, the Swiss National Science Foundation, and the Medic Foundation. We apologize to those colleagues whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rüegg, C., Alghisi, G.C. (2010). Vascular Integrins: Therapeutic and Imaging Targets of Tumor Angiogenesis. In: Liersch, R., Berdel, W., Kessler, T. (eds) Angiogenesis Inhibition. Recent Results in Cancer Research, vol 180. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-78281-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-540-78281-0_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-78280-3
Online ISBN: 978-3-540-78281-0
eBook Packages: MedicineMedicine (R0)