Abstract
One of the most challenging reconstructions in maxillofacial surgery is that involving the condyle and ramus. Common reconstructive techniques involve either autogenous bone grafting such as costochondral rib grafting, a sliding posterior ramus border osteotomy, microvascular free fibula graft, or alloplastic reconstruction involving either stock or custom total joint replacement. None of these techniques specifically address the articular disc and some address only bone and not soft tissue. Bioengineering, which uses cells, molecules, chemistry, and scaffolds with engineering principles, is now providing novel solutions to complex biological problems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the United States alone, temporomandibular joint (TMJ) disorders (TMDs) are reported to affect close to ten million Americans annually. TMJ disorders requiring reconstruction of the ramus/condyle unit (RCU) can be the result of genetic disorders such as hemifacial microsomia and Treacher Collins syndrome; inflammatory disorders resulting in chondromalacia, arthritis, and condylar resorption; and acquired defects secondary to trauma, infection, failed surgery, and neoplasms. The underlying pathology, the anatomic defect, and the effect on function will dictate whether nonsurgical or surgical treatment is required. One of the most challenging reconstructions in maxillofacial surgery is that involving the condyle and ramus. Common reconstructive techniques involve either autogenous bone grafting such as costochondral rib grafting, a sliding posterior ramus border osteotomy, microvascular free fibula graft, or alloplastic reconstruction involving either stock or custom total joint replacement. None of these techniques specifically address the articular disc and some address only bone and not soft tissue. Bioengineering, which uses cells, molecules, chemistry, and scaffolds with engineering principles, is now providing novel solutions to complex biological problems.
2 Anatomy and Function
A brief review of the anatomy and function (although presented elsewhere in the text) is essential to understand the challenges inherent in designing an engineered graft that precisely replicates the defect. The temporomandibular joint is one of the most intricate functional joints in the human body. It is a ginglymoarthrodial joint, with both the ability for hinging movement (ginglymoid) in one plane and at the same time gliding movement (arthrodial) in another plane. Adding to its functional and reconstruction complexity is that it is the only joint in the body in which movement of one joint is always synchronous with the contralateral joint. Interestingly, unlike most other human joints that are composed of hyaline cartilage, the TMJ’s articulating zone is made of dense fibrous connective tissue and fibrocartilage. The only other regions of the body that have similar fibrocartilage is the meniscus of the knee and annulus fibrosis of intervertebral discs. The articular surfaces of both the temporal bone and condyle are lined with the fibrous connective tissue. The head of the condyle is composed of four distinct layers—the most superficial is the articular zone composed of dense fibrous connective tissue; the next is the proliferative zone, which is mostly cellular and reparative and housing the stem cell niche; the third layer is the fibrocartilaginous zone providing support and resistance; and the deepest zone is the calcified cartilage zone, comprised of chondrocytes and chrondroblasts [1].
The condyle is separated from the roof of the glenoid fossa of the squamous portion of the temporal bone by a thin articular disc, thereby creating two joint spaces. The articular disc functionally serves as a non-ossified bone and is composed of dense fibrous connective tissue [1]. The disc enables the joint to do its complicated movements and also contributes to the reconstructive challenge of this anatomic structure. The articular surface of the condylar head abuts the thinnest and most central portion of the disc. The disc is attached via the main three functional ligaments of the TMJ, the capsular ligament, collateral ligaments, and temporomandibular ligament, and it maintains its position circumferentially around the entire condyle. Endothelial cells producing synovial fluid line the superior and inferior joint spaces—hence the TMJ is also a synovial joint.
Lastly, it is important to understand the muscle attachments in this area. The superior lateral pterygoid inserts on the articular capsule, disc, and neck of condyle. The inferior lateral pterygoid inserts primarily onto the neck of the condyle. Currently, muscle attachments are not addressed with any of the reconstruction options following condylectomy.
The normal range of motion of the mandible includes rotation within the inferior joint space to approximately 25 mm and then translation within the superior joint space to approximately 40–45 mm of opening. As the condyle slides anteriorly and posteriorly, moving in and out of the fossa, the articular disc rotates around the attachments of the discal collateral ligaments to maintain its position. Lateral and protrusive excursions through contraction of the lateral pterygoid muscles also occur. Movement should be smooth without any joint noises. At the end of the range of motion, the condyle should rest under the articular eminence with the biconcave portion of the disc sitting between the two. During function the loose synovium provides nutrition and lubrication to the articulating surfaces. It is the intimate and intricate anatomic relationship of the articular disc to the mandibular condyle via the main supportive ligaments in addition to the external pterygoid attachments that are vital to healthy and normal TMJ function.
3 Current Treatment Modalities
While autogenous reconstruction is the current gold standard, it is almost impossible to recreate the precise three-dimensional geometric shape, structure, and supporting tissues that are being replaced. Autogenous reconstruction also requires additional surgical sites to harvest tissues and carries the risk of donor site morbidity. There is still no gold standard for replacement of the disc, but options include dermis fat graft, auricular cartilage, or rotating a temporalis fascia or temporalis muscle flap to line the joint and separate the new condyle from the glenoid fossa roof [2]. Further, the literature supports that disc resection without replacement has predictable long-term success [2]. Previous attempts to use alloplasts such as silastic sheets or Teflon-proplast were dismal failures [2,3,4].
Alloplastic reconstruction is very technique sensitive, which will result in no excursive movements off of the ipsilateral side, and although failure rate is low, an infection of the joint would require its removal resulting in a significant postoperative deformity [5].
Bioengineering solutions such as the injection of growth and repair factors to serve as homing agents, the direct injection of stem cells to permit repair of diseased tissue, and the combination of scaffolds and stem cells engineered to precisely reconstruct the anatomic defect will play an increasingly important role in repair and regeneration. Recent advances in bioengineering employing stem cell technologies have brought us closer to an autologous graft (derived from recipient cells ± scaffold) that precisely reconstructs the anatomy of the bone and articulating cartilaginous surfaces. Bioengineered constructs would also limit donor site morbidity and decrease the length of stay; both of which would improve patient care and potentially decrease cost of care.
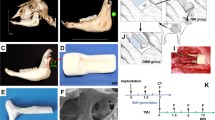
Osteochondral grafts to replace articulating surfaces like the RCU have an increased degree of complexity. We will explore here the components required for tissue engineering and its impact for reconstructive oral and maxillofacial surgery. Successful bioengineering would allow reconstructive surgeons to design scaffolds for each patient and their specific defect being treated (Fig. 15.1). We will discuss the process required for bioengineering a RCU including scaffold selection and fabrication, cell selection, seeding of scaffold with cells (±growth factors), viable tissue growth, surgical implantation into chosen animal model, and postimplantation evaluation of remodeling and breakdown (Table 15.1).
Engineering of cartilage/bone grafts. The process begins with 3D imaging of the defects for manufacturing an anatomical shape scaffold, consisting of strong mineralized region for the formation of bone and hydrogel region for the formation of cartilage. Both regions are seeded with cells and cultured in a bioreactor (also manufactured with the aid of imaging) that provides environmental control and physical stimulation, perfusion for bone, dynamic mechanical loading for cartilage
4 Scaffold Selection
A scaffold is a mechanical template that supports cell attachment, growth, and differentiation, and its main purpose is to provide the compositional, structural, and mechanical properties of native extracellular matrix (ECM) [6]. Intrinsic or external growth factors can assist the scaffold in performing its function. Scaffolds have been in use for decades in reconstructive surgery, such as for placing allogeneic bone grafts into a defect, as templates for endogenous bone formation with eventual replacement of the graft with host tissues. However, when bioengineering anatomic structures with cells, the success of such a construct naturally becomes more complex and unpredictable. Ideally, the engineered construct would simulate both the ECM and local microenvironment to support or induce tissue formation [7].
Appropriate selection of the correct material is critical to the success of any biologic scaffold. The ideal qualities of such a construct are (1) sufficient mechanical strength; (2) appropriately sized and positioned pores to allow for cell seeding, transport, and interconnectivity; (3) being non-immunogenic and biocompatible, and (4) being biodegradable to allow for future proliferation and differentiation of the cells into the desired tissue phenotype (see Table 15.1). These features allow for crucial biologic functions such as vascular infiltration and waste management, while also maintaining enough structural integrity to withstand the load-bearing function of the RCU. Equally important is finding a material that degrades and/or resorbs at a similar rate to replacement tissue formation by the recipient. For mandibular reconstruction, the scaffold must be able to withstand compressive forces during the healing phase when the graft is being replaced by host bone and remodeled. While many believe being non-immunogenic is still an important principle in material selection, some research has shown that finding a material able to produce a controlled immune response may actually enhance integration [8]. Finding the appropriate materials for craniofacial tissue engineering has been a vibrant area of research over the past decade.
5 Scaffold Types
The craniofacial engineering material armamentarium consists of natural and synthetic polymers, decellularized bone, ceramics, composite materials, silk, and electrospun nanofibers [9].
Research started with natural polymers such as polypeptides (e.g., collagen) polysaccharides (e.g., hyaluronic acid, chitosan), and silk. Collagen was a popular material as it is a predominant organic component of bone ECM and total bone protein. The benefits of natural polymeric materials include the proven ability to support the attachment, proliferation, and differentiation of cells [10, 11]. However, investigations into the material showed utility was limited by natural polymeric mechanical strength, unpredictable degradation and breakdown rates, and risk of infection [7].
Silk fibroin, derived from silkworms, has shown excellent biocompatibility, mechanical properties, and degradation patterns. Silk sponges, tubes, and fibers have been used for cartilage [12], blood vessels [13], and ligaments [14], respectively. Until recently, silk was never investigated as a scaffold for bone regeneration. However, the porosity of silk sponges behaves quite favorably as a bone scaffold allowing for cell attachment and nutrient and waste transport [15]. Further, the silk’s pore size and geometry, as well as material stiffness were important factors for bone formation with adipose derived stem cells [15]. Silks remain a viable option as a dependable scaffold in the future.
Synthetic polymers such as poly (lactic acid), poly (glycolic acid), and poly (methyl methacrylate) demonstrate greater structural stability than their natural counterparts and provide support for bone tissue formation [16]. Synthetic polymers compared to their natural counter parts are more convenient because they can be reproduced easily with targeted mechanical properties and degradation kinetics [17]. Natural bone is composed of collagen and hydroxyapatite. Bioceramics such as hydroxyapatite (HA) and tricalcium phosphate (TCP) have been used for bone regeneration. Hyaluronic acid alone has also been used in CAD-CAM designs for TMJ replacement with promising results in sheep [18]. When a polymer matrix is incorporated with TCP or HA, the material then becomes a hybrid/composite. The fillers enable the tissue engineer to alter the degradation and resorption kinetics of the planned complex tissue [19]. It is important to understand the degradation of the synthetic product in vivo; i.e., if acid products are produced by scaffold degradation, a prolonged inflammatory response may result.
Hydrogels such as agarose, alginate, and chitosan are important polymers for tissue engineering purposes. Cartilage matrices consist of a highly hydrated proteoglycan hydrogel embedded into a type II collagen network [20]. Hydrogels have been very successful as the material of choice for cartilage scaffolding since they support the spherical shape and normal phenotype of chondrocytes [21].
The process of electrospinning allows scientists to accurately recapitulate the bone extracellular matrix. The natural network that makes up bone is intricately interspersed with nanocrystallites such as hydroxyapatite, which allows it to function as a nanocomposite organized on the nanoscale [22]. Nanofibrous matrices have high porosity and a favorable surface area to volume ratio, which allow the material to maximize protein adsorption, cell adhesion, nutrient exchange, angiogenesis, and other critical cellular tissue functions. Further, different materials can be cross-linked to polymers by electrospinning, which would enhance the weak mechanical strength of certain polymers.
The authors’ own research focuses primarily on using decellularized bovine trabecular bone as the scaffold for RCU bioengineering with promising translational successes [23,24,25]. We use an already FDA-approved decellularized bovine trabecular bone and utilize image-guided micromilling to craft a scaffold into an anatomically correct shape for the target host defect [24]. The xenograft has intrinsic adhesion molecules for the cells. However, care must be taken to ensure no antigenic proteins remain after decellularization.
6 Scaffold Fabrication
Scaffolds can be fabricated via fiber bonding, solvent casting, freeze-drying, salt leaching, and phase separation among other more traditional mechanisms. Both computer-aided design (CAD) and computer-aided manufacturing (CAM) provided an important progressive step in scaffold fabrication. CAD-CAM 3D printing or micromilling from existing materials and electrospinning now allow engineers the precision to craft most infrastructures they require.
When preparing autologous derived grafts (grafts made for hosts using their own stem cells), the TMJ can be imaged via a computed tomography (CT) scanner similar to a patient preparing for virtual surgical planning for orthognathic surgery. Subsequently, depending on the clinician’s and engineer’s material of choice, one option is to either 3D print the scaffold with a synthetic polymer or micromill it from an existing block. We recently reported successful use of micromilling a large decellularized cancellous bone block from bovine femurs. The scaffold was milled to the custom geometric specifications based on the CT images of each study animal (Fig. 15.1) [24]. This can hopefully be done clinically as well, by imaging a patient defect or mirror-imaging their “healthy TMJ” if applicable and preparing a unique autologous graft for that patient. Regardless of what technique is being used, the geometry, pore size, and dispersal are critical for cell seeding. CAD technology has allowed groups to precisely fabricate a scaffold to mimic exact bone defects needed to be reconstructed with improved internal architecture.
Following the fabrication of the construct, it must be cultivated with the cells of choice for adhesion and future development of the desired tissue. This step is critical and frequently a problem for research laboratories. The bioreactor must maintain appropriate conditions for tissue growth and maturation prior to implantation. The provision of precise interstitial flows and physiologic functions during this culture period is highly technique sensitive, and laboratory dependent, yet ultimately crucial for biologic success. Ideally, a bioreactor should be capable of coordinating biological, mechanical, and physiological stimuli in a spatially and temporally controlled manner to support a desired cell and tissue growth [20].
7 Stem Cells and Growth Factors
There are two basic types of stem cells: embryonic and adult. Embryonic stem cells are harvested from embryos in the blastocyst stage of development and are capable of dividing indefinitely and, under appropriate stimulation and/or culture medium, can differentiate into all cell types of all three germ layers (termed pluripotent) [26, 27]. However, due to ethical concerns, the use of embryonic stem cells in the United States has been controversial and therefore limited.
Scientists have been forced to focus their efforts on harvesting other cell lineages that would be of similar pluripotent and multipotent utility. Adult stem cells are undifferentiated cells that reside in a stem cell niche among differentiated cells until they are called upon to initiate repair. Most human tissues have delineated reservoirs of stem cells used for repair—neural, hematopoietic, gastrointestinal, and mesenchymal [27]. For example, transcription cofactor YAP activates mesenchymal stem cells (MSCs) residing in the synovium to initiate repair of damaged cartilage [28]. Future therapy may be directed to providing the appropriate cues to initiate this mechanism of repair in damaged joints and elsewhere.
MSCs are multipotent cells that give rise to a variety of tissue types: bone, cartilage, vascular, and adipose tissues [29]. Multipotent cells are more limited than pluripotent cells because they are not able to produce cells of all three germ layers. For completeness, it is worth mentioning that some researchers have demonstrated MSCs to have pluripotential when reprogrammed [30,31,32]. Regardless, this cell line is the one of most interest to bioengineers and clinicians alike focusing on craniofacial reconstruction [30, 33]. MSCs have been the cell line of choice due to little or no ethical limitations, availability, minimal immunogenicity, and ability to produce the relevant tissues. During the harvest of either a cancellous or corticocancellous autogenous bone graft, MSCs are naturally harvested.
Original cell-based bioengineered TMJ grafts used mature osteoblasts and chondrocytes to seed the constructs [34]. Now investigations have advanced to employing MSCs that can be harvested with ease from adipose or bone marrow tissues [24, 35]. Bone marrow tissues were the original source of MSCs and previously the “standard of care” for obtaining the multipotent cells [36]. Now MSCs can be isolated from adipose [37], umbilical cord blood [38], peripheral blood [39], dental pulp [40], exfoliated deciduous teeth [41], dermis [42], amniotic fluid [43], and tumors [44]
We have found success using both bone marrow MSCs and adipose derived stem cells (ASCs) for bioengineering of the RCU [23,24,25]. Despite bone marrow-derived MSCs having higher osteogenic potential, ASCs have sufficient osteogenic capacity and similar in vitro self-renewal and are widely available with easy harvest from any subcutaneous source of fat (sourced commonly from elective liposuction aspirates) when compared to traditional bone marrow harvests [45]. Thus, ASCs have come to the forefront of the bone regeneration research community.
There have been countless laboratory and clinical successes using ASCs for bone regeneration, which we will describe later in this chapter. Recently we reported a successfully tissue-engineered autologous facial bone reconstruction using recipient animal subcutaneous fat as a source for ASCs [24, 46]. ASCs have a vast amount of differentiation capabilities and need to be cultured in lineage-specific media. They can be predictably cultured to differentiate toward chondrocytes and osteoblasts [45]. Osteogenic media may include 1,25-dihydroxyvitamin D3, ascorbate-2-phosphate, and bone morphogenetic protein (BMP)-2 (BMP-2) [47]. Also important for any bioengineered RCU would be the formation of a cartilage cap through the utilization of the chondrogenic potential of cells. ASCs demonstrate chondrogenic potential if its media contains other supplements such as transforming growth factor beta 1 (TGF-β1), insulin, dexamethasone, ascorbate-2-phosphate, BMP-6, and a high-density pellet culture [35].
The placement of some of the above osteogenic growth factors, molecules, or cells alone into defects can initiate cell homing for repair. The placement of human recombinant BMP-2 is the most obvious example of this. BMPs are able to initiate, promote, and support chondrogenesis and osteogenesis [48]. BMP-2 has been demonstrated to recruit mesenchymal stem cells that then differentiate into bone. It does this by stimulating transcription of core binding alpha-1 (Cbaf-1/RunX2) and Osterix, which are responsible for activating osteoblastic specific genes (e.g., alkaline phosphatase (ALP); osteopontin; osteonectin; bone sialoprotein, collagen type I) [49]. BMP-2 has been used to reconstruct both maxillary and mandibular defects such as alveolar clefts and continuity defects following ablative surgery for benign disease [50]. However, the use of BMP-2 for creation of a new condyle has not been reported. In maxillofacial surgery, BMP-2 is FDA approved only for sinus lifts and socket preservation and is not approved in children. Other uses of BMP-2 are necessarily off-label.
Ontogeny of osteoblast and regulatory control of osteoblast lineage progression and phenotypic features. Sequence and stages of the osteoblast lineage from a self-renewing, pluripotent mesenchymal stem cell to terminally differentiated osteocyte is diagrammatically illustrated. The characteristic feature of each developmental stage is indicated below the cell morphology. Next row summarizes the key transcription factor and co-regulatory protein involved in genetic control of osteoblast differentiation. Factors that negatively regulate Runx2 activity and osteoblast differentiation are indicated in red. Several physiologic mediators influencing osteoblast development, including transforming growth factor β (TGFβ), the bone morphogenetic proteins (BMPs), and fibroblast growth factors (FGFs), Wnt/β-catenin signaling, and hormones, are also indicated. Secretory molecules, receptor, and signal transducer that inhibit osteoblast maturation are highlighted in red. Last row summarize phenotypic marker genes expressed at different developmental stages of osteoblast differentiation. The understanding of these markers allows scientists to evaluate the stage of MSC induction. Reprinted from Oral and Maxillofacial Surgery Clinics of North America, 22, Genetic and Transcriptional Control of Bone Formation, 283–293, (2010), with permission from Elsevier
It is important to understand some of the genes and proteins involved with bone production because the osteogenic potential of the ASCs can be measured in frequent time points by measuring mRNA expression of the well-known bone markers. Early in the growth process proteins Runx-2, Osterix, or the gene ALP can be quantified, compared to more mature stages when collagen type I can be found. Some studies have found expression of Runx-2 in their culture media as early as 1 and 4 days [51, 52]. Researchers depend on the presence of these proteins to determine experimental efficacy and success (Fig. 15.2).
Morphology and structure of regenerated RCU. (a) Condyle regeneration was assessed using μCT 3D reconstruction and (b) Movat’s pentachrome staining at low magnification (top; 1-cm scale) and high magnification (bottom; 2-mm scale) of the condylectomy site. The dashed circumferences indicate the remaining graft regions, with the red trabecular structure representing the remaining scaffold material. (c) μCT 3D reconstruction of the graft-host interface and Movat’s pentachrome staining were used to assess integration of the implanted graft with the host bone. For acellular grafts, the mineralized host bone (hb) and the graft structures (g) were separated by soft fibrous tissue (f). In contrast, host bone extended into the tissue-engineered bone graft. In the proximity of the new bone, osteoclastic resorption (white arrowheads) was detected on the implanted scaffold with the lining of osteoblasts (black arrowheads), indicating active ossification. Scale bars, 1 mm (4×) and 100 μm (40×). From Bhumiratana S, Bernhard JC, Alfi DM, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8(343):343–83. Reprinted with permission from AAAS
8 Clinical Investigations and Applications to the Maxillofacial Skeleton
Now that we have reviewed the scientific and engineering components of what is required for craniofacial bioengineering, we can now begin to discuss promising areas of translational investigation and preliminary research results. While much work remains for optimization of tissue engineering, the reconstructive surgeon is closer than ever to having this in their surgical armamentarium.
One of the earliest reports of using autologous stem cells for bone regeneration in a human was reported in 2004, when a 7-year-old girl suffered widespread calvarial defects after trauma and cranial surgeries. Due to limited autogenous cancellous bone, the team utilized autologous adipose stem cells and mixed them with milled cancellous bone and autologous fibrin glue manufactured from the patient’s plasma. They reported a great yield of stem cells and marked ossification after 3 months but concede that it is impossible to determine to what degree the stem cells were responsible for regarding the regeneration [53]. Nonetheless, the results were promising and helped show proof of concept for ASCs use in a pediatric human subject who has limited autogenous bone sources.
Warnke PH et al. reported a successful outcome of a custom bone implant through a bone-muscle-flap technique [54]. A CT was taken of the patient’s mandibular defect following a 7 cm, subtotal mandibulectomy. With the use of CAD technology, a Teflon construct was milled to the exact specifications of the planned reconstruction geometrical shape. Ultimately, they used pre-bent titanium mesh; filled it with bone mineral block grafts and particles, BMP-7, collagen type I, and autologous aspirate from iliac crest; and then implanted it into the patient’s latissimus dorsi muscle as an in vivo bioreactor. They eventually implanted the graft into the mandibular defect, and it proved viable for 15 months until the patient unfortunately died from an unrelated comorbidity [55]. These early human reports provide hope that bioengineered constructs can be translated to human application.
9 Ramus Condyle Unit
The earliest report investigating cell-based TMJ engineering was in 2001. Investigators used a polyglycolic and polylactic acid as a scaffold and seeded them with mature osteoblasts and chondrocytes [34]. Scaffolds were implanted subcutaneously for 12 weeks in a non-load-bearing region of nude mice. This study demonstrated not only trabecular bone formation but also a bone-cartilage interface representative of articulating joints.
Other proof-of-concept studies were published regarding MSCs and scaffolds for the mandible and RCU. Abukawa, H, et al. isolated porcine MSCs and cultured them with osteogenic supplements [56]. A porcine mandibular condyle was then used as a model to fabricate poly-DL-lactic-co-glycolic acid (PLGA) scaffolds. Once the osteoblasts were differentiated, they were transferred to the scaffold and cultured for 6 weeks in a rotational oxygen-permeable bioreactor. Evaluation of the constructs showed promising radiographic radiodensity, and histology proved that bone existed on the entire surface of the scaffold [56]. The same group then published the first report in our literature of using autologous MSCs from a Yucatan mini-pig and biodegradable scaffold for actual implantation into a mandible [57]. Porcine MSCs were isolated from the ilium and seeded onto poly-DL-lactic-co-glycolic acid (PLGA) scaffolds. Compared to controls, iatrogenic full thickness bony defects (2 × 2 cm) showed filling with hard tissue that were uniformly radiodense with indistinct interfaces between native bone and implanted constructs [57].
Additional studies aimed to engineer osteochondral grafts in the shape of human TMJs [58,59,60,61,62]. Since the synovial joint head has a combination of fibrocartilage and bone, this model may have high utility in not only reconstruction but also long-term success of any implanted scaffold. Groups have applied the principles that we have outlined throughout the chapter to achieve this—a bilayered hydrogel or scaffold, mixed with lineage-specific growth factors for chondrocytes and osteoblasts, seeded with MSCs and either evaluated in vitro or in vivo. As an example, Re’em et al. used a bilayered affinity binding alginate scaffold. TGF-β1 (for chondrocytes) and BMP-4 (for osteoblasts) were affinity bound to two distinct layers of the hydrogel, and the entire complex was subsequently seeded with MSCs isolated from human donors. After evaluation they determined that both cartilage and bone formed. Further, when implanting an acellular two-layer hydrogel in situ, they found tissue growth after 4 weeks [62]. However, this required the use of growth factors.
Sheehy et al. described a novel approach to osteochondral constructs [60]. They describe the difficulty investigators have in maintaining MSC-derived cartilage from resisting hypertrophy and ultimate endochondral ossification compared to fully differentiated chondrocytes [63, 64]. However, this limitation for MSC engineered cartilage formation can be employed for in vivo bone regeneration. This route may be more advantageous because of the natural conditions that cells typically endure during endochondral ossification. For example, hypertrophic chondrocytes are already designed to withstand hypoxic conditions that occur during the early implantation stage of a tissue-engineered scaffold in vivo and also release natural angiogenic and mineralization factors that promote bone growth [65]. Farrell, E, et al used a bilayered hydrogel with chondrogenically primed cells, MSCs, in one layer and stable cartilage, chondrocytes, on top. They reported success in finding enhanced chondrogenesis in the cartilage layer and mineralization of the MSC-seeded layer when cultured in a hypertrophic medium with osteogenic supplements [65].
Recently Vunjak-Novakovic’s lab successfully engineered autologous grafts for facial bone reconstruction. Bhumiratana et al. describe the process in which they grew an anatomically precise RCU and repaired a large defect in the jaw of a Yucatan mini-pig without using BMPs or other growth factors. Instead, they used the decellularized native bovine bone matrix to induce osteogenic differentiation of ASCs [24]. By utilizing computer-aided micromilling guided by three-dimensional reconstructions of CT images of each individual pig jaw, precise anatomically shaped scaffolds were customized to each animal. All scaffolds were cultured for 3 weeks with autologous porcine ASCs in an anatomically shaped, perfused bioreactor system with tight control of exchange of nutrients, metabolites, and oxygen [23]. Fourteen mini-pigs had their left condyle resected to create a standardized defect and were then either reconstructed with a tissue-engineered scaffold, cell-free scaffold, or not reconstructed. Ultimately, through sequential CT-imaging, sacrifice, and histological and bone marker assay analysis, the investigators demonstrated that over 6 months of implantation, the engineered grafts reestablished the entire RCU, integrated with host bone, and formed extensively vascularized bone-like tissue that was significantly different than both control groups (Fig. 15.3).
The bioengineered RCU successfully reconstructed a load-bearing joint. Multiple previously described proof-of-concept studies, such as those looking into scaffold material, bioreactor design, cell seeding, stem cell selection, and stem cell differentiation, were all combined and in one successful investigation. The investigation also mimicked the logistics of a future commercial process. Grafts were grown and implanted at two locations greater than 1200 miles apart. This would allow surgeons to send images and patient information to a centralized bioengineering center, which would then fabricate and return a custom bioengineered scaffold loaded with cells to the treating surgeon.
To summarize, as we focus on temporomandibular joint reconstruction, there are a few principles to highlight when reviewing replacement of the TMJ in the literature moving forward. First, it is important to create an osteochondral construct. Cartilage is believed to be necessary for maintaining a stable functional joint as it enables friction-free physiologic activity. Further, both cell layers—cartilaginous and bony—need to be able to repair themselves and have cells capable to regenerate as a healthy joint would. To complement the accurate cellular makeup of two tissues, correct geometry of the anatomy to allow for precise joint mechanics is of equal importance. This depends on anatomically shaped scaffolds, bioreactors, and the advent of CAD with either 3D printing or micromilling. For any future RCU construct to be successful, patients will have to be imaged using computed tomography and then have scaffolds fabricated via solid free-form fabrication (SFF) [66]. This technique simply uses the patient-specific imaging and allows engineers to design scaffolds with the specific internal architecture of the target, in our case, the TMJ. This helps optimize mechanical properties that are of most importance when fabricating load-bearing, stratified osteochondral joints [67].
10 Articular Disc
Although we have discussed briefly the principles of bioengineering cartilage as it pertains to osteochondral constructs, the process of engineering the disc itself is unique and worth reviewing. Cartilage responds poorly to injury due to its avascular and acellular makeup. Unfortunately, damage to cartilage is often progressive, leading to subchondral bone remodeling, and ultimately osteoarthritis. However, due to its avascular nature, the tissue is also immunoprivileged and therefore engineered cartilage replacements do not generate large immune responses [68, 69]. In addition, the ECM of cartilage is dense and does not enable cells from within the matrix to repair damage at a distant site, further adding to the reparative challenge of cartilaginous tissue.
Load-bearing joints and their cartilaginous tissues have received a great deal of attention primarily in the orthopedic community. It is important to remember that only the TMJ disc and the meniscus in the knee are composed of fibrocartilage. Fibrocartilage differs from hyaline cartilage by its histomorphology and the ratio and amounts of collagen type I and II, with the TMJ disc nearing a ratio close to one (Col I/II), with hyaline cartilage close to zero (Col I/II) [70]. Nonetheless, many of the principles of cartilage reconstruction from orthopedics are still transferrable to the TMJ.
The cell choice for engineering cartilaginous tissues has proven to be more difficult than for engineering bone. MSC-derived chondrocytes have a tendency to hypertrophy, mineralize, and undergo endochondral ossification, which is not acceptable for cartilage replacement applications. For cartilage tissue alone, bone marrow, synovium, and periosteum are the best sources for MSC-induced chondrogenesis [71]. Synovium-derived MSCs, referred to as SDSCs, are believed to have the greatest potential to produce cartilaginous ECM when supplemented appropriately [72,73,74,75]. SDSCs can undergo chondrogenesis in vitro when combined with specific growth factors such as basic fibroblast growth factor (bFGF), and insulin-like growth factor I, TGF-β1 [72]. Until recently, MSCs were much more difficult to use in cartilage tissue engineering than juvenile chondrocytes, until a breakthrough finding revealed that a condensation step needed to be added, forming condensed mesenchymal bodies [76]. The key for TMJ-targeted tissue will be to find the best combination to produce in vivo conditions of collagen type I.
In 1991 the first pilot study on TMJ disc growth was reported by using TMJ cartilage from New Zealand white rabbits and mixing them with collagen type I to inject into a collagen matrix in vitro [77]. A few years later, another group demonstrated true hyaline cartilage growth in the shape of a TMJ disc and biomaterial polymer success and explored the biomechanical nature of their constructs [78]. These early investigations frequently harvested mature chondrocytes from newborn calves as their cell source. However the TMJ disc is composed of fibrocartilage, not hyaline cartilage.
A decade after the first investigation of biomaterials for disc replacement, researchers found that, unlike other joint engineering success, TMJ chondrocytes (isolated from porcine discs) prefer PGA non-woven meshes when compared to alginate hydrogels [79]. There has been more recent success using an alternative material, poly (glycerol sebacate) (PGS) as a scaffold material in growing fibrocartilage through their experimental process, where they found both cell seeding time and density were important variables for success [80].
There is an alternative approach to cell-based, scaffold models for TMJ disc replacement. Brown BN, et al followed up on their original pilot study by more thoroughly investigating the use of an acellular, scaffold-based approach for disc replacement [81, 82]. They creatively used decellularized porcine urinary bladder tissue (urinary bladder matrix (UBM)) alone as a scaffold without any isolated chondrocytes, MSCs, or SDSCs, to serve as an interpositional graft and inductive template for reconstruction of the disc in vivo [81]. They prepared the UBM and layered it onto a hard plastic mold, which mimicked the approximate TMJ disc size. Following complete, bilateral disc removals on ten adult female mongrel dogs, each subject received one graft and had the contralateral side left alone. Animals were sacrificed at 6 months, and analysis indicated that multiple tissue types formed throughout the scaffold, and histology showed architecture highly analogous to native disc tissues [82]. If reproducible, this approach to replacement would serve as a stock packaged option for replacement during TMJ surgery.
In recent years, multiple studies exploring in vitro fibrocartilage for the TMJ disc have been performed finding answers to important clinical questions. It is believed that either costal chondrocytes or articular chondrocytes are both superior cell sources for disc engineering than TMJ disc cells [83]. When comparing IGF, TGF-β1, and bFGF, TGF-β1 demonstrated the greatest ability to produce ECM, glycosaminoglycans (GAGs), and collagen [84, 85]. Scaffoldless constructs may be better than cell-seeded scaffold constructs [70]. Yet, all current methods are still inferior in strength and chemical makeup to native TMJ disc tissue [70, 85,86,87]. Perhaps a more focused stem cell approach may be the next wave of fibrocartilage research for TMJ disc investigators.
11 Future Directions and Challenges
Significant advances in the field of tissue engineering as it pertains to the RCU have been made. As with all translational research, there is still much to be done moving forward.
Ideally a standardized scaffold for the RCU could be developed. For example, either decellularized bovine bone or one synthetic option such as PGA would be optimized and available for all researchers to then proceed with further research focusing on other aspects. Also, many studies have done only in vitro or ex vivo synthesis on a small scale. Groups need to ensure they are able to not only scale up their tissues but to employ methods like CAD/CAM to ensure their constructs are anatomically precise and unique.
ASCs seem to be a promising cell type that is easily accessible and useful for hard tissue. As we have discussed, some groups use growth factors and others do not. A standard cocktail for media would help standardize experiments across all laboratories trying to answer the same clinical question. In view of the current controversies surrounding the use of growth factors, the ideal culture medium would be designed without them.
Creating an osteochondral construct with a bilayered system of bone and cartilage that would be able to repair itself and thrive in vivo still remains to be seen. Most studies that have done animal implantation have sacrificed the animals at around 6 months postimplantation. True long-term viability data is needed; can these grafts survive long term as a load-bearing joint?
Another challenge in maxillofacial reconstruction is the need to replace a large volume of soft tissue and bone. The workhorse of maxillary and mandibular reconstruction of cancer patients who have undergone ablative surgery is the microvascular free fibula graft. The use of an engineered bone construct without an adequate vascularized soft tissue bed will be unsuccessful. One technique that has been reported would be to implant the graft into a muscle bed and then bring the muscle with feeding and draining vessels and the graft to the recipient site. However, this requires two surgeries and a second surgical site at the time of tissue transfer. Ultimately an engineered construct of bone enveloped into a soft tissue of appropriate size with blood vessels for anastomosis would be ideal and allow for wide applicability of engineering techniques for facial reconstruction in acquired and congenital disease.
An important question is if the disc and RCU engineering and implantation can be coupled together. There does not seem to be any investigations at this point that have resulted in implanting a bioengineered RCU and articular disc simultaneously. Using bioreactors for osteochondral constructs allowing for the formation of both tissues would be groundbreaking. Perhaps, fabrication of a dual-compartment bioreactor would allow precise control of two separate environments, one chondrogenic and one osteogenic.
Also, no current studies address muscle reattachment of the external pterygoids. Alloplastic total joint replacement does not allow for lateral or protrusive movement because of the inability of the lateral pterygoid muscle to attach to the alloplast. This is another important area of research for total ramus condyle reconstruction utilizing engineered biologic constructs.
A possible augmenting area of bioengineering is gene therapy. Gene therapy depends on the transfer of genetic material into living cells in order to regenerate tissue, treat a disease process, or silence the unwanted gene expression. Through viral transfection or non-viral physical and chemical means, manipulated genetic material is taken up by the host cells that begin to express the transfected proteins of the selected gene (e.g., BMP-2, bFGF, etc.). For example, if increased levels of BMP-2 in vivo help bone regeneration from TMJ osteoarthritis, transfected cells can be injected locally into the joint space or necessary area, BMP-2 will be upregulated in the local environment, and bone regeneration will occur. The possibilities of gene therapy, if controlled, can be endless. They do come with risk, however, as viral infection is one of the more common ways of transfecting target cells. The possibility of gene silencing and editing strategies with methylation and miRNA, respectively, will hopefully expand gene therapy utility in the future without the risk for infection.
Some investigations for bone regeneration have already demonstrated great promise with this modality. In rabbits, orbital bony defects were repaired with BMP-2- and VEGF- transfected rabbit BMSCs [88]. Maxillofacial-derived stem cells, when transfected with osteogenic gene BMP-2 via adenoviral vector, also showed high utility in treating a mandibular bony defect with high expression levels of the desired growth factor [89]. This is an avenue worth exploring to repair bony defects throughout the cranio-maxillofacial skeleton and possibly pairing with RCU engineering.
12 Conclusion
Numerous studies over the past decade provided opportunities for what the future of TMJ bioengineering may hold. Anywhere from stock disc replacement to custom, anatomical autologous condylar reconstruction will broaden the reconstructive surgeon’s armamentarium to help the patients in need. We look forward to an integrated use of biomaterials, bioactive factors, and cells toward serving the patients’ needs.
References
Okeson J. Management of temporomandibular disorders and occlusion. St. Louis: Elsevier; 2008.
Dimitroulis G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int J Oral Maxillofac Surg. 2011;40(6):561–8.
Wagner JD, Mosby EL. Assessment of Proplast-Teflon disc replacements. J Oral Maxillofac Surg. 1990;48(11):1140–4.
Chuong R, Piper MA. Cerebrospinal fluid leak associated with proplast implant removal from the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1992;74(4):422–5.
Mercuri LG, Urban RM, Hall DJ, Mathew MT. Adverse local tissue responses to failed temporomandibular joint implants. J Oral Maxillofac Surg. 2017;75(10):2076–84.
Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–32.
Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4(160):160rv12.
Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88.
Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis. 2010;16(8):709–16.
Meinel L, Karageorgiou V, Fajardo R, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32(1):112–22.
Mano JF, Silva GA, Azevedo HS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4(17):999–1030.
Hofmann S, Knecht S, Langer R, et al. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006;12(10):2729–38.
Lovett M, Eng G, Kluge JA, et al. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis. 2010;6(4):217–24.
Altman GH, Horan RL, Lu HH, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131–41.
Correia C, Bhumiratana S, Yan LP, et al. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012;8(7):2483–92.
Petrie C, Tholpady S, Ogle R, Botchwey E. Proliferative capacity and osteogenic potential of novel dura mater stem cells on poly-lactic-co-glycolic acid. J Biomed Mater Res A. 2008;85(1):61–71.
Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–8.
Ciocca L, Donati D, Fantini M, et al. CAD-CAM-generated hydroxyapatite scaffold to replace the mandibular condyle in sheep: preliminary results. J Biomater Appl. 2013;28(2):207–18.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43.
Grayson WL, Chao PH, Marolt D, Kaplan DL, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 2008;26(4):181–9.
Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–24.
Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61(12):1065–83.
Grayson WL, Frohlich M, Yeager K, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3299–304.
Bhumiratana S, Bernhard JC, Alfi DM, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8(343):343ra83.
Frohlich M, Grayson WL, Marolt D, et al. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16(1):179–89.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9.
Roelofs AJ, Zupan J, Riemen AHK, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017;8:15040.
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85(11):966–79.
Kang SK, Putnam LA, Ylostalo J, et al. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004;117(Pt 18):4289–99.
Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells. 2008;26(10):2713–23.
Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65(8):1640–7.
Weng Y, Cao Y, Silva CA, Vacanti MP, Vacanti CA. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg. 2001;59(2):185–90.
Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells. 2014;6(3):312–21.
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47.
Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36(4):585–97.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–30.
Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12.
Haniffa MA, Wang XN, Holtick U, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179(3):1595–604.
Sessarego N, Parodi A, Podesta M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93(3):339–46.
Yan XL, Fu CJ, Chen L, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132(1):153–64.
Noel D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314(7):1575–84.
Williams KJ, Picou AA, Kish SL, et al. Isolation and characterization of porcine adipose tissue-derived adult stem cells. Cells Tissues Organs. 2008;188(3):251–8.
Halvorsen YD, Franklin D, Bond AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7(6):729–41.
Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247–52.
Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310(1–2):52–62.
Herford AS, Boyne PJ, Rawson R, Williams RP. Bone morphogenetic protein-induced repair of the premaxillary cleft. J Oral Maxillofac Surg. 2007;65(11):2136–41.
Liu Q, Cen L, Yin S, et al. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29(36):4792–9.
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32(6):370–3.
Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364(9436):766–70.
Warnke PH, Wiltfang J, Springer I, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27(17):3163–7.
Abukawa H, Terai H, Hannouche D, et al. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61(1):94–100.
Abukawa H, Shin M, Williams WB, et al. Reconstruction of mandibular defects with autologous tissue-engineered bone. J Oral Maxillofac Surg. 2004;62(5):601–6.
Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82(12):951–6.
Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87(5):936–44.
Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9(3):5484–92.
Chen J, Chen H, Li P, et al. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32(21):4793–805.
Re’em T, Witte F, Willbold E, Ruvinov E, Cohen S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater. 2012;8(9):3283–93.
Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–66.
Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A. 2012;18(11–12):1161–70.
Farrell E, van der Jagt OP, Koevoet W, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15(2):285–95.
Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–24.
Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10(9–10):1376–85.
Ng KW, Lima EG, Bian L, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16(3):1041–51.
McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411–20.
Lowe J, Almarza AJ. A review of in-vitro fibrocartilage tissue engineered therapies with a focus on the temporomandibular joint. Arch Oral Biol. 2017;83:193–201.
Tan AR, Hung CT. Concise review: mesenchymal stem cells for functional cartilage tissue engineering: taking cues from chondrocyte-based constructs. Stem Cells Transl Med. 2017;6(4):1295–303.
Sampat SR, O'Connell GD, Fong JV, et al. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17(17–18):2259–65.
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–9.
Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 2009;15(1):75–86.
Kim JH, Lee MC, Seong SC, Park KH, Lee S. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;17(7–8):991–1002.
Bhumiratana S, Eton RE, Oungoulian SR, et al. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014;111(19):6940–5.
Thomas M, Grande D, Haug RH. Development of an in vitro temporomandibular joint cartilage analog. J Oral Maxillofac Surg. 1991;49(8):854–6; discussion 57.
Puelacher WC, Wisser J, Vacanti CA, et al. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J Oral Maxillofac Surg. 1994;52(11):1172–7; discussion 77–8.
Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10(11–12):1787–95.
Hagandora CK, Gao J, Wang Y, Almarza AJ. Poly (glycerol sebacate): a novel scaffold material for temporomandibular joint disc engineering. Tissue Eng Part A. 2013;19(5–6):729–37.
Brown BN, Chung WL, Pavlick M, et al. Extracellular matrix as an inductive template for temporomandibular joint meniscus reconstruction: a pilot study. J Oral Maxillofac Surg. 2011;69(12):e488–505.
Brown BN, Chung WL, Almarza AJ, et al. Inductive, scaffold-based, regenerative medicine approach to reconstruction of the temporomandibular joint disk. J Oral Maxillofac Surg. 2012;70(11):2656–68.
Anderson DE, Athanasiou KA. Passaged goat costal chondrocytes provide a feasible cell source for temporomandibular joint tissue engineering. Ann Biomed Eng. 2008;36(12):1992–2001.
Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333(3):439–47.
Kalpakci KN, Kim EJ, Athanasiou KA. Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering. Acta Biomater. 2011;7(4):1710–8.
MacBarb RF, Chen AL, Hu JC, Athanasiou KA. Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials. 2013;34(38):9980–9.
Hagandora CK, Tudares MA, Almarza AJ. The effect of magnesium ion concentration on the fibrocartilage regeneration potential of goat costal chondrocytes. Ann Biomed Eng. 2012;40(3):688–96.
Xiao C, Zhou H, Liu G, et al. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater. 2011;6(1):015013.
Steinhardt Y, Aslan H, Regev E, et al. Maxillofacial-derived stem cells regenerate critical mandibular bone defect. Tissue Eng Part A. 2008;14(11):1763–73.
Acknowledgments
The authors gratefully acknowledge funding of this work by NIH (grants DE016525 and EB002520).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Eisig, S.B., Forman, M., Vunjak-Novakovic, G. (2019). Bioengineered Constructs of the Ramus/Condyle Unit. In: Connelly, S.T., Tartaglia, G.M., Silva, R.G. (eds) Contemporary Management of Temporomandibular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-99909-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-99909-8_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99908-1
Online ISBN: 978-3-319-99909-8
eBook Packages: MedicineMedicine (R0)