Abstract
Angiogenesis in the stroke brain has been increasingly recognized as a key player in tissue repair, endogenous neurogenesis, and functional recovery. Important findings in the last decade have shown that promoting the formation of a mature and functional vascular network in a wound that is dynamic in space and time represents one of the biggest challenges in pro-repair therapies after stroke. Recent preclinical studies of therapeutic angiogenesis have been introducing tissue engineering-based systems that allow precisely controlled delivery of provascular drugs directly to the site of damage, without inducing the commonly associated side effects.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
According to the American Heart Association, 15 million people suffer ischemic stroke worldwide each year [5]. Of these, five million die and five million are permanently disabled. These sobering statistics demonstrate stroke’s global impact and emphasize the importance of deepening our understanding in order to design efficient treatments. To date, the overwhelming majority of brain repair research focuses on developing therapies that promote tissue repair through neuronal replacement with neuronal-derived progenitor cell transplantation or pro-neuronal drugs administration [23]. However, extensive and unsuccessful clinical trials show that this approach does not lead to long-term development of all brain cell types or the establishment of functional interactions between the different networks. Both experimental and clinical studies have reported that angiogenesis and neurogenesis in the ischemic brain work in tandem [26] and that enhanced vessel formation and restored perfusion after stroke reduces the severity of damage [31], promotes tissue repair [24], and significantly extends survival of stroke patients [20]. Therefore, developing pro-angiogenic regenerative therapies offers tremendous potential in stroke treatment. Preclinical studies using provascular growth factor and progenitor cells show promising results in enhancing revascularization and tissue repair after stroke. The therapeutic outcome, however, is often limited by poor survival of transplanted cells, uncontrolled cell differentiation, non-sustained delivery of growth factors, and ineffective engraftment with the host tissue. A tissue engineering approach provides an alternative for treating the damaged brain, as it provides multifunctional solutions to overcome the limitations of conventional approaches. In this chapter, we focus on the limitations of current pro-angiogenic therapies using vascular growth factors and some recent advances using engineered biomaterials and drug delivery systems for brain tissue repair.
7.2 The Neurovascular Damage after Stroke
The neuron has traditionally been viewed as the centerpiece of the mammalian central nervous system (CNS ) because of its fundamental role in neurotransmission. Thus, the classical approach of neurorepair was solely based on neuroprotection and neuron cell replacement. Over the past decade, remarkable advances have been made in understanding the mechanisms of neurorepair, such as neurogenesis (formation of new neurons), angiogenesis (formation of new blood vessels), gliogenesis (formation of new glial cells), and re-myelinization, among other processes. These discoveries changed the classical view of brain repair, providing greater understanding of the brain as a whole and supporting the hypothesis that saving neurons alone may not be sufficient [8]. This concept of a “neurovascular unit” defines tissue remodeling as a highly dynamic process between vascular, neuronal, and glial cells. After stroke, these cells respond to injury in a coordinated and synergistic manner, releasing molecular signals and trophic factors that mutually influence each other to create a pro-repair environment where tissue regeneration and neurological recovery may take place [32]. In recent years, the promotion of vascular growth and remodeling in the injured brain is increasingly recognized as a particularly promising approach to treat brain illnesses [9]. This approach has brought confidence that stimulating the growth of new vessels both in and around the ischemic site may stabilize brain perfusion and promote tissue repair through neuronal survival, plasticity, and functional recovery [13, 14, 18].
7.3 Spatiotemporal Dynamic of Brain Angiogenesis
At the core of an ischemic incident , the severe energy and glucose loss induces a rapid neuronal death , leaving behind a necrotic site within minutes after the stroke onset. However, the surrounding area, called penumbra, has a mild-to-moderate vascular compromise, where the energy deficit is less severe and collateral vessels maintain a certain degree of blood flow (Fig. 7.1).
Schematic of the human brain with the core of the ischemic core and the penumbra. Figure reprinted from [10] with the permission from Elsevier
This area may be rescued if blood flow is restored [10]. Increasing evidence, in both human stroke patients and animal experimental models, suggests that angiogenesis occurs in those penumbra areas [28]. Indeed, an increasing body of evidence suggests that newly formed vessels in the periphery of the injured site mediate recovery via the activation of endogenous mechanisms of plasticity [7]. In particular, it was shown that angiogenesis and neurogenesis not only work in tandem but are also causally linked. The daily administration of an angiogenesis inhibitor, in a mouse model of ischemic stroke, is associated with a significant reduction of proliferation, migration, and differentiation of endogenous neural progenitors which impairs their functional recovery [24, 26]. In addition, aggregate data supports a “cleanup hypothesis,” whereby the newly formed vessels in the penumbra serve to facilitate macrophage infiltration and clear up necrotic debris [22, 37].
7.4 Role of VEGF in Endogenous Angiogenesis
In both experimental and clinical studies, enhanced vessel formation and restored perfusion in the ischemic border correlate with improved long-term recovery and longer survival times [20]. Older patients, who tend to do worse after stroke, often show reduced new vessel formation in the penumbra [2, 12, 34]. Similarly, patients who develop dementia after stroke have reduced blood flow in cortical regions adjacent to the stroke [30]. Pro-repair factors correlated with brain angiogenesis have also been extensively assessed in experimental stroke models. One major endogenous signal, vascular endothelial growth factor (VEGF) is found in both neurons and astrocytes after cerebral ischemia [1, 39]. A positive correlation was found between the severity of damages and the concentration of VEGF in stroke patients [31]. Likewise, postmortem studies reveal an increased expression of the pro-angiogenic factor and its receptor VEGFR-2, both at mRNA and protein levels in the penumbra [17]. In addition, studies have shown that a deficit in VEGF distribution is associated with disorganized and impaired vessel formation, suggesting that ECM-binding VEGF isoforms provide essential stimulatory cues to initiate vessel branching [29].
7.5 Current VEGF-Based Therapeutic Approach
Interestingly, studies with therapeutic VEGF application revealed quite different effects, depending on the timing of administration and the route of growth factor delivery. The systemic or intracerebral administration of VEGF, within the first 48 h after stroke onset, was associated with an increased blood-brain barrier opening and subsequent edema and promoted the formation of disorganized and immature vasculature [39]. Likewise, the early administration of an antagonist for endogenous VEGF reduced the ischemia−/reperfusion-related brain edema and injury [35]. Similarly, infusing VEGF into the lateral ventricles stimulated angiogenesis and decreased infarct volume in rodent models of cerebral ischemia [33]. In transgenic mice overexpressing human VEGF 165, brain microvessel density was significantly increased, compared to wild-type before and after ischemia [36]. In addition, VEGF is associated with a reduced infarct volume when topically applied on the surface of the reperfusion site in a transient model of cerebral ischemia [15]. A completely different picture resulted from studies where VEGF was administered repeatedly [33], in delayed delivery (after day 3) [16] or when the growth factor was encapsulated in a hydrogel [11]. These studies consistently found improvement in neurological deficits following VEGF therapy, along with a reduction in ischemic injury, brain edema, and blood-brain barrier permeability for serum proteins, with an overall better effect when administered locally rather than systemically [21]. This suggests that maintaining elevated tissue levels of VEGF in the stroke site, for prolonged periods of time along with controllable spatial distribution, dosage, and duration of exposure, satisfies many of the characteristics of an ideal delivery system , by overcoming the main limitations of poor penetration across the blood-brain barrier, the clinically unviable option of repeated local injections, and the short VEGF half-life time.
7.6 Tissue Engineering Approach to Promote Brain Angiogenesis
Recent advances in our understanding of the central nervous system and the development of sophisticated biomaterials have significantly changed the landscape for developing strategies to repair the damaged brain. Tissue engineering approaches now offer the potential to design biomaterials tailored for particular applications such as hydrogels to fill out the stroke site, where necrotic tissue gives place to an empty compartmentalized cavity. These biomaterials offer a physical support for cell infiltration in a highly inflamed and damaged tissue, and present the ability to protect and enhance the beneficial effect of provascular growth factors, known to have a short half-life time and severe side effects due to its ability to promote vascular permeability [39]. Developing engineered pro-repair approaches is a scientific challenge on many levels, since (1) therapeutic agents must remain alive/active during and after brain transplantation, (2) the success of a therapy is widely dependent on the interaction between the transplanted/injected material and the host tissue, (3) the material degradation rate and composition must be finely tuned to allow tissue growth, and (4) the success of a pro-repair therapy relies on the simultaneous activation of axonal sprouting, vascular growth, neurogenesis, and modulation of the injury-induced inflammation, each presenting unique biological challenges.
In recent years, several experimental studies developed injectable hydrogels and growth factor delivery systems to promote poststroke angiogenesis through VEGF administration. A 2010 study from Emerich D.F. et al. describes the effect of an injectable VEGF-loaded alginate-based hydrogel on poststroke functional recovery [11]. Behavioral testing shows that the performance of rats, receiving VEGF gels in the striatum 15 min prior to stroke, was significantly improved relative to a blank gel with or without VEGF. This study strengthens previous evidence that VEGF exerts neuroprotective properties when upregulated prior to injury.
Zhang et al. attempt to restore tissue integrity in a rat model of brain damage by implanting a porous polydimethylsiloxane-tetraethoxysilane (PDMS-TEOS) hybrid with or without VEGF [38]. Despite a low infiltration of vessels in the injected materials, the VEGF-loaded gel was associated with a significant increase of proliferating endothelial cells within the implanted material (Fig. 7.2). However, the use of a non-injectable material does not meet the clinical need for a minimally invasive therapy.
Transplantation of a porous PDMS-TEOS material with VEGF in a rat model of cortical brain damage. Double immunofluorescence study showing proliferating cells (a) endothelial cells (b) and a merge image of both stainings (c) within the implanted material. Figure reprinted from [38] with the permission from Elsevier
A similar approach was used by Ju et al. with a hyaluronic acid (HA)-based hydrogel mixed with poly(lactic-co-glycolic) acid (PLGA) microspheres containing VEGF and angiopoietin-1 [19]. The authors report that the implantation of the HA-PLGA composite in the cortical lesion significantly increased blood vessel density and reduced inflammation in the peri-infarct. Although this material demonstrated potential for promoting angiogenesis , the HA-PLGA composite’s non-injectability reduces its potential for clinical translation. Interestingly, an injection of the same VEGF-releasing PLGA particles in the stroke cavity was associated with poor vascular effect, suggesting that the presence of a matrix-derived scaffold is required to provide a structural support to growing vessels [6]. Recently, Oshikawa et al. developed an implantable porous laminin-based sponge material that immobilizes histidine-tagged VEGF via affinity interactions [27]. The authors report that the laminin-VEGF transplantation into the injured cortex significantly increased vessel density in and around the transplant, compared with laminin gel with and without soluble VEGF. However, this non-injectable material is invasive and would require further modification to be clinically translatable.
In order to fill the need for an injectable scaffold that provides both structural support and delivers multiple provascular factors in a sustained manner, we developed a delivery system where VEGF is encapsulated in protease-specific, cleavable peptides, in which degradation rate controls the VEGF release in the brain [40]. We showed that the combination of VEGF nanocapsules and HA hydrogel with fibronectin-derived RGD peptide can be injected directly into the stroke cavity, which promotes greater vessel formation and pericyte recruitment, in and around the lesion site, compared with empty HA and HA + soluble VEGF (Fig. 7.3).
Temporal control of HA-VEGF nanocapsule delivery in a mouse model of ischemic stroke. (a) Representative fluorescent images of blood vessels (Glut-1) and pericytes (PDGFR-β) in (+) and around the lesion site injected with HA-RGD alone (control), with soluble VEGF (VEGF), or with nanocapsules of VEGF (n(VEGF)) and (b) quantitative analysis. Anova with Tukey test’s posttest, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars = 100 μm. Figure reprinted from [40] with the permission from Elsevier
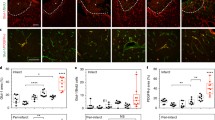
Recently, we showed that displaying VEGF in a clustered conformation by immobilizing covalently the growth factor on nanoparticles of heparin and controlling the discrete distribution on the particle’s surface modulates vascular sprouting and endothelial proliferation in vitro [4]. In addition, the severe inflammatory response after stroke is widely regarded as the cause that impedes axonal growth in the damaged central nervous system [3]. Thus, we hypothesized that the brain administration of a dual-function engineered biomaterial with both immunomodulatory and angiogenic properties, directly to the stroke cavity, can promote tissue formation de novo by modulating poststroke inflammatory response and vessel growth. For this, male adult mice were subjected to an ischemic stroke and injected with a hyaluronic acid-based hydrogel containing heparin nanoparticles and different clusterization densities of bound VEGF onto heparin particle’s surface [25]. We found that the highly clustered VEGF lead to the highest degree of vascular growth in and around the stroke site (Fig. 7.4). Moreover, the addition of naked heparin particles generates a vascularized network of regenerated functional axonal connections that leads to recovery and reduces the poststroke inflammation. These results are lost with the absence or reduction of bound VEGF where only the immunomodulator effect is observed and with the absence or reduction of heparin particles where angiogenesis is no longer associated with axonal sprouting.
(a) Schematic representing a brain injectable hydrogel composed of hyaluronic acid (HA), RGD motif peptide, and metalloproteinase-sensitive cross-linker. The gel was loaded with highly clustered VEGF (hcV) covalently bound onto heparin nanoparticle’s surface and injected in a mouse model of ischemic stroke. (b) Vascular (Glut-1, red) and axonal growth (neurofilament NF200, green) was evaluated 16 weeks after gel implantation and compared with control groups treated with an empty gel, or gel loaded with soluble VEGF (Vs), low clusters of VEGF (lcV), or hcV and endostatin (angiogenesis inhibitor). The results show a significantly increased tissue growth in the hcV group, with a close association of axonal and vascular networks (c, d). Data are presented using a minimum-to-maximum box plot. Each dot in the plots represents one animal, and p values were determined by one-way ANOVA with Tukey’s post hoc test. **p < 0.01, ****p < 0.0001. Scale bar, 100 μm. Figure reprinted from [25] with the permission from Elsevier
7.7 Conclusion
The use of pro-angiogenic materials, for focal and controlled delivery of vascular growth factors to the brain, is an emerging discipline in the field of brain repair. Although these materials have demonstrated potential in activating endogenous endothelial cells in and around the damaged tissue, their ability to form vascular structure de novo in the injected site is limited. Future work in this area needs to focus on fine-tuning the optimal morphological features and to design macro- and microarchitecture that mimics the native matrix and environment. In addition, these materials would be best as an injectable therapy to increase their likelihood of clinical translation in the future and also provide a physical support with mechanical properties similar to brain tissue. Furthermore, long-term studies are needed to fully understand the biological effect of the hydrogels and their degradation over time. Finally, further investigation on dual therapies combining pro-angiogenic and immunomodulator properties is needed to determine the role of inflammation on angiogenesis-induced tissue repair. Taken together, a tissue engineering approach to provascular therapies presents challenges that need to be overcome, before provascular materials are a valuable alternative for the clinical treatment of brain damage.
References
Abe, K., Setoguchi, Y., Hayashi, T., & Itoyama, Y. (1997). Dissociative expression of adenoviral-mediated E. coli LacZ gene between ischemic and reperfused rat brains. Neuroscience Letters, 226, 53–56.
Allen, C. M. (1984). Predicting the outcome of acute stroke: A prognostic score. Journal of Neurology, Neurosurgery, and Psychiatry, 47, 475–480.
Anderson, M. A., Burda, J. E., Ren, Y., Ao, Y., O’Shea, T. M., Kawaguchi, R., et al. (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature, 532, 195–200.
Anderson, S. M., Siegman, S. N., & Segura, T. (2011). The effect of vascular endothelial growth factor (VEGF) presentation within fibrin matrices on endothelial cell branching. Biomaterials, 32, 7432–7443.
Benjamin, E. J., Blaha, M. J., Chiuve, S. E., Cushman, M., Das, S. R., Deo, R., et al. (2017). Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation, 135, e146–e603.
Bible, E., Qutachi, O., Chau, D. Y. S., Alexander, M. R., Shakesheff, K. M., & Modo, M. (2012). Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials, 33, 7435–7346.
Castellanos, M., Sobrino, T., & Castillo, J. (2006). Evolving paradigms for neuroprotection: Molecular identification of ischemic penumbra. Cerebrovascular Diseases, 21(Suppl 2), 71–79.
Chopp, M., Li, Y., & Zhang, J. (2008). Plasticity and remodeling of brain. Journal of the Neurological Sciences, 265, 97–101.
del Zoppo, G. J., & Mabuchi, T. (2003). Cerebral microvessel responses to focal ischemia. Journal of Cerebral Blood Flow and Metabolism, 23, 879–894.
Dirnagl, U., Iadecola, C., & Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends in Neurosciences, 22, 391–397.
Emerich, D. F., Silva, E., Ali, O., Mooney, D., Bell, W., Yu, S. J., et al. (2010). Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplantation, 19, 1063–1071.
Granger, C. V., Hamilton, B. B., & Fiedler, R. C. (1992). Discharge outcome after stroke rehabilitation. Stroke, 23, 978–982.
Greenberg, D. A. (1998). Angiogenesis and stroke. Drug News & Perspectives, 11, 265–270.
Greenberg, D. A., & Jin, K. (2007). Regenerating the brain. International Review of Neurobiology, 77, 1–29.
Hayashi, T., Abe, K., & Itoyama, Y. (1998). Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. Journal of Cerebral Blood Flow and Metabolism, 18, 887–895.
Herz, J., Reitmeir, R., Hagen, S. I., Reinboth, B. S., Guo, Z., Zechariah, A., et al. (2012). Intracerebroventricularly delivered VEGF promotes contralesional corticorubral plasticity after focal cerebral ischemia via mechanisms involving anti-inflammatory actions. Neurobiology of Disease, 45, 1077–1085.
Issa, R., Krupinski, J., Bujny, T., Kumar, S., Kaluza, J., & Kumar, P. (1999). Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Laboratory Investigation, 79, 417–425.
Jin, K., Mao, X. O., & Greenberg, D. A. (2006). Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via rho kinase signaling. Journal of Neurobiology, 66, 236–242.
Ju, R., Wen, Y., Gou, R., Wang, Y., & Xu, Q. (2014). The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplantation, 23(Suppl 1), S83–S95.
Krupinski, J., Kaluza, J., Kumar, P., Kumar, S., & Wang, J. M. (1994). Role of angiogenesis in patients with cerebral ischemic stroke. Stroke, 25, 1794–1798.
Ma, Y., Zechariah, A., Qu, Y., & Hermann, D. M. (2012). Effects of vascular endothelial growth factor in ischemic stroke. Journal of Neuroscience Research, 90, 1873–1882.
Manoonkitiwongsa, P. S., Jackson-Friedman, C., McMillan, P. J., Schultz, R. L., Lyden, P. D., et al. (2001). Angiogenesis after stroke is correlated with increased numbers of macrophages: The clean-up hypothesis. Journal of Cerebral Blood Flow and Metabolism, 21, 1223–1231.
Moshayedi, P., Nih, L. R., Llorente, I. L., Berg, A. R., Cinkornpumin, J., Lowry, W. E., et al. (2016). Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials, 105, 145–155.
Nih, L. R., Deroide, N., Leré-Déan, C., Lerouet, D., Soustrat, M., Levy, B. I., et al. (2012). Neuroblast survival depends on mature vascular network formation after mouse stroke: Role of endothelial and smooth muscle progenitor cell co-administration. The European Journal of Neuroscience, 35, 1208–1217.
Nih, L. R., Gojgini, S., Carmichael, S. T., & Segura, T. (2018). Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nature Materials, 17, 642–651.
Ohab, J. J., Fleming, S., Blesch, A., & Carmichael, S. T. (2006). A neurovascular niche for neurogenesis after stroke. The Journal of Neuroscience, 26, 13007–13016.
Oshikawa, M., Okada, K., Kaneko, N., Sawamoto, K., Ajioka, I., et al. (2017). Affinity-immobilization of VEGF on laminin porous sponge enhances angiogenesis in the ischemic brain. Advanced Healthcare Materials, 6(11), 28488337.
Rodriguez-Yanez, M., Castellanos, M., Blanco, M., Mosquera, E., & Castillo, J. (2006). Vascular protection in brain ischemia. Cerebrovascular Diseases, 21(Suppl 2), 21–29.
Ruhrberg, C., Gerhardt, H., Golding, M., Watson, R., Ioannidou, S., Fujisawa, H., et al. (2002). Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes & Development, 16, 2684–2698.
Schmidt, R., Schmidt, H., & Fazekas, F. (2000). Vascular risk factors in dementia. Journal of Neurology, 247, 81–87.
Slevin, M., Krupinski, J., Slowik, A., Kumar, P., Szczudlik, A., & Gaffney, J. (2000). Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke, 31, 1863–1870.
Slevin, M., Kumar, P., Gaffney, J., Kumar, S., & Krupinski, J. (2006). Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clinical Science, 111, 171–183.
Sun, Y., Jin, K., Xie, L., Childs, J., Mao, X. O., Logvinova, A., et al. (2003). VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of Clinical Investigation, 111, 1843–1851.
Szpak, G. M., Lechowicz, W., Lewandowska, E., Bertrand, E., Wierzba-Bobrowicz, T., & Dymecki, J. (1999). Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathologica, 37, 264–268.
van Bruggen, N., Thibodeaux, H., Palmer, J. T., Lee, W. P., Fu, L., Cairns, B., et al. (1999). VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. The Journal of Clinical Investigation, 104, 1613–1620.
Wang, Y., Kilic, E., Kilic, U., Weber, B., Bassetti, C. L., Marti, H. H., et al. (2005). VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain, 128, 52–63.
Yu, S. W., Friedman, B., Cheng, Q., & Lyden, P. D. (2007). Stroke-evoked angiogenesis results in a transient population of microvessels. Journal of Cerebral Blood Flow and Metabolism, 27, 755–763.
Zhang, H., Hayashi, T., Tsuru, K., Deguchi, K., Nagahara, M., Hayakawa, S., et al. (2007). Vascular endothelial growth factor promotes brain tissue regeneration with a novel biomaterial polydimethylsiloxane-tetraethoxysilane. Brain Research, 1132, 29–35.
Zhang, Z., Zhang, L., Jiang, Q., Zhang, R., Davies, K., Powers, C., et al. (2000). VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. The Journal of Clinical Investigation, 106, 829–838.
Zhu, S., Nih, L., Carmichael, S. T., Lu, Y., & Segura, T. (2015). Enzyme-responsive delivery of multiple proteins with spatiotemporal control. Advanced Materials, 27, 3620–3625.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nih, L.R., Carmichael, S.T., Segura, T. (2018). Pro-Angiogenic Regenerative Therapies for the Damaged Brain: A Tissue Engineering Approach. In: Gerecht, S. (eds) Biophysical Regulation of Vascular Differentiation and Assembly. Biological and Medical Physics, Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-99319-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-99319-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99318-8
Online ISBN: 978-3-319-99319-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)