Abstract
Cortical neuronal networks consist of excitatory glutamatergic principal cells (PCs) and GABAergic inhibitory interneurons (INs). Although INs form a minority of the cortical neuron population, they control key aspects of cortical network function by providing feedforward and feedback inhibition, controlling the formation of PC assemblies, defining the excitability of neuronal networks and the timing of the activation of PCs, and promoting synchrony of fast neuronal network oscillations (Freund and Buzsáki 1996; McBain and Fisahn 2001; Klausberger and Somogyi 2008; Sohal 2016; Strüber et al. 2017). INs are highly diverse and can be subdivided into several types on the basis of various criteria, such as intrinsic physiological properties, neurochemical marker content, morphological features, including the laminar distribution of the axon, and finally the postsynaptic target profile of their output (Freund and Buzsáki 1996; Hosp et al. 2014; Savanthrapadian et al. 2014; Yuan et al. 2017). On the basis of synaptic targets, INs have been classified into two major groups, perisomatic- and dendrite-targeting cells.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Overview
Cortical neuronal networks consist of excitatory glutamatergic principal cells (PCs) and GABAergic inhibitory interneurons (INs). Although INs form a minority of the cortical neuron population, they control key aspects of cortical network function by providing feedforward and feedback inhibition, controlling the formation of PC assemblies, defining the excitability of neuronal networks and the timing of the activation of PCs, and promoting synchrony of fast neuronal network oscillations (Freund and Buzsáki 1996; McBain and Fisahn 2001; Klausberger and Somogyi 2008; Sohal 2016; Strüber et al. 2017). INs are highly diverse and can be subdivided into several types on the basis of various criteria, such as intrinsic physiological properties, neurochemical marker content, morphological features, including the laminar distribution of the axon, and finally the postsynaptic target profile of their output (Freund and Buzsáki 1996; Hosp et al. 2014; Savanthrapadian et al. 2014; Yuan et al. 2017). On the basis of synaptic targets, INs have been classified into two major groups, perisomatic- and dendrite-targeting cells.
Transmission at perisomatic GABAergic synapses is characterized by fast time course and large peak amplitudes (Bartos et al. 2001, 2002; Strüber et al. 2015). Fast GABAA receptor (GABAAR)-mediated perisomatic inhibition can precisely determine the timing and the frequency of action potential discharge in PCs (Cobb et al. 1995; Miles et al. 1996; Pouille and Scanziani 2001). In contrast, inhibitory signaling mediated by dendritic GABAergic contacts has slower time course and shows high degree of diversity (Pearce 1993; Miles et al. 1996; Vida et al. 1998; Banks et al. 1998; Szabadics et al. 2007; Savanthrapadian et al. 2014). The slower time course of dendritic inhibitory (DI) signals is partially due to electrotonic attenuation, when examined in somatic recordings; however, differences in the kinetics of the underlying conductance, as a consequence of differential GABAAR expression, are likely to contribute significantly. Dendritic inhibition plays a major role in regulating local linear or nonlinear integration of excitatory synaptic inputs, activation of dendritic voltage-gated conductances, synaptic plasticity, and dendritic spike generation (Miles et al. 1996; Makara et al. 2009; Müller et al. 2012). Indeed, recent in vivo examinations show that dendritic inhibition is involved in shaping the activity of hippocampal place cells important for spatial navigation (Royer et al. 2012) and certain forms of learning (Lovett-Barron et al. 2014).
In addition to GABAARs, metabotropic GABABRs mediate a slower form of inhibition by synaptically released GABA (Solís and Nicoll 1992; Isaacson et al. 1993; Scanziani 2000; Booker et al. 2013, 2017b). Activation of GABABRs generates a slow inhibitory postsynaptic potential (IPSP) postsynaptically and inhibits transmitter release from the axon terminals presynaptically. Finally, besides these forms of phasic inhibition, extrasynaptic GABA receptors mediate “tonic” inhibition (Nusser and Mody 2002; Scimemi et al. 2005; Glykys and Mody 2006). Tonic inhibition controls the excitability of the cell and the gain in the input-output relationship during synaptic excitation as a function of ambient GABA levels.
In this chapter we review characteristics of GABAAR-mediated inhibitory transmission at perisomatic and dendritic synapses, as well as GABABR-mediated pre- and postsynaptic inhibition in hippocampal networks.
GABAAR-Mediated Synaptic Inhibition in Hippocampal Circuits
GABAAR-Mediated Perisomatic Inhibition
A major factor which determines the influence of a given IN on its target cell is the location of the synapses on the surface of the target cell. Inhibitory synapses located close to the soma have a large impact on the generation of action potentials at the output of neurons (Miles et al. 1996; Jonas et al. 2004). These synapses therefore can precisely control timing and frequency of action potentials. Consequently, soma-near “phasic” inhibition underlies important cortical network functions such as the synchronization of neuronal activity and the generation of neuronal network oscillations (Cobb et al. 1995; Pouille and Scanziani 2001; Mann et al. 2005; Mittmann et al. 2005; Vida et al. 2006; Doischer et al. 2008). The primary sources of perisomatic inhibition are basket cells (BCs). These INs show characteristic physiological, pharmacological, and immunohistochemical properties (Freund and Buzsáki 1996; Freund 2003; see also chapter “Morphology of Hippocampal Neurons”). On the basis of the expression profile of Ca2+-binding proteins and neuropeptides, two types of soma-inhibiting cells have been distinguished: parvalbumin (PV)- and cholecystokinin (CCK)-expressing cells (Freund and Buzsáki 1996; Hefft and Jonas 2005; Elgueta et al. 2015). Another IN type, the so-called chandelier or axo-axonic (AA) cells, innervate the axon initial segment of PCs (Somogyi et al. 1985; Soriano et al. 1990; Buhl et al. 1994, 1995), and therefore these INs are in an optimal position to control the initiation of action potentials in their postsynaptic targets. While BCs form synaptic contacts onto both pyramidal cells (PyCs) and other INs, including BCs (Bartos et al. 2001, 2002), AA cells selectively target PCs (Buhl et al. 1994, 1995). The contribution of PV-expressing INs (PV-INs) in neuronal network synchronization has been broadly accepted on the basis of single-unit recordings of PV-INs during spatial exploration demonstrating their strong phase relationship of individual action potentials to single gamma cycles in anesthetized (Tukker etal. 2007; Klausberger and Somogyi 2008) and freely moving rodents (Katona etal. 2014). Moreover, optogenetic approaches allowing light-mediated recruitment of PV-INs or their presynaptic PCs in cortical networks showed that gamma power increased upon PV-IN activation (Cardin et al. 2009; Sohal et al. 2009; Cardin 2016).
PV-BCs
GABAergic transmission at PV-BC output synapses is characterized by rapid time course, large peak conductance, and high reliability of transmitter release (Fig. 1; Kraushaar and Jonas 2000; Bartos et al. 2001, 2002; Glickfeld and Scanziani 2006; Glickfeld et al. 2008; Savanthrapadian et al. 2014). Paired whole-cell patch-clamp recordings from presynaptic PV-expressing BCs and postsynaptic PCs in acute hippocampal slices revealed that the time course of unitary GABAAR-mediated inhibitory postsynaptic currents (IPSCs) is extremely fast in all hippocampal areas. In dentate gyrus granule cells (GCs), the rise time (20–80%) is 0.2–0.3 ms, and decay time constant is 3.2–3.5 ms at near-physiological temperatures (Bartos et al. 2002; Table 1).
Morphological, physiological, and synaptic characteristics of perisomatic and dendritic INs in rodent dentate gyrus. (A) left, confocal image stacks of pairs of synaptically connected GABAergic INs intracellularly labeled with biocytin and visualized with streptavidin conjugated with Alexa Fluor 647 (white labeling). (A 1) perisomatic INs with axon in the granule cell layer (gcl). Right, basket cells (BCs) are parvalbumin (PV)-positive as revealed by antibody labeling. From top to bottom, green, biocytin labeling, arrows point to both somata; red, PV expression; colocalization of both markers. (A 2) commissural-associational path cells (HICAPs) with axon collaterals mainly located in the inner molecular layer (iml). Right, a single intracellularly labeled HICAP identified as cholecystokinin (CCK)-positive. Inset, characteristic discharge pattern of the HICAP cell (0.7 nA, 1 s; scale bars, 200 ms, 50 mV). (A 3) hilar perforant path-associated cells (HIPPs) with axon located predominantly in the outer molecular layer (oml). Right, both neurons co-express somatostatin (SOM; arrows). (B) passive and active membrane properties of identified BCs (B 1), HICAPs (B 2), and HIPPs (B 3). Top traces in B1–B3, voltage trajectories of cell pairs shown in A (1 s, −100, −50, 300–800 pA). Summary graphs in B 1 –B 3 show left the input resistance (Rin) of the recorded cell types and right the half duration of single action potentials. Each circle represents a single data point, and colored circles with lines represent mean values ± SEM. C, unitary IPSCs (uIPSCs) recorded at pairs shown in A. A presynaptic action potential (top) evokes uIPSCs in the postsynaptic cell. Single uIPSCs (6 traces) are shown superimposed (middle), and the average uIPSC (30 traces) at −70 mV is depicted below. Schematic illustration represents the recoded neuron types; In C 3bottom, average uIPSCs shown in C1–C3 were peak normalized and superimposed. ***p ≤ 0.001, **p ≤ 0.01, * p ≤ 0.05; 1, significantly different to HICAP; 2, different to HIPP. (Adapted from Savanthrapadian et al. 2014; with permission, © The Society for Neuroscience)
Highly specialized pre- and postsynaptic mechanisms underlie the rapid time course of unitary IPSCs at PV-BC output synapses. Presynaptically, GABA release is initiated by a brief and precisely timed Ca2+ transient. This is reflected by the highly synchronous time course of GABA release at BC-GC synapses (Hefft and Jonas 2005). The high level of synchrony is further realized by the tight coupling of the Ca2+ source (P/Q - type Ca2+ channels) and the sensor (Bucurenciu et al. 2008). Postsynaptically, fast inhibitory signaling at PV-BC synapses is largely mediated by α1 subunit-containing GABAARs as revealed by postembedding immunogold-labeling studies (Nyíri et al. 2001; Klausberger et al. 2002). Analysis of recombinant GABAARs showed that α1β1γ2 channels deactivate faster than α2β1γ2 channels (Lavoie et al. 1997). Consistent with these findings, bath application of the benzodiazepine type I receptor agonist zolpidem, which has a high affinity to GABAARs containing the α1 subunit (Thomson et al. 2000; Cope et al. 2005), results in a marked prolongation of the decay time constant of PV-BCs IPSCs (Thomson et al. 2000; Doischer et al. 2008).
The peak amplitude of the inhibitory conductance at PV-BC output synapses shows considerable variability across hippocampal areas (Table 1). Nevertheless, peak amplitudes are generally high (up to 9.5 nS), which can be explained by the large number of synaptic contact sites between the coupled neurons. In the CA1 area, for example, the number of contacts per unitary connection is between 10 and 12 (Buhl et al. 1995). Consistent with the high number of contact sites, the failure rate of transmission is low (Hefft and Jonas 2005; Doischer et al. 2008; Table 1). The reliable transmission at BC output synapses is further supported by the high initial probability of transmitter release, estimated to be 0.79 in the dentate gyrus (Kraushaar and Jonas 2000).
BC synapses display differences in kinetic properties depending on the identity of the postsynaptic target (Bartos et al. 2001, 2002; Doischer et al. 2008). In particular, the decay time constant was found to be by a factor of ∼2 faster in postsynaptic BCs than in PCs. The mean value of the decay time constant at BC-BC pairs in the dentate gyrus, CA1, and CA3 are 2.5, 1.7, and 1.2 ms, respectively (Bartos et al. 2001, 2002; Table 1). These target cell-specific differences in the hippocampus (Bartos et al. 2002; Glickfeld et al. 2008) could be explained by the expression of distinct postsynaptic GABAARs. PV-BCs express α1 GABAAR subunits at higher levels than PCs (Gao and Fritschy 1994; Fritschy and Möhler 1995; Klausberger et al. 2002). Interestingly, however, other parameters of the synapses, including the rise time, the peak amplitude of the inhibitory conductance, and the failure rate of synaptic transmission, show no differences between BC-BC and PV-BC-PC synapses (Bartos et al. 2001 2002; Table 1).
Previous paired recordings have been performed between two closely spaced neurons due to the high connectivity among closely spaced cells. Recent investigations, however, indicate that the amplitude and time course of perisomatic inhibition depends on the axonal distance between the pre- and postsynaptic neuron (Strüber et al. 2015, 2017). In particular in the dentate gyrus, PV-INs effectively control the activity of large neuron populations by their wide axonal arborization. Here, synaptic signals become weaker with axonal distance between presynaptic BC and its target GC due to lower contact numbers. The decay of uIPSCs also declines more slowly with distance, resulting from changes in GABAAR subunit composition (Strüber et al. 2015). Indeed, antibody labeling revealed that closely spaced GCs are contacted by BC-mediated GABAergic synapses expressing less α2 subunits postsynaptically, whereas the α2 content increases at more distant target GC (Strüber et al. 2015). Interestingly, this form of distance-dependent perisomatic inhibition was independent of the target cell and observed at both postsynaptic GCs as well as PV-INs (Strüber et al. 2017).
Morphological analysis revealed that many BC-IN synapses are on proximal apical dendrites because the cell body of these cells is often located below the somatic layer. The dendritic position of the synapses leads to attenuation and deceleration of the synaptic current (Johnston and Brown 1983; Rall and Segev 1985; Major et al. 1993; Doischer et al. 2008). Therefore, to determine the real-time course of the inhibitory conductance, rise and decay time constants of the inhibitory postsynaptic conductance were estimated using passive cable models of reconstructed BC-IN pairs (Bartos et al. 2001). In these simulations, the mean value for the 20–80% rise time was found to be 0.17 ± 0.04 ms and the decay time constant 1.8 ± 0.6 ms (Bartos et al. 2001). These values were by a factor of 1.8 and 1.4 faster than the experimentally obtained ones, indicating a considerable electrotonic deceleration of the evoked IPSCs for these proximally positioned dendritic synapses.
CCK-BCs
Information on kinetic properties, synaptic strength, and precision in transmitter release at CCK-BC inhibitory output synapses is limited (Table 1). Paired whole-cell patch-clamp recordings showed that uIPSCs at CCK-BC to PC synapses in CA1 have a 10–90% rise time of 0.73 ± 0.05 ms and a decay time constant of 6.8 ± 0.2 ms (Neu et al. 2007), indicating that synaptic inhibition at CCK-BC output synapses might be slower than at PV-BC output synapses (Bartos et al. 2002). Furthermore, paired recordings at CCK-BC to GC synapses in the dentate gyrus revealed average uIPSCs with slow 20–80% rise times and decay time constants (∼0.9 ms and ∼22 ms, respectively; Table 1; Harney and Jones 2002; Hefft and Jonas 2005). However, these recordings have been performed at room temperature (20–22 °C) and therefore cannot be directly compared with data obtained at PV-BC output synapses measured at near-physiological temperatures (Bartos et al. 2002). While PV-BCs display a fast-spiking (FS) discharge pattern, CCK-BCs have been shown to be regular-spiking (RS) (Freund 2003). Whole-cell recordings of presynaptic RS-BCs and postsynaptic PCs in CA1 revealed uIPSCs with moderately fast time course with a 10–90% rise time of 0.86 ± 0.09 ms and a decay time constant of 8.3 ± 1.22 ms (Table 1; Glickfeld and Scanziani 2006, Glickfeld et al. 2008). These values were, however, not significantly different to the ones obtained at FS-BC to PCs synapses with a 10–90% rise time of 0.66 ± 0.06 ms and a decay time constant of 7.03 ± 1.03 ms in the same experiments (Table 1; Glickfeld and Scanziani 2006; Glickfeld et al. 2008). Although the time course of IPSCs at FS and RS output synapses are not significantly different, they seem to be mediated by different postsynaptic GABAAR subunits. Putative CCK/vasoactive intestinal polypeptide (VIP)-immunopositive BC synapses show several-fold lower α1, but higher α2 subunit content than PV-positive synapses (Nyíri et al. 2001; Klausberger et al. 2002). Finally, CCK-IN to GC synapses show a higher level of transmission failures than PV-BC to GC synapses, pointing to major differences in the release probability among the two BC types (Table 1).
In addition to differences in the kinetics, strength, reliability, and transmitter release at CCK-IN output synapses are characterized by a marked asynchrony (Maccaferri et al. 2000; Hefft and Jonas 2005; M. Bartos, unpublished observation). The less precisely timed GABA release results in a slow rise time of unitary IPSCs observed at CCK-IN to principal cell synapses in the dentate gyrus and CA1 (Maccaferri et al. 2000; Hefft and Jonas 2005). The standard deviation of the first latency distribution, which can be used as a measure for synchrony of release, is significantly larger at CCK-IN to GC than at PV-BC to GC synapses (CCK-INs: 0.95 ± 0.3 ms versus 0.26 ± 0.06 ms). In summary, the properties of the inhibitory output differ markedly between CCK- and PV-BCs. While PV-BC output synapses are characterized by fast, strong, precisely timed transmission, CCK-BC synapses are slower, are weaker, and show asynchronous signaling.
Similar to PV-BCs, CCK-BCs do also target other INs including CCK-BCs. While neuroanatomical studies indicate that the mutual connectivity is comparable to that of PV-BCs, and the total inhibitory input is stronger (Mátyás et al. 2004), functionally, perisomatic inhibition appears to be weaker in CCK-INs. Recordings from the two types in the CA1 area revealed that IPSCs evoked by minimal stimulation in the cell body layer had large peak amplitudes in PV-immunopositive cells, but small in CCK-BCs (Glickfeld et al. 2008). In comparison to ISPCs recorded simultaneously in PyCs, the ratio of the amplitudes was close to 1 in PV- but only 0.14 ± 0.05 in CCK-BCs, pointing to a target cell-dependent difference in the strength perisomatic inhibition (Glickfeld et al. 2008).
Although HICAPs of the dentate gyrus do not have the classical axonal arbors as BCs located in the GC layer, they are CCK-positive (Savanthrapadian et al. 2014), giving rise to the hypothesis that dentate gyrus CCK-expressing BCs are homologous to HICAP cells.
Chandelier or Axo-Axonic (AA) Cells
In contrast to PV-BCs, information on the functional properties at AAs output synapses is scarce. Data from paired recordings in the dentate gyrus showed that these INs evoke fast GABAAR-mediated IPSPs in GCs (Buhl et al. 1994). Properties of the currents underlying the effect of AAs were examined in paired whole-cell patch-clamp recordings in the CA3 area. Results showed that unitary AA ISPCs have larger amplitude (463.3 ± 61.8 pA) and a moderately fast time course (rise time, 1.1 ± 0.1 ms; decay time constant 11.0 ± 0.6 ms at room temperature; Szabó et al. 2010). Recordings from neocortical AA cells and synaptically coupled PyCs suggested that the effect of this IN type is not inhibitory but excitatory (Szabadics et al. 2006). Results from the hippocampus, however, indicate that AA cells predominantly mediate hyperpolarization in the postsynaptic PC population (Glickfeld et al. 2009). Indeed, a hyperpolarizing effect of GABAergic synapses located at the AA segment controls ectopic backpropagation of action potentials in PC axons and thereby lowers the invasion of the soma by antidromic action potentials to maintain the functional polarization of PCs during network oscillations (Dugladze et al. 2012).
Dynamic Properties of Perisomatic Inhibition
When two action potentials are elicited in the presynaptic BC in short succession, the amplitude of the second IPSC elicited in the postsynaptic cell is smaller than that of the first. This phenomenon is called paired-pulse depression (PPD) (Kraushaar and Jonas 2000; Bartos et al. 2001 2002). Coefficient of variation analysis (Malinow and Tsien 1990) suggests a presynaptic locus for PPD (Bartos et al. 2001). In fact, PPD is independent of the identity of the postsynaptic neuron: the extent of PPD of PV-BCs evoked ISPCs was found to be similar at in PV-BC and PCs (∼31 % and ∼33 %, respectively; Bartos et al. 2002). Under conditions of prolonged activity, synaptic transmission at BC-BC and BC-GC synapses show rapid and marked initial depression but subsequently stabilize at a lower level (Kraushaar and Jonas 2000; Bartos et al. 2001) demonstrating that GABAergic transmission is extremely stable at BC output synapses.
CCK-BC output synapses express PPD to a similar extent as PV-BCs in the dentate gyrus (Hefft and Jonas 2005). During repetitive stimulation (10 action potentials, 50 Hz), however, the onset of depression was slower at CCK-INs than at PV-BCs (Hefft and Jonas 2005). Interestingly, in the CA3 and CA1 area high-frequency trains elicited in CCK-BCs result in facilitation of IPSCs in PCs (Losonczy et al. 2004; Földy et al. 2006; Neu et al. 2007). Release at these synapses is tightly controlled by endocannabinoids through CB1 receptors resulting in a low release probability when the cells are quiescent (Földy et al. 2006).
Dendritic GABAAR-Mediated Inhibition
Dendritic inhibition is mediated by a highly heterogeneous population of INs. Some of these INs, such as neurogliaform cells (NGFCs), perforant path- and Schaffer collateral-associated INs of the CA1 area, or MOPP cells of the dentate gyrus, mediate exclusively feedforward inhibition (Vida et al. 1998; Price et al. 2008; Elfant et al. 2008); others, such as O-ML and HIPP INs, provide feedback inhibition (Han et al. 1993; Blasco-Ibáñez and Freund 1995); and yet another group, such as CA1 bistratified cells, are involved in both types of inhibitory microcircuits. Dendritic inhibition controls excitation of cells by glutamatergic inputs, voltage-dependent activation of NMDA receptors, and synaptic plasticity (Staley and Mody 1992; Davies et al. 1991; Mott and Lewis 1991; Miles et al. 1996). Furthermore, dendritic inhibition modulates the activation of voltage-gated channels, the generation of slow Ca2+ spikes, and the backpropagation of action potentials (Miles et al. 1996; Buzsáki 1996).
The large electrotonic distance between synapse location and the site of somatic action potential generation, as well as the low-pass filtering properties of passive membranes will result in attenuation of synaptically evoked IPSCs (Johnston and Brown 1983; Rall and Segev 1985; Major et al. 1993; Häusser and Roth 1997). Dendritic inhibition will thereby have a slower, tonic rather than fast, “phasic” inhibitory effect at the soma. Thus, in contrast to perisomatic inhibition which determines spike timing and synchronizes the activity of PCs, dendritic inhibition may offset the input-output relation of postsynaptic target cells (Mitchell and Silver 2003).
Information on the functional properties of GABAergic transmission of identified DIs is limited. The following section summarizes data on properties of GABAAR-mediated transmission at the output synapses of morphologically-identified DIs from the CA1 and the dentate gyrus available in the literature (Hosp et al. 2014; Savanthrapadian et al. 2014).
Neurogliaform Cells
NGFCs form a dense axonal plexus in the stratum (str.) lacunosum-moleculare of CA1 (Vida et al. 1998; Price et al. 2005, 2008) and the molecular layer (Armstrong et al. 2011). GABAAR-mediated inhibitory signaling at NG output synapses is characterized by slow time course and small peak amplitude (Price et al. 2005, 2008; Szabadics et al. 2007; Armstrong et al. 2011). Paired whole-cell patch-clamp recordings of presynaptic NGFCs and postsynaptic PCs in acute hippocampal slice preparations at near-physiological temperature (30–34 °C) revealed a decay time constant of the unitary IPSCs of 50 ± 4.9 ms and an underlying peak conductance of ∼0.48 nS (Table 2; Price et al. 2008). Similarly, paired recordings of synaptically interconnected NGFCs showed slow kinetics with an average decay time constant of 42.05 ± 21.03 ms (Table 2; Price et al. 2005). NGFCs additionally communicate with other types of INs, thereby forming networks of synaptically connected GABAergic cells. Inhibition at these NGFC to non-NGFC synapses is also characterized by a long decay time constant (37.4 ± 11.86 ms; Table 2; Price et al. 2005). In the dentate gyrus, faster kinetics have been observed at NGFCs targeting GCs, but they were still slower than at BC-GC synapses with a rise time (10–90%) of 5.8 ± 1.1 ms, an amplitude of 8.01 ± 1.22 pA (Vhold −50 mV), and a decay time constant of 14.7 ± 3.6 ms (Armstrong et al. 2011). Thus, synaptic inhibition by NGFCs has a very slow time course, independent of the nature of the target cell, suggesting that this neuron type is the source of the slow dendritic inhibition observed in earlier studies (Pearce 1993; Banks et al. 1998). The slow time course of the inhibitory conductance stems from several structural and functional characteristics, including spillover of GABA from the synapses formed by the dense axonal arbor and the properties of the GABA receptors on the postsynaptic membrane (Szabadics et al. 2007).
The dynamic properties of GABAergic transmission at NGFC output synapses during repetitive presynaptic activation were characterized by a marked depression (5 Hz trains of 4 presynaptic action potentials). The peak amplitude of the second IPSC in such a train of presynaptic activity was reduced by ∼40% at NGFC to PyC synapses (Price et al. 2008) and by ∼25% at NGFC-NGFC synapses (Price et al. 2005). Thus, GABA release at NGFC output synapses is strongly depressing.
Stratum Oriens Interneurons (SO-IN)
Functional synaptic communication has been identified between CA1 INs with their somata and dendrites in str. oriens and postsynaptic PCs. SO-INs are highly diverse, but one of the most abundant types is the so-called oriens lacunosum-moleculare (O-LM) IN with axonal projection to the str. lacunosum-moleculare (Maccaferri et al. 2000). Electron microscopy revealed that the axon terminals of O-LM cells form symmetrical synapses with the distal apical dendrites, mainly shaft, but also dendritic spines, of PyCs and other INs (Gulyás et al. 1993; Sik et al. 1995; Katona et al. 1999). Unitary IPSCs originating from O-LM cells and recorded at the soma of postsynaptic PCs at near-physiological temperature (∼30 °C) have small peak amplitudes (∼0.43 nS) and slow time courses with a 10–90% rise time of 6.2 ± 0.6 ms and a decay time constant of 20.8 ± 1.7 ms (Table 2; Maccaferri et al. 2000). These dendritic inputs are markedly slower than perisomatic inhibitory synapses, but considerably faster than “slow” dendritic inhibition mediated by NGFCs.
Similarly to O-LM to PC connections, GABAAR-mediated inhibition between presynaptic O-LM and postsynaptic SL-INs is slow and weak with a decay time constant of 23 ± 5 ms and a peak conductance of 1.34 nS (Table 2, Elfant et al. 2008). Short-term plasticity was characterized by ∼40% PPD at 100 ms inter-spike intervals. Finally, paired-pulse modulation resulted always in synaptic depression independent of the type of the postsynaptic IN (Elfant et al. 2008). In contrast, similar activity patterns failed to influence the second IPSC in PyCs (93 ± 4%; Maccaferri et al. 2000), raising the possibility of target cell-specific differences in presynaptic properties of these synapses.
Interneuron-Specific Cells (IS-3)
IS-3 cells co-express the VIP and calretinin in the hippocampal area CA1 and are located with their soma in the str. oriens/alveus (O/A). They innervate several O/A INs including O-LM, bistratified cells, BCs, and oriens-oriens INs with preferential innervation of O-LM cells through dendritic synapses (Tyan et al. 2014). The amplitude of uIPSCs was small and the time course slow with low release probability (Table 2), which was reflected in a high failure rate (59.7%). An O-LM cell was contacted by an IS-3 cell via multiple contact sites. Recruitment of converging inhibitory inputs from IS-3 cells onto target O-LM neurons controlled their firing rate and the timing of action potential generation, indicating that dendritic inhibition provided by IS-3 cells is required for precise activity-dependent recruitment of O-LM cells and thus feedback inhibition in the network.
Mossy Fiber-Associated Interneurons (MFA)
MFA INs have dense axonal arborization co-aligned with mossy fibers (MFs) in the str. lucidum of CA3 and the hilus (Vida and Frotscher 2000). Their dendrites are located in the strata radiatum and oriens, indicating that these INs are innervated by associational and commissural fibers and thus primarily mediate feedback inhibition. Output synapses of MFA INs are found on proximal dendritic shafts and to a lesser degree on somata of PyCs. IPSCs recorded at postsynaptic PCs had fast 20–80% rise times of 0.28 ± 0.08 ms and decay time constants of 4.6 ± 1.2 ms (Vida and Frotscher 2000; Losonczy et al. 2004; Table 2). The unitary peak conductance was high with an estimated 5 nS. Thus, in comparison to other DIs, GABAergic transmission at MFA output synapses is characterized by fast time courses and high strength. Dynamic properties of MFA synapses are similar to CCK-BCs in that they have a low initial release probability, show a remarkable frequency-dependent facilitation, and transmit with high reliability during high-frequency trains (Vida and Frotscher 2000; Losonczy etal. 2004).
Dendritic Inhibitory Interneurons in the Dentate Gyrus
The dense layer-specific axonal distribution of the various DI cells indicates the formation of GABAergic synapses located on the entire somato-dendritic domain of INs and PCs in the dentate gyrus (Han et al. 1993; M. Bartos, unpublished data). Unitary inhibitory events elicited by the activation of DI cells recorded at the soma of GCs and INs are slower and weaker than perisomatic inhibition (Fig. 1; Savanthrapadian et al. 2014). The difference is in part due to electrotonic attenuation of the synaptic events, but properties of postsynaptic GABAARs are likely to be of different subunit composition (Table 2). Paired recordings of presynaptic DIs and postsynaptic target cells at near-physiological temperature (32–34 °C) revealed unitary IPSCs with the following parameters:
-
uIPSCs at HICAP-HICAP synapses were induced after a 1.8-fold longer synaptic latency (2.2 ± 0.1 ms) with a 10.9-fold smaller peak amplitude (12.9 ± 3.9 pA vs. BC-BC in mice 140.2 ± 30.8 pA) and slower time course (rise time 0.7 ± 0.09 ms, decay 6.4 ± 0.8 ms). The coefficient of variation (CV) in the synaptic latency was three times higher at HICAP-HICAP than BC-BC synapses (0.43 ± 0.08 vs. 0.14 ± 0.007). However, the decay time constant of the unitary IPSCs is slower (3.95 ± 0.75 ms) than at BC-BC synapses (2.5 ± 0.2 ms). Similarities in the functional synaptic properties are also evident between BC-BC and HIPP-HIPP connections. Inhibitory signals at HIPP-HIPP synapses are evoked after a similar mean synaptic latency (1.5 ± 0.16 ms) with a low CV (0.2 ± 0.04). The rise time of uIPSCs is also short (0.5 ± 0.08 ms) similar to BC-BC paired recordings, indicating a highly synchronous GABA release. Some differences in the synaptic signaling are also apparent. First, the percentage of failures is 29.7-fold higher than at BC-BC synapses (50.4 ± 6.1%). Second, the amplitude of uIPSCs was 5.6 times smaller (25.0 ± 11.3 pA) than at BC-BC synapses. Finally, the decay is 2.8-fold slower (10.9 ± 1.9 ms).
Although HIPP cells project their main axonal arbors to the outer molecular layer of the dentate gyrus, some axon fibers are located in the hilus. Indeed, the short latency at HIPP-HIPP signaling can be explained by the synapse location close to the soma of the target cell as revealed by single-cell reconstructions (Savanthrapadian et al. 2014). HICAP-HICAP pairs with axon collaterals in the inner molecular layer form their contact sites at apical dendrites (Savanthrapadian et al. 2014). During trains of 10 action potentials at 50 Hz, HICAP-HICAP synapses express multiple-pulse facilitation (MPF) with a mean uIPSC10/uIPSC1 ratio of 1.6 ± 0.4. Signals at HIPP-HIPP synapses have a biphasic response with an initial strongly facilitating phase (uIPSC5/uIPSC1 ratio 2.4 ± 0.3) followed by a second phase characterized by a decline in subsequent uIPSC size (uIPSC10/uIPSC1 ratio 1.4 ± 0.5; Savanthrapadian et al. 2014).
Reversal Potential (Esyn) of Synaptically Evoked IPSCs
Several lines of evidence indicate that GABAAR-mediated synaptic inhibition on mature INs is not hyperpolarizing as previously assumed but “shunting” (Alger and Nicoll 1979; Andersen et al. 1980; Martina et al. 2001; Chavas and Marty 2003; Vida et al. 2006). Shunting inhibition is defined as an inhibitory effect, which only minimally affects the membrane potential, but counteracts excitation by short-circuiting the underlying currents. In this scenario, the reversal potential of synaptically evoked IPSCs (Esyn) is close to the resting membrane potential (Vrest), e.g., in the voltage range between Vrest and the threshold for action potential generation (Bartos et al. 2007; Sauer et al. 2012). To determine Esyn, whole-cell recordings from BCs were performed in the perforated-patch configuration in the dentate gyrus of rats and PV-EGFP-expressing mice (Vida et al. 2006). The ionophore gramicidin preserves the intracellular Cl- concentration during recording, thus allowing a realistic assessment of Esyn (Kyrozis and Reichling 1995). To determine Esyn, IPSCs were evoked by extracellular stimulation in the PC layer at varying holding potentials. Synaptically evoked IPSCs reversed on average at −52 ± 1.9 mV (Vida et al. 2006). This value was more positive than the corresponding Vrest of −58.4 ± 1.4 mV, but more negative than the threshold potential (Table 3), indicating that inhibition is shunting in BCs. Similarly, perforated-patch recordings from CA3 str. pyramidale, str. oriens, and str. lucidum INs revealed that GABAAR-mediated synaptic inhibition is shunting or slightly hyperpolarizing with average Esyn values 4–5 mV more positive than the corresponding Vrest (Table 3; Lamsa and Taira 2003; Banke and McBain 2006). These results have been further confirmed in CA1 str. radiatum INs by using cell-attached recordings of GABAAR-mediated effects (Esyn: −69.1 ± 1 mV; Vrest: −66 ± 1 mV; Tyzio et al. 2008).
In contrast to INs, Esyn of synaptically evoked IPSCs in PCs seems to be diverse (Table 3). Synaptic inhibition in GCs is shunting with an Esyn ∼1–10 mV more positive than the resting membrane potential (Table 3, Overstreet-Wadiche et al. 2005; Sauer et al. 2012). Noninvasive recording of unitary field potentials which reflect the postsynaptic effect of GABA release from an identified presynaptic BC confirmed the shunting nature of perisomatic inhibition in the dentate gyrus (Sauer et al. 2012). Importantly, these results did not depend on the age of the animal, excluding a late developmental change in Esyn at this synapse (Sauer et al. 2012). Synaptically evoked IPSCs in CA3 PCs reverse at ∼−73 mV, which is ∼10 mV more negative than the corresponding Vrest of ∼−63 mV (Table 3), reflecting hyperpolarizing inhibition in these neurons (Banke and McBain 2006; Lamsa and Taira 2003). In contrast, somatic cell-attached recordings from CA3 PyCs reveal shunting or even depolarizing inhibition with an Esyn of −75.3 ± 0.9 mV and a corresponding Vrest of −78 ± 2 mV (Table 3; Tyzio et al. 2008). Similarly, in CA1, some discrepancy persists about the nature of GABAergic inhibition. Perforated-patch recordings from CA1 PyCs indicated shunting or slightly hyperpolarizing inhibition (Table 3, Riekki et al. 2008). In contrast, unitary field recording with distinct IN types including BCs, AA, O-LM, and bistratified cells as the presynaptic neuron was always hyperpolarizing (Glickfeld et al. 2009; Sauer et al. 2012). Differences in the excitation state of the recorded cells (Lamsa and Taira 2003), modulation of Cl− transporters (Woodin et al. 2003) or differences in membrane properties between neurons in different brain areas might explain the variability in the obtained Esyn values.
In summary, synaptic GABAAR-mediated synaptic inhibition onto INs is largely shunting, independent of the nature of the recorded IN type or the hippocampal area. However, synaptic inhibition in PCs is diverse and varies from hyperpolarizing to shunting and even depolarizing inhibition.
GABABR-Mediated Inhibition in Hippocampal Circuits
Early electrophysiological and pharmacological studies demonstrated that, in addition to fast ionotropic GABAARs, slow-acting metabotropic GABABRs are also involved in the mediation of the effects of GABA in the hippocampus. In CA1 PyCs, extracellular stimulation in the str. radiatum elicits a biphasic IPSP consisting of a fast and a slow component (Newberry and Nicoll 1984; Dutar and Nicoll 1988b). While the fast component of the compound IPSP is blocked by the GABAAR antagonist bicuculline, the slow component persists under bicuculline application (Newberry and Nicoll 1984; Dutar and Nicoll 1988b) and can be blocked by the GABABR antagonist CGP35348 (Fig. 2a; Solís and Nicoll 1992). Similarly, stimulation in dendritic layers elicits slow GABABR-mediated inhibitory responses in CA3 PyCs, dentate gyrus GCs, and various types of INs (Thompson and Gähwiler 1992; Otis et al. 1993; Khazipov et al. 1995; Mott et al. 1999; Booker et al. 2013, 2017b). Results of these studies, thus, indicate a widespread and abundant postsynaptic localization of GABABRs in the dendrites of PCs and INs (Sibbe and Kulik 2017; Kulik et al. 2017).
GABABR-mediated effects in hippocampal neurons. (a) Pharmacological dissection of the monosynaptic IPSP reveals the fast GABAA and the slow GABABR-mediated IPSP components in a CA1 PyC. B,C Kinetics (b) and reversal potential (c) of the GABABR-mediated slow IPSC in dentate gyrus GCs. (d) Activation of GABAB autoreceptors contribute to depression of IPSCs in response to paired stimuli (top trace, arrow). CGP 35348, a GABAB receptor antagonist relieves presynaptic inhibition and increases the amplitude of the second response (bottom trace, arrow). (a adapted from Solís and Nicoll 1992; b and c adapted from Otis et al. 1993; d adapted from Davies and Collingridge 1993 with permission, © The Society for Neuroscience and The Physiological Society)
GABABRs are also expressed presynaptically where they modulate release of various neurotransmitters and neuromodulators (Sibbe and Kulik 2017; Kulik et al. 2017). Presynaptic receptors are commonly subdivided into autoreceptors and heteroreceptors (Bettler et al. 2004) depending on whether they control the release of GABA from inhibitory terminals (Booker et al. 2013, 2017a) in a feedback manner (Davies et al. 1991) or act at the axon terminals of other transmitter systems (e.g., glutamatergic axons; Vogt and Nicoll 1999; Kulik et al. 2002, 2003; Guetg et al. 2009; Oláh et al. 2009).
Structure and Signaling Through Metabotropic GABABRs
GABABRs belong to the family of seven transmembrane domain G protein-coupled receptors (GPCRs) (Kaupmann et al. 1997). Two different genes, encoding the GABAB1 – which occurs in alternatively spliced forms designated GABAB1a-n – and the GABAB2, subunits have so far been identified (Kaupmann et al. 1997; Isomoto et al. 1998; Pfaff et al. 1999; Schwarz et al. 2000; Wei et al. 2001; Lee et al. 2010). As regards GABAB1, the GABAB1a and GABAB1b transcripts are the most abundant throughout the central nervous system (CNS), exhibiting differences in the extracellular NH2-terminal domain (Kaupmann et al. 1997). Although there are indications that splice variants exist for GABAB2 (GABAB2a-c) (Billinton et al. 2001), more recent results suggest that GABAB2b and GABAB2c transcripts may represent artifacts arising during cDNA synthesis and/or PCR amplification (Bettler et al. 2004).
For their surface localization, efficient coupling to the physiological effectors and formation of fully functional receptors, assembly of heterodimeric complexes made up of GABAB1 and GABAB2 subunit isoforms is required (Jones et al. 1998; White et al. 1998; Kaupmann et al. 1998a; Marshall et al. 1999; Kuner et al. 1999; Bettler et al. 2004; Pin and Bettler 2016). The independently regulated GABAB(1a;2) and GABAB(1b;2) receptor subtypes differentially compartmentalize and fulfill distinct pre- vs. postsynaptic functions, respectively, in cortical PCs and INs (Pérez-Garci et al. 2006; Vigot et al. 2006; Shaban et al. 2006; Guetg et al. 2009). In the heterodimers, the GABAB1 protein contains the ligand-binding domain, whereas the GABAB2 subunit interacts with G proteins (Kaupmann et al. 1998a).
Recent biochemical and electrophysiological studies, prompted by the diversity in properties and characteristics of GABABR responses, provided compelling evidence that a further variety of molecularly and functionally distinct types of GABABRs exist (Schwenk et al. 2016; Bettler and Fakler 2017; Fritzius et al. 2017). The diversity of the native GABABRs originates from the co-assembly of the GABABR subunits with numerous types of auxiliary proteins (Gassmann and Bettler 2012; Schwenk et al. 2016). One of the most abundant and best studied constituents of the GABABR macromolecular complex is the K+ channels tetramerization domain-containing (KCTD) family of proteins (Schwenk et al. 2010; Bartoi et al. 2010; Metz et al. 2011; Turecek et al. 2014). Four KCTD proteins, designated KCTD8, KCTD12, KCTD12b, and KCTD16, associate with the GABAB2 subunit and determine the kinetics and pharmacology, including agonist potency and G protein signaling, as well as the desensitization of the receptor response in both dendritic and axonal compartments of the cells (Schwenk et al. 2010; Adelfinger et al. 2014; Rajalu et al. 2015; Booker et al. 2017b). Two recent elegant pharmacological studies provided direct evidence for the involvement of both KCTD12 and KCTD16 in determining phenotypes of behavioral activity, emotionality, as well as neuronal excitability (Cathomas et al. 2015, 2017). Furthermore, high-resolution functional proteomics more precisely identified the building blocks of GABABRs: the receptor core is assembled from GABAB1, GABAB2, KCTD proteins, and a distinct set of G protein subunits, whereas the periphery of the receptor’s nanoenvironment is formed by transmembrane proteins (Schwenk et al. 2016). These peripheral constituents can operate as effectors (Schwenk et al. 2016); can be, via interaction with sushi domains, mediators of cellular processes that direct the trafficking of GABAB(1a;2) receptors into the appropriate compartment of neurons (Tiao et al. 2008); or may represent linkers between GABABRs and cellular signaling processes (Sakaba and Neher 2003; Pettem et al. 2013).
As GPCRs, effects of GABABR complexes are mediated by second messenger cascades regulating the activity of adenylyl cyclase and phospholipase A2, as well as effector channels such as G protein-coupled inwardly rectifying K+ (Kir3) channels or low and high-voltage-activated Ca2+ (Cav) channels (Marshall et al. 1999; Pérez-Garci et al. 2006; Gassmann and Bettler 2012; Sibbe and Kulik 2017; Kulik et al. 2017; Booker et al. 2018). Accordingly, application of pertussis toxin, an adenosyl transferase, which inactivates several types of Gi proteins, or GDPβ-S, a structural analog of GDP, which competes with GTP for the G protein-binding site, abolishes both pre- and postsynaptic effects of GABABRs in hippocampal PyCs (Andrade et al. 1986; Dutar and Nicoll 1988b; Thompson and Gähwiler 1992; Sodickson and Bean 1996).
Postsynaptic Slow Inhibition Mediated by GABABRs
Functional properties of postsynaptic GABABR-mediated responses are markedly different from those of fast GABAAR-dependent signaling. First, GABAB IPSCs have a much slower time course (Fig. 2b). Pharmacologically isolated slow IPSCs have a long onset latency (∼12–20 ms, Otis et al. 1993; Table 4), reflecting the multiple steps leading up to the activation of the receptors and their G protein-mediated coupling to the effectors. Furthermore, GABAB IPSCs have very slow rise and decay. In dentate gyrus GCs, the rise of the IPSC could be described by a fourth-order exponential with an activation time constant of ∼45 ms and the decay by a biexponential function with time constants of ∼110 and ∼516 ms (Otis et al. 1993; Fig. 2b; Table 4). Similarly slow kinetics of GABAB IPSCs have been reported in CA1 PyCs (Davies et al. 1990; Ling and Benardo 1994; Degro et al. 2015) and in various types of INs (Khazipov et al. 1995; Mott et al. 1999; Booker et al. 2013, 2017b; Table 4).
Second, ionic mechanisms of GABABR-mediated postsynaptic effects are also different from those of GABAARs. Slow IPSPs and baclofen-induced currents reverse close to the estimated equilibrium potential of K+ ions, in the range between −90 and −100 (Fig. 2c and Table 4), indicating that GABABRs activate a K+-selective conductance (Gähwiler and Brown 1985; Davies et al. 1990; Thompson and Gähwiler 1992; Ehrengruber et al. 1997; Booker et al. 2013; Degro et al. 2015). Furthermore, application of Ba2+, an inhibitor of inwardly rectifying K+ (Kir) channels, abolishes these effects (Gähwiler and Brown 1985; Thompson and Gähwiler 1992; Sodickson and Bean 1996). In fact, the channels mediating the GABAB responses have been identified as the Kir3 channel subfamily (Lüscher et al. 1997; Kaupmann et al. 1998b). Kir3 channels comprise four subunits (Kir3.1-3-4; Dascal 1997) and form homotetrameric or heterotetrameric complexes (Krapivinsky et al. 1995; Inanobe et al. 1995; Kofuji et al. 1995; Spauschus et al. 1996; Slesinger et al. 1996; Liao et al. 1996; Wischmeyer et al. 1997). In the hippocampus, Kir3 channels are thought to be mainly composed of the Kir3.1 and Kir3.2 subunits (Lesage et al. 1995; Duprat et al. 1995; Leaney 2003). The Kir3.2 subunit is an essential part of the functional channel, determining its assembly and surface localization (Inanobe et al. 1999; Ma et al. 2002; Lujan et al. 2009), whereas Kir3.3 protein contains a lysosomal-targeting motif that reduces its surface expression and accumulation (Ma et al. 2002). In Kir3.2 knockout animals, expression of Kir3.1 is reduced, and slow inhibitory postsynaptic responses are abolished (Liao et al. 1996; Signorini et al. 1997; Lüscher et al. 1997). In good agreement with the proposed coupling of GABABRs and Kir3 channels, immunocytochemical investigations revealed robust colocalization of the two proteins in dendrites of CA PyCs and various types of INs (Kulik et al. 2006; Booker et al. 2013; Degro et al. 2015; Booker et al. 2017b).
In addition to regulating Kir3 channels, dendritic GABABRs have been shown to inhibit Kir2 channels (Rossi et al. 2006) and modulate Cav channels in cortical PCs and INs. Recent studies provided evidence that GABABRs can inhibit Cav1.2 (L-type), Cav2.1 (P/Q-type), Cav2.2 (N-type), and Cav2.3 (R-type) channel-mediated dendritic spikes in prefrontal cortical neurons (Chalifoux and Carter 2011) and in layers 2/3 and 5 somatosensory neocortical PyCs (Pérez-Garci et al. 2006, 2013; Larkum et al. 2007). GABABRs have also been shown to inhibit Cav1.2 channels in dendrites of hippocampal somatostatin-expressing INs (SOM-INs) and abolish thereby the induction of long-term potentiation (LTP) at their excitatory input synapses (Booker et al. 2018).
Presynaptic Inhibition of Synaptic Transmission by GABABRs
Presynaptic GABABRs play an important role in regulating synaptic transmission at both excitatory and inhibitory synapses. At excitatory synapses, their activation results in depression of glutamatergic synaptic responses (Lei and McBain 2003). Evidence for presynaptic effects of GABA has been obtained at various hippocampal afferent pathways including the hippocampal CA3-CA1 and MF-CA3 PyC synapses (Vogt and Nicoll 1999; Vigot et al. 2006; Guetg et al. 2009). At these synapses, it has been shown that synaptically released GABA suppresses Schaffer collateral and MF responses through GABABRs, predominantly via GABAB(1a;2) receptors (Vigot et al. 2006; Guetg et al. 2009). Thus, GABA spilling over from local inhibitory synapses can regulate glutamatergic transmission by heterosynaptic inhibition at various cortical synapses (Vogt and Nicoll 1999; Oláh et al. 2009; Urban-Ciecko et al. 2015). As many DI cells have axons co-aligned with afferent pathways (Gulyás et al. 1993; Han et al. 1993; Vida et al. 1998; Vida and Frotscher 2000; see chapter “Morphology of Hippocampal Neurons”), this mechanism enables INs to provide input-specific presynaptic control through heterosynaptic inhibition to the main afferent systems to the hippocampal areas (Sohal and Hasselmo 1998).
Presynaptic action of GABABRs is primarily dependent on G protein-mediated inhibition of the Ca2+ conductance (Bettler et al. 2004). Paired recordings from calyx of Held terminals and postsynaptic neurons in the medial nucleus of the trapezoid body provided direct evidence that activation of the receptors by baclofen has no effect on presynaptic K+ conductances but inhibits Ca2+ currents in these terminals (Takahashi et al. 1998). It has been further demonstrated that the presynaptic effect of baclofen is also blocked by GDPβ-S (Takahasi et al. 1998). In the hippocampus, direct patch-clamp recordings from presynaptic elements cannot be routinely performed, with the exception of the large MF terminals on CA3 PyCs (e.g., Geiger and Jonas 2000). Nevertheless, overwhelming evidences suggest that the main mechanisms underlying presynaptic GABABR responses at hippocampal synapses also involve Cav channels (Bettler et al. 2004; Ulrich and Bettler 2007; Laviv et al. 2011; Vertkin et al. 2015). Presynaptic depression of EPSPs by baclofen is unaffected by Ba2+ in cultured hippocampal PyCs (Thompson and Gähwiler 1992), and remains also unchanged in slices from Kir3.2 (GIRK2) knockout mice (Lüscher et al. 1997). In contrast, Ca2+ currents evoked in cultured PyCs and INs are highly sensitive to baclofen (Scholz and Miller 1991). Most compellingly, the inhibitory effects of baclofen on fast presynaptic Ca2+ transients and field EPSPs show similar time course in CA1 PyCs (Wu and Saggau 1995). Thus, presynaptic inhibition of excitatory transmission by GABABRs depends on reduction in Ca2+ conductance rather than activation of K+ currents in the hippocampus (Gassmann and Bettler 2012). However, the involved Ca2+ channel types seem to differ at the various synapses. In CA1 PyCs GABABR-mediated presynaptic inhibition is occluded by the application of ω-conotoxin, a selective Cav2.2 Ca2+ channel blocker, but not affected by ω-agatoxin, a blocker of Cav2.1 channels (Wu and Saggau 1995). In contrast, in CA3 str. radiatum INs ω-conotoxin and ω-agatoxin occlude presynaptic inhibitory effects of baclofen on miniature EPSCs to an equal degree (Lei and McBain 2003). However, evidence exists that the control of transmitter release by GABABR not only acts through Ca2+ channels reducing presynaptic release itself but may also lead to altered short-term plasticity independent of the change in release probability (Lei and McBain 2003; Booker etal. 2017a), pointing to more direct interactions with the release machinery.
At hippocampal GABAergic synapses, activation of presynaptic GABABRs, both GABAB(1a;2) and GABAB(1b;2) receptor types (Vigot et al. 2006), results in reduced inhibitory transmission (Doze et al. 1995; Poncer et al. 2000; Booker et al. 2017a). Consequently, repetitive stimulation leads to the attenuation of IPSCs (“autoinhibition,” Davies et al. 1991; Mott and Lewis 1991; Fig. 2d). This dynamic modulation of inhibitory transmission has an important function in regulating the induction of LTP in the hippocampus (Davies et al. 1991; Mott and Lewis 1991). The molecular mechanism of GABABR-dependent presynaptic inhibition in GABAergic terminals has extensively been investigated. In an early study, effects of baclofen on unitary IPSPs in synaptically coupled pairs of cultured hippocampal neurons were not affected by pre-treatment with pertussis toxin (Harrison 1990). Others have reported that pertussis toxin abolished the baclofen-induced depression of IPSPs in cultured CA3 PyCs (Thompson and Gähwiler 1992). Similarly, findings about the ionic mechanism of presynaptic GABABRs have remained somewhat controversial. Thompson and Gähwiler (1992) have shown that extracellular Ba2+ reduces presynaptic depression of IPSPs by baclofen in cultured CA3 PyCs, suggesting a contribution of K+ channels, plausibly of the Kir3 type. Furthermore, baclofen decreases the frequency of both spontaneous IPSCs and action potential- and Ca2+-independent miniature IPSCs recorded in the presence of tetrodotoxin (Lei and McBain 2003). Increased frequency of miniature IPSCs by elevated levels of extracellular KCl is blocked by Cd2+, and the additional application of baclofen leads to a further reduction in the IPSC frequency (Lei and McBain 2003). Thus, in GABAergic terminals the coupling of presynaptic GABABRs may, at least partially, utilize pertussis toxin-insensitive G proteins and activate K+ channels or directly affect the synaptic release machinery in addition to the inhibition of Cav channels. Furthermore, there is also evidence that the modulation of K+ and Cav channels is not the only mechanism by which GABA can regulate transmitter release: whole-cell recordings from CA1 PyCs demonstrated that inhibition of GABA release by GABABRs is reduced by an activator of protein kinase C (PKC) (Jarolimek and Misgeld 1997).
Cellular and Subcellular Localization of Postsynaptic GABABRs
Consistent with the physiological and pharmacological data, in situ hybridization (Kaupmann et al. 1998a; Bischoff et al. 1999) and autoradiography (Bowery et al. 1987; Chu et al. 1990) confirmed the abundant expression of GABABR subunits in hippocampal PCs and INs. Subsequent immunocytochemical studies (Fritschy et al. 1999; Sloviter et al. 1999; Margeta-Mitrovic et al. 1999; Kulik et al. 2002, 2003) further revealed the cellular and subcellular distribution and localization of the GABABR subunits. At the light microscopic level, the immunostaining for the two subunits, GABAB1 and GABAB2, showed very similar patterns of distribution in the hippocampus (Fig. 3a, b). In the CA areas and the dentate gyrus, immunoreactivity was most intense over the dendritic layers. The str. lacunosum-moleculare of CA3 showed the strongest labeling for the proteins, whereas in CA1 the immunoreactivity for the receptor subunits was generally weak to moderate. In the dentate gyrus, the immunolabeling was weak in the hilus and moderate in the molecular layer (Fritschy et al. 1999; Margeta-Mitrovic et al. 1999; Kulik et al. 2003).
Cellular and subcellular distribution of GABABRs in the hippocampus. (a and b) Light micrographs show the distribution of immunoreactivity for GABAB1 and GABAB2 in the hippocampus. Strong labeling for the subunits was observed in dendritic layers of CA areas and dentate gyrus. Strong immunoreactivity for GABAB1, but not for GABAB2, was detected in somata of CA1 pyramidal cells and INs. (c–e), Electron micrographs show pre-embedding immunogold labeling for the receptor subunits in pre- and postsynaptic compartments of pyramidal cells. Immunogold particles for GABAB1 and GABAB2 were detected on the synaptic membranes (arrowheads in c and e) of the axon terminals (T), as well as on the extrasynaptic membranes (arrows in c–e) of dendritic spines (s) and dendritic shafts (Den) of pyramidal cells. (f and g) Histograms illustrate the distribution of immunoparticles for GABAB1 and Kir3.2 relative to symmetrical and asymmetrical synapses on dendrites of CA1 pyramidal cells. Note the enrichment of both molecules in the vicinity of asymmetrical, putative glutamatergic synapses on dendritic spines (g), but not around symmetrical, putative inhibitory synapses on shafts (f). Scale bars: a and b, 200 μm; c–e, 0.2 μm (a adapted from Kulik et al. 2003; f and g adapted from Kulik et al. 2006 with permission, © Society for Neuroscience)
At the subcellular level, the immunolabeling for both GABAB1 and GABAB2 was observed in postsynaptic and, to a lesser extent, presynaptic compartments of PCs (Fig. 3c–e). Postsynaptically, the majority of the receptor subunits were localized to the extrasynaptic plasma membrane of dendritic spines and shafts of PyCs and dentate GCs (Kulik et al. 2003). Quantitative analysis further revealed an enrichment of GABABRs around excitatory synapses on dendritic spines, and an even distribution on dendritic shafts of PyCs contacted by GABAergic axon terminals (Fig. 3f, g; Kulik et al. 2003). Interestingly, the effector Kir3 channels displayed a very similar cellular and subcellular distribution (Koyrakh et al. 2005; Kulik et al. 2006). Moreover, GABABRs and Kir3 channels were found to be co-clustered around excitatory synapses on dendritic spines of PyCs (Fig. 3g; Kulik et al. 2006) indicating the functional association of these two proteins in this subcellular compartment. The enrichment of the GABABR-Kir3 channel complexes in spines implies their intimate involvement in the control of synaptic integration and plasticity. Indeed, GABABR-mediated inhibition has been shown to act as a break on NMDA receptor-mediated responses and thereby reduced synaptic plasticity in PyCs (Otmakhova and Lisman 2004; Malenka and Bear 2004). Conversely, activation of NMDA receptors and Ca2+/calmodulin-dependent protein kinase II (CaMKII), as well as rise in postsynaptic Ca2+ results in LTP of the GABABR-Kir3 channel-mediated slow IPSPs, which parallel the time course of LTP of excitatory transmission (Huang et al. 2005). The functional significance of this IPSC potentiation is to sharpen the coincidence detection of synchronous excitatory inputs (Huang et al. 2005), a hallmark for learning and memory. Moreover, activation of NMDA receptors along with CaMKII can regulate the surface expression and function of GABABRs: prolonged activation of glutamate receptors results in endocytosis and subsequent degradation of GABABRs (Terunuma et al. 2010; Guetg et al. 2010).
Presynaptically, the immunolabeling for GABABRs is substantially weaker. Nevertheless, immunoreactivity has been consistently found in glutamatergic axon terminals forming asymmetrical synaptic contacts (Kulik et al. 2003). The labeling intensity was higher in these boutons than in putative inhibitory terminals making symmetrical synapses (see below). The receptor subunits were mainly detected at the extrasynaptic plasma membrane and occasionally over the presynaptic membrane specialization (Fig. 3c, e; Kulik et al. 2003). Recent results further showed that, while postsynaptic receptors are mainly composed of the GABAB1b and GABAB2 subunits (GABAB(1b;2) receptors), terminals of excitatory afferents, including the Schaffer collaterals and the MFs, preferentially contain receptors made up of GABAB1a and GABAB2 subunits (GABAB(1a;2) receptors, Vigot et al. 2006; Guetg et al. 2009). Interestingly, receptors with the latter subunit composition have a higher sensitivity for baclofen and GABA and can mediate heterosynaptic inhibition of glutamatergic transmission by synaptically released GABA (Guetg et al. 2009).
Functional GABABRs in Hippocampal Inhibitory Interneurons
In addition to labeling in PCs, immunoreactivity for GABABR subunits was also found in various subpopulations of INs. At the light microscopic level, strong immunostaining for the GABAB1 subunit, but not for the GABAB2 subunit, is present in cell bodies of a subset of GABAergic INs scattered throughout the hippocampus (Fritschy et al. 1999; Margeta-Mitrovic et al. 1999; Sloviter et al. 1999; Kulik et al. 2003). Electron microscopic investigation demonstrated that the strong somatic immunoreactivity for GABAB1 can be ascribed to the abundance of the protein in the endoplasmic reticulum (Kulik et al. 2003), conceivably reflecting a reserve pool of the receptor subunit. Fluorescence colocalization studies showed that INs with high somatic GABAB1 include PV-, CCK-, SOM-, neuropeptide Y-, calbindin-, and calretinin-containing cells (Sloviter et al. 1999; Booker et al. 2013, 2017b). Ultrastructural analysis further demonstrated that immunoreactivity for GABABRs is present postsynaptically along the extrasynaptic plasma membrane of dendritic shafts of NGFCs, PV-, CCK-, and SOM-expressing cells (Fig. 4a, b, f, g; Price et al. 2005; Booker et al. 2013, 2017b, 2018). Postsynaptic GABABRs were found to activate Kir3 channels in PV- and CCK-expressing BCs producing substantial slow IPSCs (Fig. 4c, d, h; Booker et al. 2013, 2017b), consistent with the expression of both the receptor and Kir3 channels on their dendritic membrane surface. Interestingly, in DI PV- and CCK-positive INs slow GABABR IPSCs were consistently smaller than in BCs suggesting IN type-specific expression of the functional receptors (Fig. 4e, h, i). Indeed, on archetypal dendrite-inhibiting SOM-INs postsynaptic GABABRs failed to produce substantial inhibitory currents, despite the presence of immunolabeling for both the channel and the receptor on the dendrites of these INs. Instead, in SOM-INs GABABR activation selectively inhibited Cav1.2 channels, leading to reduced Ca2+ influx and loss of LTP at excitatory synapses onto these INs (Booker et al. 2018).
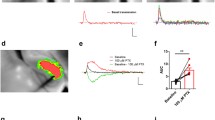
Postsynaptic dendritic localization and effects of GABABRs in hippocampal PV- and CCK-expressing INs. (a and f) Electron micrographs showing immunoreactivity for GABAB1 (immunoparticles, arrows) in dendritic shafts (Den) of PV- (a, peroxidase reaction end product) and CCK-positive INs (f, peroxidase) contacted by presynaptic boutons (b) in the CA1 str. radiatum. (b and g), Summary bar charts of the surface density of immunoparticles in dendrites of principal cells (PC), PV- (b) and CCK-positive INs (g). (c) Pharmacologically isolated slow GABABR IPSCs in a PV BC evoked by a single stimulus (top trace) or trains of 3 (middle) and 5 stimuli (bottom) elicited via an extracellular electrode. Inset shows the PV immunolabeling (left, green pseudocolor) in the biocytin-filled cell body (right, in blue). (d) Inwardly rectifying voltage dependence of the baclofen-induced current (IWC) in a PV BC. Inset, summary of the reversal potential (ER) of the baclofen-induced currents for PCs and BCs. (e) Summary chart of the baclofen-induced IWC measured in PCs, PV-positive BCs and dendritic inhibitory (DI) INs. (h) Slow GABABR/Kir3-mediated IPSC in CCK-expressing INs elicited by 200 Hz train of 5 stimuli applied via an extracellular electrode. Slow IPSCs were recorded from a BC and a DI Schaffer collateral-associated cell (SCA), a perforant path-associated cell (PPA) and a lacunosum projecting cell (LA). The selective GABABR antagonist CGP fully blocked the IPSCs in BC and SCA cells (bottom traces). (i) Summary bar chart of the GABABR-mediated IPSC amplitudes recorded from CCK IN types. Bar charts show mean ± SEM overlain by data from individual cells (open circles). Note that the mean IPSC in both PV and CCK DI cells was significantly smaller than in BCs. Scale bars: 0.2 μm (a–e from Booker et al. 2013, f–J from Booker et al. 2017b. Reproduced with permission, © Society for Neuroscience and Wiley-Blackwell)
Presynaptic GABABRs were also observed in GABAergic axon terminals, albeit at lower levels than in excitatory boutons (Kulik et al. 2003). Similar to glutamatergic terminals, the labeling was mainly found on the extrasynaptic and, to a lower degree, on synaptic membrane segments of inhibitory boutons. IN type specific data is scarce, however, in two recent studies PV- and CCK-positive putative BC terminals in the str. pyramidale of the CA1 area were investigated comparatively (Booker et al. 2013, 2017a). Results obtained from quantitative SDS-digested freeze-fracture replica (SDS-FRL) immunoelectron microscopic analysis demonstrated a differential expression in these two bouton populations, while GABABRs were present at high densities on virtually all CB1-positive putative CCK terminals (Fig. 5a, b, e, f), they showed markedly lower densities and were expressed by only 40% of M2-positive putative PV boutons (Fig. 5c–f; Booker et al. 2017a). This expression pattern was in good agreement with the differential strength of presynaptic inhibition at these synapses: GABA release was dramatically (80–90%) inhibited by the receptor activation at CCK BC synapses, whereas it was reduced only moderately (∼50%) at PV BC synapses (Fig. 5g–i).
Presynaptic axonal localization and effects of GABABRs in hippocampal CCK- and PV-positive INs. (a–d) Electron micrographs of freeze-fracture replicas showing the surface distribution of GABAB1 (5 nm particles, arrows) in CB1-positive (10 nm particles) putative CCK (a and b) and M2-positive (10 nm particles) putative PV boutons (b; c and d). (e) Summary bar chart of the proportion of GABAB1 on double-labeled CB1 receptor – or M2 receptor – containing the axon terminals. (f) Quantification of the density of immunoparticles for GABAB1 on CB1 receptor- and M2 receptor-positive axon terminals in comparison to PC dendrites. Statistics shown: *** P < 0.0001, Fisher’s exact test and 1-way ANOVA with multiple comparisons. (g) Action potentials elicited in the CCK BC (upper traces) evoked unitary IPSCs in the PC (lower traces) under control conditions (Ctrl, left panel), during bath application of the GABABR agonist baclofen (10 μM, middle) and the antagonist CGP (10 μM, right). (h) Summary chart of the IPSC amplitudes under control conditions, during baclofen and CGP application. (i) Summary bar chart of the normalized IPSC amplitudes for a comparison of baclofen-induced inhibition at CCK and PV BC synapses; recovery in CGP is shown for both types on the right. Statistics: ns P ≥ 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. Abbreviations: cf, cross-fractured; S, soma; den, dendritic shaft. Scale bars, 0.2 μm. (Reproduced from Booker et al. 2017a, © The Authors)
Activation of GABABRs by Spillover of GABA from Inhibitory Synapses
Conditions for the activation of GABABRs differ dramatically from those of GABAARs (Farrant and Nusser 2005; Kulik et al. 2017). Whereas GABAAR-mediated responses are readily observed at low-stimulus intensities and in paired recordings of synaptically coupled neurons, strong and/or repetitive stimulation is required to elicit GABABR-mediated postsynaptic effects (Newberry and Nicoll 1985; Isaacson et al. 1993), indicating that recruitment of a number of GABAergic neurons and the release of larger amount of GABA is necessary for the activation of the receptors. It was estimated that the simultaneous recruitment of ∼2–20 INs is required to induce slow GABAB IPSPs (Scanziani 2000).
These differences between the two receptor types appear to contradict the fact that the affinity of GABABRs for GABA is ∼16-fold higher than that of GABAARs. Sodickson and Bean (1996) showed that the EC50 for the activation of GABABR-mediated potassium conductance by GABA is much lower (1.6 mM) than for the activation of GABAAR-mediated chloride conductance (25 mM). However, this apparent discrepancy can be explained by the differential localization of the two types: while GABAARs are clustered primarily in synapses opposite to the GABA release sites (Nusser and Somogyi 1997; Farrant and Nusser 2005), GABABRs are preferentially localized to the extrasynaptic membrane at some distance from GABAergic synapses (Kulik et al. 2003; Fig. 6). Thus, for the activation of GABABRs GABA needs to flow out from the inhibitory synapses and diffuses through the extracellular space before it can reach the receptors (“spillover hypothesis,” Isaacson et al. 1993). In order to achieve sufficiently high concentration of GABA at the location of the receptors, release from larger number of GABAergic terminals is required in a given volume (Newberry and Nicoll 1985; Dutar and Nicoll 1988a; Otis et al. 1993; Brown et al. 2007). Recent studies have, however, provided evidence that single NGFCs can produce activation of GABABRs in hippocampal and neocortical PCs (Tamás et al. 2003; Price et al. 2005, 2008; Oláh et al. 2009). A plausible explanation for this observation is that the highly dense focal axonal arbor of NGFCs enables this IN type to produce substantial volume transmission and evoke “unitary” GABABR-mediated responses in neighboring PyCs and other INs. Indeed, another study provides further evidence that GABA can spillover from synapses of NGFCs and activate extrasynaptic GABAARs and plausibly also GABABRs (Szabadics et al. 2007). However, in the presence of GABA uptake blockers, other IN types can also elicit GABABR-mediated currents in PCs and INs (Scanziani 2000; Booker et al. 2013), indicating that under physiological conditions efficient uptake mechanisms control activation of metabotropic GABA receptors in cortical networks.
Activation of the pre- and postsynaptic GABABRs by GABA spillover from inhibitory terminals. Abbreviations: GABA – GABAergic terminal, Glu – glutamatergic terminals, blue circles represent GABA molecules released from the inhibitory terminal. (Modified from Kulik et al. 2003 with permission, © Society for Neuroscience)
In summary, under physiological conditions, GABABR-mediated responses are evoked upon concerted high activity of GABAergic INs. Such synchronous activity of large sets of INs occurs during network oscillations (see chapter “Cell Type-Specific Activity During Hippocampal Network Oscillations In Vitro”) and can, indeed, lead to the activation of GABABRs (Scanziani 2000). Conversely, GABABR-mediated effects can dynamically influence the frequency of the oscillations (Scanziani 2000; Booker et al. 2013). Thus, GABABRs can serve an important regulatory mechanism during rhythmic oscillatory and other population patterns in vivo. Such a role was further substantiated in recent set of experiments using in vitro models of cortical Up and Down state population activity: in these models activation of GABABRs promoted the termination of Up states recorded in the entorhinal cortex (Mann et al. 2009). This function of the receptors was contrasted by the role of GABAAR-mediated inhibition, which was necessary for balancing persistent Up state activity (Mann et al. 2009). Interestingly, both pre- and postsynaptic GABABRs were involved in the termination of Up states, but in a differential manner: while presynaptic receptors containing the GABAB1a subunit contributed to spontaneous ending of Up states (Craig et al. 2013), postsynaptic GABABRs containing the GABAB1b subunit were found to be essential for afferent input-dependent active termination of Up states (Craig et al. 2013).
Future Perspectives
Although our understanding of the functional and dynamic properties of synaptic ionotropic GABAAR- and the extrasynaptic, predominantly dendritic metabotropic GABABR-mediated signaling and the underlying mechanisms increased substantially, several fundamental questions remained unanswered. What are the functional and dynamic characteristics of dendritically located GABAergic synapses? How does dendritic inhibition influence local integration of excitatory afferent inputs and thereby the input-output relation of target cells? How does dendritic inhibition contribute to neuronal network function such as information processing and network synchronization? Which other IN types express GABABRs and how do they contribute to signaling at the level of the individual neurons and the network? What are the additional constituents of the pre- and postsynaptic GABABR nanoenvironment? How dynamic is the functional and structural interaction of GABABRs with auxiliary proteins, neurotransmitter receptors, and effector ion channels?
Answering these questions is challenging and requires new experimental strategies and concepts. First, paired whole-cell patch-clamp recordings from synaptically connected INs in combination with light and electron microscopic analyses are required to determine synaptic properties, identity of recorded IN types, as well as the number and location of inhibitory synapses. Second, voltage-sensitive dye imaging in combination with whole-cell recordings are needed to examine dendritic integration of inhibitory and excitatory inputs. Third, in vivo whole-cell recordings from IN types together with biochemical and high-resolution quantitative immunoelectron microscopy would be useful to examine the kinetic and dynamic characteristics of synaptic inputs and receptor-associated network in dependence on the activity state of cortical circuits. Finally, a combined experimental and computational approach based on optogenetic analysis of synaptic and intrinsic physiological properties would be important to model integrative properties of the various IN types and to develop complex networks in order to identify the functional contribution of individual cell types and their synapses to network activity and information processing and network synchronization.
References
Adelfinger L, Turecek R, Ivankova K, Jensen AA, Moss SJ, Gassmann M, Bettler B (2014) GABAB receptor phosphorylation regulates KCTD12-induced K+ current desensitization. Biochem Pharmacol 91:369–379
Alger BE, Nicoll RA (1979) GABA-mediated biphasic inhibitory responses in hippocampus. Nature 281:315–317
Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM (1980) Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol (Lond) 305:279–296
Andrade R, Malenka RC, Nicoll RA (1986) A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 234:1261–1265
Armstrong C, Szabadics J, Tamás G, Soltesz I (2011) Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward gamma-aminobutyric acidetic modulators of entorhinal-hippocampal interplay. J Comp Neurol 519:1476–1491
Banke TG, McBain CJ (2006) GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci 26:11720–11725
Banks MI, Li TB, Pearce RA (1998) The synaptic basis of GABAA,slow. J Neurosci 18:1305–1317
Bartoi T, Rigbolt KT, Du D, Köhr G, Blagoev B, Kornau HC (2010) GABA receptor constituents revealed by tandem affinity purification from transgenic mice. J Biol Chem 285:20625–20633
Bartos M, Vida I, Frotscher M, Geiger JRP, Jonas P (2001) Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci 21:2687–2698
Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, Jonas P (2002) Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A 99:13222–13227
Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56
Bettler B, Fakler B (2017) Ionotropic AMPA-type glutamate and metabotropic GABAB receptors: determining cellular physiology by proteomes. Curr Opin Neurobiol 45:16–23
Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84:835–867
Billinton A, Ige AO, Bolam JP, White JH, Marshall FH, Emson PC (2001) Advances in the molecular understanding of GABAB receptors. Trends Neurosci 24:277–282
Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B (1999) Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol 412:1–16
Blasco-Ibáñez JM, Freund TF (1995) Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci 7:2170–2180
Booker SA, Gross A, Althof D, Shigemoto R, Bettler B, Frotscher M, Hearing M, Wickman K, Watanabe M, Kulik Á, Vida I (2013) Differential GABAB-receptor-mediated effects in perisomatic- and dendrite-targeting parvalbumin interneurons. J Neurosci 33:7961–7974
Booker SA, Althof D, Degro CE, Watanabe M, Kulik A, Vida I (2017a) Differential surface density and modulatory effects of presynaptic GABAB receptors in hippocampal cholecystokinin and parvalbumin basket cells. Brain Struct Funct 222:3677–3690
Booker SA, Althof D, Gross A, Loreth D, Müller J, Unger A, Fakler B, Varro A, Watanabe M, Gassmann M, Bettler B, Shigemoto R, Vida I, Kulik A (2017b) KCTD12 auxiliary proteins modulate kinetics of GABAB receptor-mediated inhibition in cholecystokinin-containing interneurons. Cereb Cortex 27:2318–2334
Booker SA, Loreth D, Gee AL, Watanabe M, Kind PC, Wyllie DJA, Kulik A, Vida I (2018) Postsynaptic GABABRs inhibit L-type calcium channels and abolish long-term potentiation in hippocampal somatostatin interneurons. Cell Rep 22:36–43
Bowery NG, Hudson AL, Price GW (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383
Brown JT, Davis CH, Randall AD (2007) Synaptic activation of GABAB receptors regulates neuronal network activity and entrainment. Eur J Neurosci 25:2982–2990
Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P (2008) Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57:536–545
Buhl EH, Halasy K, Somogyi P (1994) Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368:823–828
Buhl EH, Cobb SR, Halasy K, Somogyi P (1995) Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci 7:1989–2004
Buzsáki G (1996) The hippocampo-neocortical dialogue. Cereb Cortex 6:81–92
Cardin JA (2016) Snapshots of the brain in action: local circuit operations through the lens of gamma-oscillations. J Neurosci 36:10496–10504
Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459:663–667
Cathomas F, Stegen M, Sigrist H, Schmid L, Seifritz E, Gassmann M, Bettler B, Pryce CR (2015) Altered emotionality and neuronal excitability in mice lacking KCTD12, an auxiliary subunit of GABAB receptors associated with mood disorders. Transl Psychiatry 5:e510
Cathomas F, Sigrist H, Schmid L, Seifritz E, Gassmann M, Bettler B, Pryce CR (2017) Behavioural endophenotypes in mice lacking the auxiliary GABAB receptor subunit KCTD16. Behav Brain Res 317:393–400
Chalifoux JR, Carter AG (2011) GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci 31:4221–4232
Chavas J, Marty A (2003) Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci 23:2019–2031
Chu DCM, Albin RL, Young AB, Penney JB (1990) Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience 34:341–357
Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P (1995) Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378:75–78
Cope DW, Halbsguth C, Karayannis T, Wulff P, Ferraguti F, Hoeger H, Leppä E, Linden AM, Oberto A, Ogris W, Korpi ER, Sieghart W, Somogyi P, Wisden W, Capogna M (2005) Loss of zolpidem efficacy in the hippocampus of mice with the GABAA receptor gamma2 F77I point mutation. Eur J Neurosci 21:3002–3016
Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ (2013) Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol 591:835–843
Dascal N (1997) Signalling via the G protein-activated K+ channels. Cell Signal 9:551–573
Davies CH, Collingridge GL (1993) The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. J Physiol 472:245–265
Davies CH, Davies SN, Collingridge GL (1990) Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol 424:513–531
Davies CH, Starkey SJ, Pozza MF, Collingridge GL (1991) GABA autoreceptors regulate the induction of LTP. Nature 349:609–611
Degro CE, Kulik A, Booker SA, Vida I (2015) Compartmental distribution of GABAB receptor-mediated currents along the somatodendritic axis of hippocampal principal cells. Front Synaptic Neurosci 7:6
Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M (2008) Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci 28:12956–12968
Doze VA, Cohen GA, Madison DV (1995) Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol 74:43–53
Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T (2012) Segregation of axonal and somatic activity during fast network oscillations. Science 336:1458–1461
Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunski M, Romey G, Barhanin J (1995) Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun 212:657–663
Dutar P, Nicoll RA (1988a) A physiological role for GABAB receptors in the central nervous system. Nature 332:156–158
Dutar P, Nicoll RA (1988b) Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron 1:585–591
Ehrengruber MU, Doupnik CA, Xu Y, Garvey J, Jasek MC, Lester HA, Davidson N (1997) Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci U S A 94:7070–7075
Elfant D, Pál BZ, Emptage N, Capogna M (2008) Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur J Neurosci 27:104–113
Elgueta C, Köhler J, Bartos M (2015) Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J Neurosci 35:4131–4139
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6:215–229
Földy C, Neu A, Jones MV, Soltesz I (2006) Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci 26:1465–1469
Freund TF (2003) Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26:489–495
Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470
Fritschy J-M, Möhler H (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194
Fritschy J-M, Meskenaite V, Weinmann O, Honer M, Benke D, Möhler H (1999) GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci 11:761–768
Fritzius T, Turecek R, Seddik R, Kobayashi H, Tiao J, Rem PD, Metz M, Kralikova M, Bouvier M, Gassmann M, Bettler B (2017) KCTD hetero-oligomers confer unique kinetic properties on hippocampal GABAB receptor-induced K+ currents. J Neurosci 37:1162–1175
Gähwiler BH, Brown DA (1985) GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A 82:1558–1562
Gao B, Fritschy J-M (1994) Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci 6:837–853
Gassmann M, Bettler B (2012) Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci 13:380–394
Geiger JR, Jonas P (2000) Dynamic control of presynaptic Ca2+ inflow by fast-activating K+ channels in hippocampal mossy fiber boutons. Neuron 28:927–939
Glickfeld LL, Scanziani M (2006) Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci 9:807–815
Glickfeld LL, Atallah BV, Scanziani M (2008) Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci 28:1824–1832
Glickfeld LL, Roberts JD, Somogyi P, Scanziani M (2009) Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci 12:21–23
Glykys J, Mody I (2006) Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor apha5 subunit-deficient mice. J Neurophysiol 95:2796–2807
Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, Bräuner-Osborne H, Shigemoto R, Kretz O, Frotscher M, Kulik A, Bettler B (2009) The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci 29:1414–1423
Guetg N, Aziz SA, Holbro N, Turecek R, Rose T, Seddik R, Gassmann M, Moes S, Jenoe P, Oertner TG, Casanova E, Bettler B (2010) NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc Natl Acad Sci U S A 107:13924–13929
Gulyás AI, Miles R, Hájos N, Freund TF (1993) Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci 5:1729–1751
Han Z, Buhl E, Lörinczi Z, Somogyi P (1993) A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5:396–410
Harney SC, Jones MV (2002) Pre- and postsynaptic properties of somatic and dendritic inhibition in dentate gyrus. Neuropharmacology 43:584–594
Harrison NL (1990) On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol 422:433–446
Häusser M, Roth A (1997) Estimating the time course of the excitatory synaptic conductance in neocortical pyramidal cells using a novel voltage jump method. J Neurosci 17:7606–7625
Hefft S, Jonas P (2005) Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8:1319–1328
Hosp JA, Strüber M, Yanagawa Y, Obata K, Vida I, Jonas P, Bartos M (2014) Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus 24:189–203
Huang CS, Shi S-H, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY (2005) Common molecular pathways mediate long-term potentation of synaptic excitation and slow synaptic inhibition. Cell 123:105–118
Inanobe A, Ito H, Ito M, Hosoya Y, Kurachi Y (1995) Immunological and physical characterization of the brain G protein-gated muscarinic potassium channel. Biochem Biophys Res Commun 217:1238–1244
Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y (1999) Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci 19:1006–1013
Isaacson JS, Solís JM, Nicoll RA (1993) Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10:165–175
Isomoto S, Kaibara M, Sakurai-Yamashita Y, Nagayama Y, Uezono Y, Yano K, Taniyama K (1998) Cloning and tissue distribution of novel splice variants of the rat GABAB receptor. Biochem Biophys Res Commun 253:10–15
Jarolimek W, Misgeld U (1997) GABAB receptor-mediated inhibition of tetrodotoxin-resistant GABA release in rodent hippocampal CA1 pyramidal cells. J Neurosci 17:1025–1032
Johnston D, Brown TH (1983) Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol 50:464–486
Jonas P, Bischofberger J, Fricker D, Miles R (2004) Interneuron diversity series: fast in, fast out – temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci 27:30–40
Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396:674–679
Katona I, Acsády L, Freund TF (1999) Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience 88:37–55
Katona I, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P (2014) Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82:872–886
Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B (1997) Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature 386:239–246
Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B (1998a) GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396:683–687
Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaff T, Karschin A, Bettler B (1998b) Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci U S A 95:14991–14996
Khazipov R, Congar P, Ben-Ari Y (1995) Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors. J Neurophysiol 74:2126–2137
Klausberger T, Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321:53–57
Klausberger T, David J, Roberts B, Somogyi P (2002) Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci 22:2513–2521
Kofuji P, Davidson N, Lester HA (1995) Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci U S A 92:6542–6546
Koyrakh L, Luján R, Colón J, Karschin C, Kurachi Y, Karschin A, Wickman K (2005) Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci 25:11468–11478
Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE (1995) The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 374:135–141
Kraushaar U, Jonas P (2000) Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci 20:5594–5607
Kulik A, Nakadate K, Nyíri G, Notomi T, Malitschek B, Bettler B, Shigemoto R (2002) Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci 15:291–307
Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M (2003) Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci 23:11026–11035
Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R (2006) Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci 26:4289–4297
Kulik A, Booker SA, Vida I (2017) Differential distribution and function of GABABRs in somato-dendritic and axonal compartments of principal cells and interneurons in cortical circuits. Neuropharmacology 136:80–91. https://doi.org/10.1016/j.neuropharm.2017.10.018 pii: S0028-3908(17)30485-9
Kuner R, Köhr G, Grünewald S, Eisenhardt G, Bach A, Kornau HC (1999) Role of heteromer formation in GABAB receptor function. Science 283:74–77
Kyrozis A, Reichling DB (1995) Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods 57:27–35
Lamsa K, Taira T (2003) Use-dependent shift from inhibitory to excitatory GABAA receptor action in SP-O interneurons in the rat hippocampal CA3 area. J Neurophysiol 90:1983–1995
Larkum ME, Waters J, Sakmann B, Helmchen F (2007) Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. J Neurosci 27:8999–9008
Laviv T, Vertkin I, Berdichevsky Y, Fogel H, Riven I, Bettler B, Slesinger PA, Slutsky I (2011) Compartmentalization of the GABAB receptor signaling complex is required for presynaptic inhibition at hippocampal synepses. J Neurosci 31:12523–12532
Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE (1997) Activation and deactivation rates of recombinant GABAA receptor channels are dependent on alpha-subunit isoform. Biophys J 73:2518–2526
Leaney JL (2003) Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci 18:2110–2118
Lee C, Mayfield RD, Harris RA (2010) Intron 4 containing novel GABAB1 isoforms impair GABAB receptor function. PLoS One 5:e14044
Lei S, McBain CJ (2003) GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol 546:439–453
Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Roemy G, Barhanin J, Lazdunski M (1995) Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270:28660–28667
Liao YJ, Jan YN, Jan LY (1996) Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 16:7137–7150
Ling DS, Benardo LS (1994) Properties of isolated GABAB-mediated inhibitory postsynaptic currents in hippocampal pyramidal cells. Neuroscience 63:937–944
Liu YC, Cheng JK, Lien CC (2014) Rapid dynamic changes of dendritic inhibition in the dentate gyrus by presynaptic activity patterns. J Neurosci 34:1344–1357
Losonczy A, Biró AA, Nusser Z (2004) Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A 101:1362–1367
Lovett-Barron BV, Kaifosh P, Kheirbek MA, Danielsaon N, Zaremba JD, Readron TR, Turi GF, Hen R, Zemelman BV, Losonczy A (2014) Dendritic inhibition in the hippocampus supports fear learning. Science 343:857–863
Lujan R, Maylie J, Adelman JP (2009) New sites of action for GIRK and SK channels. Nat Rev Neurosci 10:475–480
Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19:687–695
Ma D, Zerrangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY (2002) Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron 33:715–729
Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P (2000) Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol 524:91–116
Major G, Evans JD, Jack JJB (1993) Solutions for transients in arbitrarily branching cables: II. Voltage clamp theory. Biophys J 65:450–468
Makara JK, Losonczy A, Wen Q, Magee JC (2009) Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nat Neurosci 12:1485–1487
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21
Malinow R, Tsien RW (1990) Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346:177–180
Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O (2005) Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron 45:105–117
Mann EO, Kohl MM, Paulsen O (2009) Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci 29:7513–7518
Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI (1999) Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol 405:299–321
Marshall FH, White J, Main M, Green A, Wise A (1999) GABA(B) receptors function as heterodimers. Biochem Soc Trans 27:530–535
Martina M, Royer S, Paré D (2001) Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol 86:2887–2895
Mátyás F, Freund TF, Gulyás AI (2004) Immunocytochemically defined interneuron populations in the hippocampus of mouse strains used in transgenic technology. Hippocampus 14:460–481
McBain CJ, Fisahn A (2001) Interneurons unbound. Nat Rev Neurosci 2:11–23
Megías M, Emri Z, Freund TF, Gulyás AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102:527–540
Metz M, Gassmann M, Fakler B, Schaeren-Wiemers N, Bettler B (2011) Distribution of the auxiliary GABAB receptor subunits KCTD8, 12, 12b, and 16 in the mouse brain. J Comp Neurol 519:1435–1454
Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16:815–823
Mitchell SJ, Silver RA (2003) Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38:433–445
Mittmann W, Koch U, Häusser M (2005) Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol 563:369–378
Mott DD, Lewis DV (1991) Facilitation of the induction of long-term potentiation by GABAB receptors. Science 252:1718–1720
Mott DD, Li Q, Okazaki MM, Turner DA, Lewis DV (1999) GABAB-receptor-mediated currents in interneurons of the dentate-hilus border. J Neurophysiol 82:1438–1450
Müller C, Beck H, Coulter D, Remy S (2012) Inhibitory control of linear and supralinear dendritic excitation in CA1 pyramidal neurons. Neuron. 75:851–864
Neu A, Földy C, Soltesz I (2007) Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol 578:233–247
Newberry NR, Nicoll RA (1984) Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. Nature 308:450–452
Newberry NR, Nicoll RA (1985) Comparison of the action of baclofen with γ-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol 360:161–185
Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87:2624–2628
Nusser Z, Somogyi P (1997) Compartmentalised distribution of GABAA and glutamate receptors in relation to transmitter release sites on the surface of cerebellar neurones. Prog Brain Res 114:109–127
Nyíri G, Freund TF, Somogyi P (2001) Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13:428–442
Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G (2009) Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461:1278–1281
Otis TS, De Koninck Y, Mody I (1993) Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol 463:391–407
Otmakhova NA, Lisman JE (2004) Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 92:2027–2039
Overstreet-Wadiche L, Bromberg DA, Bensen AL, Westbrook GL (2005) GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol 94:4528–4532
Pearce RA (1993) Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron 10:189–200
Pérez-Garci E, Gassmann M, Bettler B, Larkum ME (2006) The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somato-sensory pyramidal neurons. Neuron 50:603–616
Pérez-Garci E, Larkum ME, Nevian T (2013) Inhibition of dendritic Ca2+ spikes by GABAB receptors in cortical pyramidal neurons is mediated by a direct Gi/o-β-subunit interaction with Cav1 channels. J Physiol 591:1599–1612
Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, Rudenko G, Wang YT, Brose N, Craig AM (2013) The specific α-neuroxin interactor calsyntenin-3 promotes excitatory and inhibitory synapses development. Neuron 80:113–128
Pfaff T, Malitschek B, Kaupmann K, Prézeau L, Pin JP, Bettler B, Karschin A (1999) Alternative splicing generates a novel isoform of the rat metabotropic GABA(B)R1 receptor. Eur J Neurosci 11:2874–2882
Pin JP, Bettler B (2016) Organization and functions of mGlu and GABAB receptor complexes. Nature 540:60–68
Poncer JC, McKinney RA, Gähwiler BH, Thompson SM (2000) Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol 1:123–130
Pouille F, Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293:1159–1163
Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M (2005) Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci 25:6775–6786
Price CJ, Scott R, Rusakov DA, Capogna M (2008) GABAB receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci 28:6974–6982
Rajalu M, Fritzius T, Adelfinger L, Jacquier V, Besseyrias V, Gassmann M, Bettler B (2015) Pharmacological characterization of GABAB receptors with auxiliary KCTD subunits. Neuropharmacology 88:145–154
Rall W, Segev I (1985) Space-clamp problems when voltage clamping branched neurons with intracellular microelectrodes. In: Smith TG Jr, Lecar H, Redman SJ, Gage P (eds) Voltage and patch clamping with microelectrodes. American Physiological Society, Bethesda, pp 191–215
Riekki R, Pavlov I, Tornberg J, Lauri SE, Airaksinen MS, Taira T (2008) Altered synaptic dynamics and hippocampal excitability but normal long-term plasticity in mice lacking hyperpolarizing GABAA receptor-mediated inhibition in CA1 pyramidal neurons. J Neurophysiol 99:3075–3089
Rossi P, Mapelli L, Roggeri L, Gall D, de Kerchove d’Exaerde A, Schiffmann SN, Taglietti V, D’Angelo E (2006) Inhibition of constitutive inward rectifier currents in cerebellar granule cells by pharmacological and synaptic activation of GABA receptors. Eur J Neurosci 24:419–432
Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15:769–775
Sakaba T, Neher E (2003) Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature 424:775–778
Sauer JF, Strüber M, Bartos M (2012) Interneurons provide circuit-specific depolarization and hyperpolarization. J Neurosci 32:4224–4229
Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M (2014) Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci 34:8197–8209
Scanziani M (2000) GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron 25:673–681
Scholz KP, Miller RJ (1991) GABAB receptor-mediated inhibition of Ca2+ currents and synaptic transmission in cultured rat hippocampal neurones. J Physiol 444:669–686
Schwarz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, Maki RA (2000) Characterization of γ-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J Biol Chem 275:32174–32181
Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, Seddik R, Tiao JY, Rajalu M, Trojanova J, Rohde V, Gassmann M, Schulte U, Fakler B, Bettler B (2010) Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature 465:231–235
Schwenk J, Pérez-Garci E, Schneider A, Kollewe A, Gauthier-Kemper A, Fritzius T, Raveh A, Dinamarca MC, Hanuschkin A, Bildl W, Klingauf J, Gassmann M, Schulte U, Bettler B, Fakler B (2016) Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat Neurosci 19:233–242
Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC (2005) Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci 25:10016–10024
Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Lüthi A (2006) Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9:1028–1035
Sibbe M, Kulik A (2017) GABAergic regulation of adult hippocampal neurogenesis. Mol Neurobiol 54:5497–5510
Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M (1997) Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A 94:923–927
Sik A, Penttonen M, Ylinen A, Buzsáki G (1995) Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci 15:6651–6665
Slesinger PA, Patil N, Liao J, Jan YN, Jan LY, Cox DR (1996) Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron 16:321–331
Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG (1999) Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology 38:1707–1721
Sodickson DL, Bean BP (1996) GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci 16:6374–6385
Sohal VS (2016) How close are we to understanding what (if anything) γ oscillations do in cortical circuits? J Neurosci 36:10489–10495
Sohal VS, Hasselmo ME (1998) GABAB modulation improves sequence disambiguation in computational models of hippocampal region CA3. Hippocampus 8:171–193
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702
Solís JM, Nicoll RA (1992) Pharmacological characterization of GABAB-mediated responses in the CA1 region of the rat hippocampal slice. J Neurosci 12:3466–3472
Somogyi P, Freund TF, Hodgson AJ, Somogyi J, Beroukas D, Chubb IW (1985) Identified axo-axonic cells are immunoreactive for GABA in the hippocampus and visual cortex of cat. Brain Res 332:143–149
Soriano E, Nitsch R, Frotscher M (1990) Axo-axonic chandelier cells in the rat fascia dentata: Golgi-electron microscopy and immunocytochemical studies. J Comp Neurol 293:1–25
Spauschus A, Lentes KU, Wischmeyer E, Dissmann E, Karschin C, Karschin A (1996) A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. J Neurosci 16:930–938
Staley KJ, Mody I (1992) Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol 68:197–212
Strüber M, Jonas P, Bartos M (2015) Strength and duration of perisomatic GABAergic inhibition depend on distance between synaptically connected cells. Proc Natl Acad Sci U S A 112:1220–1225
Strüber M, Sauer JF, Jonas P, Bartos M (2017) Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nat Commun 8:758
Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G (2006) Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311:233–235
Szabadics J, Tamás G, Soltesz I (2007) Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci U S A 104:14831–14836
Szabó GG, Holderith N, Gulyás AI, Freund TF, Hájos N (2010) Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus. Eur J Neurosci 31:2234–2246
Takahashi T, Kajikawa Y, Tsujimoto T (1998) G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci 18:3138–3146
Tamás G, Lörincz A, Simon A, Szabadics J (2003) Identified sources and targets of slow inhibition in the neocortex. Science 299:1902–1905
Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A (2010) Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci U S A 107:13918–13923
Thompson SM, Gähwiler BH (1992) Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol 451:329–345
Thomson AM, Bannister AP, Hughes DI, Pawelzik H (2000) Differential sensitivity to zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci 12:425–436
Tiao JY, Bradaia A, Biermann B, Kaupmann K, Metz M, Haller C, Rolink AG, Pless E, Barlow PN, Gassmann M, Bettler B (2008) The sushi domains of secreted GABAB1 isoforms selectively impair GABAB heteroreceptor function. J Biol Chem 283:31005–31011
Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47:803–815
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T (2007) Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27:8184–8189
Turecek R, Schwenk J, Fritzius T, Ivankova K, Zolles G, Adelfinger L, Jacquier V, Besseyrias V, Gassmann M, Schulte U, Fakler B, Bettler B (2014) Auxiliary GABAB receptor subunits uncouple G protein βγ subunits from effector channels to induce desensitization. Neuron 82:1032–1044
Tyan L, Chamberland S, Magnin E, Camiré O, Francavilla R, David LS, Deisseroth K, Topolnik L (2014) Dendritic inhibition provided by interneuron-specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J Neurosci 34:4534–4547
Tyzio R, Minlebaev M, Rheims S, Ivanov A, Jorquera I, Holmes GL, Zilberter Y, Ben-Ari Y, Khazipov R (2008) Postnatal changes in somatic γ-aminobutyric acid signalling in the rat hippocampus. Eur J Neurosci 27:2515–2528
Ulrich D, Bettler B (2007) GABAB receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol 17:298–303
Urban-Ciecko J, Fanselow EE, Barth AL (2015) Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr Biol 25:722–731
Vertkin I, Styr B, Slomowitz E, Ofir N, Shapira I, Berner D, Fedorova T, Laviv T, Barak-Broner N, Greitzer-Antes D, Gassmann M, Bettler B, Lotan I, Slutsky I (2015) GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal network. Proc Natl Acad Sci U S A 112:3291–3299
Vida I, Frotscher M (2000) A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci U S A 97:1275–1280
Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH (1998) Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506:755–773
Vida I, Bartos M, Jonas P (2006) Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49:107–117
Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Luján R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Müller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B (2006) Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50:589–601
Vogt KE, Nicoll RA (1999) Glutamate and γ-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A 96:1118–1122
Wei K, Jia Z, Wang YT, Yang J, Liu CC, Snead OC III (2001) Cloning and characterization of a novel variant of rat GABA-BR1 with a truncated C-terminus. Brain Res 89:103–110
White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH (1998) Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396:679–682
Wischmeyer E, Döring F, Wischmeyer E, Spauschus A, Thomzig A, Veh R, Karschin A (1997) Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol Cell Neurosci 9:194–206
Woodin MA, Ganguly K, Poo MM (2003) Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron 39:807–820
Wu LG, Saggau P (1995) GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol 485:649–657
Yuan M, Meyer T, Benkowitz C, Svanthrapadian S, Ansel-Bollepalli L, Foggetti A, Wulff P, Alcami P, Elgueta C, Bartos M (2017) Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. Elife 6:e21105. https://doi.org/10.7554/eLife.21105
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bartos, M., Sauer, JF., Vida, I., Kulik, Á. (2018). Fast and Slow GABAergic Transmission in Hippocampal Circuits. In: Cutsuridis, V., Graham, B., Cobb, S., Vida, I. (eds) Hippocampal Microcircuits. Springer Series in Computational Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-99103-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-99103-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99102-3
Online ISBN: 978-3-319-99103-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)