Abstract
The endovascular treatment of intracranial aneurysms has significantly evolved over the past two decades, demonstrating a rapidly improving level of safety and efficacy. In fact, coil embolization with or without device assistance has become the first-line treatment for aneurysms in many centers, especially for aneurysms with a favorable dome-to-neck ratio (>2). However, wide-necked aneurysms are harder to be treated by primary coiling and usually require balloon remodeling or stent reconstructions to adequately cover the aneurysm neck and to avoid coil protrusion and possible compromise of the parent vessel. Stent reconstructions should be individually considered based on cerebral vasculature, aneurysm characteristics, armamentarium available, and neurointerventionalist experience over other technical alternatives. The aim of this chapter is to describe technical nuances and clinical experience with complex stent reconstructions such as Y-stenting, X-stenting, waffle-cone technique, and T-stenting for the treatment of wide-necked aneurysms located at bifurcations. Also, a brief description regarding new technologies for this particular subset of aneurysms is included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intracranial aneurysm
- Endovascular
- Stent-assisted coiling
- Y-stenting
- S-stenting
- Waffle for intracranial aneurysm
The natural history of unruptured intracranial aneurysms (IA) has been described in multiple international studies, but some controversy remains regarding instances in which these lesions should be treated. In 1998, the International Study of Unruptured Intracranial Aneurysms (ISUIA) estimated the risks of rupture based on a retrospective cohort of 722 patients with a history of subarachnoid hemorrhage (SAH). A rupture rate of 0.5% per year was found for IAs less than 10 mm in diameter and 0.7% per year in IAs larger than 10 mm [1]. A prospective study published in 2003, the ISUIA-2, included a total of 4060 patients [2]. The observational arm consisted of 1692 subjects with a mean follow-up of 4.1 years. Outcomes showed that the risk of rupture was dependent on size and location. The highest risk of rupture was reported in the posterior circulation (including aneurysms located in the posterior communicating artery) and for large (13–24 mm) and giant (≥25 mm) aneurysms. Subsequent cohorts have reported other predictors of aneurysm rupture, which include older age, hypertension, the presence of a daughter sac, smoking, and family history of SAH [3,4,5,6,7]. Endovascular management has emerged as a feasible, safe, and effective modality for the treatment for IAs. In the setting of unruptured IAs, the results of ISUIA-1 and ISUIA-2 reported lower rates of morbidity and mortality in comparison to surgical clipping.
Regarding ruptured aneurysms, the International Subarachnoid Aneurysm Trial (ISAT) included 2143 patients with SAH in the UK [8]. The study was a randomized multicenter clinical trial designed to assess the safety and efficacy of coiling embolization versus surgical clipping. Results favored endovascular coiling over surgical clipping at 1-year follow-up with percentage dead or disabled (23.5% vs. 30.9%; p < 0.05). Recently, a longer follow-up of the ISAT cohort was published. Patients in the endovascular treatment group were more likely to be alive and independent (modified Rankin Scale 0–2) at 10 years than patients in the surgical clipping group (OR 1.34, 95%CI 1.07–1.67) [9]. A similar study performed in the USA, the Barrow Ruptured Aneurysm Trial (BRAT), evaluated the safety and efficacy of surgical clipping versus coiling embolization in acutely ruptured IAs and compared functional outcomes based on clinical and angiographic data [10]. The 1-year results favored endovascular management over surgical clipping (mRS > 2 was reported in 23.2% in the endovascular group and 33.7% in the surgical clipping arm; p < 0.05). The 3-year follow-up of the BRAT cohort reported a favorable 5.8% absolute difference in the endovascular arm compared with the outcomes in the surgical clipping arm, but the difference did not obtain statistical significance (p = 0.25). In addition, subjects in the surgical clipping arm had a significantly higher degree of aneurysm occlusion and a lower rate of recurrence and retreatment. Interestingly, younger patients and patients with lesions located in the anterior communicating complex had better outcomes with clipping at the 3-year analysis [11]. However, the limitations of single-coil embolization have become evident over time. More recently, the 6-year follow-up of the BRAT cohort was published, and the results showed that complete aneurysm occlusion was achieved in 96% (111/116) of the subjects in the surgical clipping group and in 48% (23/48) of the subjects in the endovascular group (p < 0.01) [12]. The overall retreatment rate for clipping was 4.6% (13/280) and for coiling was 16.4% (21/128). In addition, they found no significant difference in poor clinical outcomes (mRS > 2, p = 0.24) between the two treatment groups.
Primary coil embolization (with or without balloon remodeling) is particularly challenging in the treatment of large- or wide-necked aneurysms due to the risk of coil protrusion into the parent vessel and a higher rate of aneurysm recanalization. The introduction of self-expanding intracranial stents has increased the options for the treatment of this subset of aneurysms. These stents have facilitated endosaccular embolization by providing support to coils, increased packing density, and providing a scaffold for endothelialization over time. Despite the advances in endovascular technology, wide-necked aneurysms arising at vascular bifurcations remain technically difficult to treat. A wide-necked aneurysm is usually defined as a lesion with a neck size ≥4 mm or a dome-to-neck ratio <2. The apex of a bifurcation is the site of highest hemodynamic wall shear stress and wall tension in the vascular network [13, 14]. This subset of aneurysms may require complex endovascular approaches to embolize the aneurysm sac and preserve blood flow through branching vessels at the bifurcation. A single stent may be insufficient to cover the aneurysm neck, and frequently, multiple-stent reconstructions are required to achieve a complete initial occlusion. Recently a meta-analysis reviewed 38 articles that included 2446 patients with 2556 wide-necked aneurysms treated with single coiling or stent-assisted coiling; the study demonstrated the safety of these techniques, but long-term occlusion rates were found to be suboptimal [15]. Among all aneurysms, 496 were located at bifurcations. Specifically, for wide-necked bifurcation aneurysms, the authors found a long-term (>6 months) complete or near-complete occlusion rate of 71.9% (95% CI, 52.6–91.1) and recanalization and retreatment rates of 9.8% (95% CI, 7.1–12.5) and 5.2% (95% CI, 1.9–8.4), respectively.
Self-Expanding Intracranial Stents
The basic structure of a vascular stent consists of a mesh composed of thin metal struts, which results in free spaces known as cells. Stents can be classified based on their cell design construction. A closed-cell design refers to a cell that is surrounded by the strut configuration, whereas in an open-cell design, a cell is partially surrounded, and several bridging membranes interconnect struts along the device structure. Based on design and arrangements, stents have several features that should be taken into consideration when choosing the device. For instance, open-cell stents have more flexibility and better conformability for tortuous vessel anatomy, but protrusion of struts into the aneurysm, when present in an outer curve, may be more frequent, whereas closed-cell stents have a larger radial force in straight vessels, but kinking and flattening of the device may occur in sharp curves [16, 17]. Table 13.1 summarizes stent features.
The introduction of self-expanding intracranial stents in the endovascular armamentarium has allowed neurointerventionalists to treat IAs that are not amenable for simple coiling embolization. Currently, there are four intracranial stents available in the USA: the Neuroform stent (Stryker Neurovascular, Fremont, CA, USA), the Enterprise stent (Codman, Miami Lakes, FL, USA), and more recently two iterations of the low-profile visualized intraluminal support device (LVIS and LVIS Jr., Microvention, Tustin, CA, USA). Stents have different features and come in various sizes and diameters. Because there is no ideal stent for all cases, knowledge of stent characteristics is required to choose the appropriate device on a case-by-case basis and overcome anatomical limitations such as parent vessel size, tortuous anatomy, or sharply angled vessels. Table 13.2 summarizes the characteristics of different intracranial stents.
Dual-antiplatelet therapy is a crucial component to the successful placement of intravascular devices, and its efficacy relies on the ability to prevent platelet aggregation and reduce the risk of device thrombosis or thromboembolic complications [18]. While a number of different antiplatelet agents are currently available, the indication for each drug is often individualized and based on several factors including efficacy, cost, personal experience, and availability. Aspirin and clopidogrel remain the most widely accepted agents and first-line therapy in most neurovascular centers. Aspirin irreversibly inactivates platelet cyclooxygenase-1, thus ultimately blocking the production of thromboxane. It has a fast onset of action with a maximum effect at 30–60 min. Clopidogrel is a thienopyridine derivative that prevents platelet aggregation by irreversible blockage of the P2Y12-ADP receptor. It requires hepatic metabolism to produce an active metabolite. Some platelet inhibition can be seen after a single dose of 75 mg, but a steady state is seen within 7 days of continued administration. When necessary, a loading dose of 300–600 mg may achieve levels of inhibition between 40% and 50% within 5 h [19, 20]. Patient response to clopidogrel is measured using assays that analyze the level of inhibition of the P2Y12 receptor. The most widely used test is the Accumetric’s VerifyNow (San Diego, CA), which reports platelet reactivity in percent inhibition and P2Y12 Reaction Units (PRU). In general, the goal is to achieve a percent inhibition ≥30% or <210 PRU to demonstrate an adequate response [21, 22]. However, clopidogrel resistance has been estimated to occur in nearly one third of patients undergoing endovascular procedures, but mechanisms remain poorly understood with some evidence of a multifactorial component and increased resistance in individuals with genetic polymorphisms in the alleles CYP2C19 and CYP3As [23,24,25,26]. Consequently, newer P2Y12 receptor inhibitors have been developed such as ticagrelor, prasugrel, and cangrelor. Contrary to clopidogrel, ticagrelor does not require hepatic activation, and it reversibly binds with the P2Y12-ADP receptors. It has faster onset and offset compared to clopidogrel without increase in major or minor bleeding events; in fact, it has been demonstrated that a loading dose of ticagrelor (180 mg) results within 30 min approximately in the same level of platelet inhibition achieved after 8 h of a loading dose of clopidogrel (600 mg) [27, 28]. Also, it is mainly metabolized via the CYP34A enzyme that could make it advantageous in the setting of mutations in CYP2C19 and has been demonstrated to be an effective and safe alternative to patients with poor response to clopidogrel in neuroendovascular procedures [29]. Prasugrel irreversibly inhibits the P2Y12-ADP receptor, and compared to clopidogrel, it has a more potent antiplatelet effect and a lower variability in platelet response. However, rates of hemorrhagic complications have been reported in neuroendovascular procedures when using aspirin and prasugrel as dual-antiplatelet strategy [30]. Cangrelor is a novel intravenous P2Y12 inhibitor, which provides an immediate effect and that also can be rapidly reversed. This drug has been exclusively tested in percutaneous coronary interventions with a substantial reduction in ischemic events and no increased in severe bleeding compared with clopidogrel [31,32,33]. Although this drug has not been tested in neuroendovascular procedures, it seems a promising alternative in the setting of emergency cases or in which clopidogrel resistance is demonstrated.
In general, stent-assisted embolization requires placing patients on dual-antiplatelet therapy with aspirin (325 mg/day) and a thienopyridine derivative, typically clopidogrel (75 mg daily) for at least 7 days before the procedure. A bolus of clopidogrel (300–600 mg) can be used if a faster intervention is required and usually adequate platelet inhibition is obtained within 2–6 h post-loading dose [34, 35]. In our practice, we routinely check preoperative PRUs, and in the setting of poor response to clopidogrel, we switch to ticagrelor (180 mg initial dose, followed by 90 mg every 12 h with 81 mg of aspirin) [29]. The intervention is performed under conscious sedation or general anesthesia. Intravenous heparin is infused to maintain an activated clotting time greater than 200 s. Accurate vessel measurements are obtained from working angle angiograms and 3-D reconstructions. Stents are deployed under fluoroscopy, and a final angiogram is performed to evaluate immediate aneurysm embolization and parent vessel patency. Dual-antiplatelet therapy is usually maintained for 3 months followed by aspirin alone continued indefinitely.
Y-Stenting Configuration
The Y-stenting technique is a feasible reconstruction for bifurcation aneurysms. It consists of deploying the stents inside the bifurcation vessels to create an artificial aneurysm neck, which enables safe embolization by protecting the branching vessels from coil herniation [14, 36, 37]. This technique was first described for basilar apex aneurysms, but it is also feasible for aneurysms located in the anterior communicating artery (ACoA), internal carotid artery (ICA) terminus, and middle cerebral artery (MCA) bifurcation. Stent placement can be performed either through the interstices of the first device, more commonly used, or in a “kissing” fashion (parallel deployment) [38]. Chow et al. described the technique in 2004 for the treatment of a basilar apex aneurysm using two Neuroform stents and demonstrated encouraging results [39]. After this first experience, other authors have contributed to the literature reporting their case series in different aneurysm locations with overall good technical and clinical outcomes. Table 13.3 summarizes studies with at least ten cases reported using the Y-stenting technique in different bifurcation locations.
With the basic principle that the rate of thromboembolic complications is likely related to the amount of intravascular metal, our preference is to maximize the use of a single stent very often mitigating the need of a second device with the assistance of balloon remodeling to protect the non-stented branch.
Endovascular Experience per Aneurysm Location
Basilar Apex Aneurysms
The basilar apex is the most common location of aneurysms in the posterior circulation [40, 41]. The catheterization of the basilar artery (BA) is technically easy due to the straight angle of its anatomy; however, it is a rich area of perforators that has to be taken into consideration when selecting an endovascular strategy. The Y-stent configuration has been broadly reported in retrospective case series and mostly used to treat basilar apex aneurysms. Although initial case series showed high rates of periprocedural complications with this technique [42, 43], larger series have demonstrated its safety and effectiveness [44].
When considering Y-stent reconstruction for the treatment of basilar apex aneurysms, accurate measurements should be obtained from both P1 segments and proximal landing zones in the basilar trunk in order to determine the device size. Initially, open-cell stents were favored for this technique due to their inherent strut configuration, but successful reconstructions have been reported using exclusively closed-cell stents [45,46,47,48]. The vascular anatomy is paramount for the success of this technique. It is not uncommon to find that one of the posterior cerebral arteries (PCA) originates at a more acute angle in relation to the basilar trunk, which may increase the difficulty of stent deployment. Therefore, we suggest that this P1 segment should be stented first (stenting the harder branch first should be the principle).
The technique for Y-stenting consists in advancing a 6-F guide catheter into the distal segment of one of the vertebral arteries (VA) under roadmap guidance. Subsequently, a microcatheter is advanced over a 0.014-in guidewire to the most difficult P1-segment configuration. The guidewire is removed, and the stent system is brought to the PCA. The first stent is deployed from the P1 segment into the upper segment of the basilar artery. Very often, we placed a microcatheter into the aneurysm and attempt coiling with a single stent in place, with or without balloon remodeling into the so far not-stented branch (see Fig. 13.1). Very often, we can obtain successful aneurysm treatment without using a second stent.
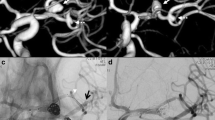
Case illustration. A 70-year-old female with an incidentally found basilar apex aneurysm was decided to be treated with a Y-stent reconstruction. Cerebral angiogram with anteroposterior (a) and lateral (b) views demonstrated a wide-necked aneurysm. After determining accurate vessel measurements, decision was made to use low-profile stents (LVIS Jr). (c) Anteroposterior fluoroscopy view demonstrating the placement of the first stent from the left P1 (white arrow) across the aneurysm neck into the basilar artery. (d) Anteroposterior fluoroscopy view depicting the Y-stent reconstruction with both stents (white arrows) placed in the posterior cerebral arteries and a coiling microcatheter placed in the aneurysm sac. (e) Cone-beam CT (Xpert CT by Phillips) reconstruction demonstrating the wall apposition of the devices in the vessels. (f) Final digital subtracted angiogram demonstrating the patency of both posterior cerebral arteries and a near-complete aneurysm occlusion
If a second stent is needed to perform a Y reconstruction, a microcatheter is carefully navigated through the first stent’s cells, and a second device is deployed with half of the stent in the contralateral P1 segment and the other half extending down within the lumen of the previously placed stent. Although coiling through the struts of both stents is a feasible option, the high metal coverage of crossing devices at the aneurysm neck can create technical difficulties. Therefore, we prefer to place a coiling microcatheter into the aneurysm sac before placing the second device. Our preference with the current commercially available devices (Neuroform, Enterprise and LVIS) is to either combine two braided devices (LVIS or LVIS Jr. more frequently) or an open-cell stent followed by a closed-cell device (Neuroform as first stent and Enterprise as second). Especially when using braided devices, we recommend to perform a cone-beam CT with reconstruction (Xpert CT by Phillips) to evaluate the stent wall apposition. Immediate angiographic occlusion has been reported as the highest predictor of long-term aneurysm occlusion [49]. One should keep in mind that when a balloon is used, as described above (typically Scepter, Microvention Terumo), a low-profile stent such as LVIS Jr. can be deployed through the lumen of the balloon.
The rate of periprocedural complications has been reported to range between 0% and 45% and derive mainly from thromboembolic events and technical events such as stent migration, stent prolapse, and coil herniation which have been reported to range from 1% to 3% [42,43,44,45, 48,49,50,51,52,53]. Spiotta et al. reported a rate of periprocedural complications of 31.6% including stent migration, artery dissection, and transient ischemic events [42]. Contrary to those results, Chalouhi et al. reported a lower rate of complications of 6.2% in the treatment of 16 basilar apex aneurysms. These authors did not find significant differences when comparing this technique with single stenting or coil embolization without stent assistance [51]. Fargen et al. reported the first multicenter experience in the treatment of 45 aneurysms, and among all lesions, 39 were located in the basilar apex [45]. The authors compared clinical and angiographic results based on the cell design stent and found no statistical difference. To date, the largest case series published using the Y-stenting technique included 188 patients with 193 bifurcation aneurysms with a low rate of procedural complication of 2.7%, mortality rate of 0.5%, and a high rate (97.8%) of complete aneurysm occlusion at 6 months [44]. Among all lesions, 22 were located at the basilar apex. Overall, the rates of aneurysm occlusion at the last follow-up range between 63% and 100% with retreatment in approximately 10% of the cases [42, 45, 48, 50, 51].
ICA Terminus Aneurysms
The ICA terminus is a unique point in the cerebral vasculature where, similar to other bifurcation locations, there is high wall shear stress. Between 2% and 9% of all intracranial aneurysms are located at the ICA terminus, and they most commonly arise at the junction of the ICA and the M1 segment [54,55,56,57]. These aneurysms typically project longitudinally following the blood flow direction. Successful surgical clipping has been reported but requires advanced surgical skills and experience since the exposure is challenging due to a large number of perforators surrounding the base or dome of the aneurysm [58]. When the dome-to-neck ratio is favorable, single-coil embolization and balloon-assisted embolization are valid strategies with overall good results [59, 60]. However, wide-necked aneurysms at the ICA bifurcation remain challenging to treat. Y-stenting reconstruction is a feasible technique, and although it has been poorly reported in this subset of aneurysms, a safe technical profile has been demonstrated [47].
The technique for stent deployment should follow the general rule of deploying the first device in the branch with the sharpest angle. A similar technique with a single stent or balloon usage on the other branch, as described for basilar apex lesions, also applies for ICA terminus aneurysms. Accurate proximal measurements of the A1 and the M1 segments should be obtained to choose the device size. In the article published by Yavuz et al., 16 aneurysms were treated using open- and closed-cell stents. Although authors did not breakdown the rate of complications per aneurysm location, their overall results demonstrated safety and efficacy of the technique at 6-month follow-up [44]. Strauss et al. treated 14 aneurysms in the anterior circulation with the Y-stenting configuration, and among all lesions, 2 were located at the ICA terminus with no technical difficulties. One of the patients was successfully treated and achieved complete aneurysm occlusion at 6 months, but the second patient died 24 h post-intervention, which was attributed to her poor preoperative status [61]. A more recent publication reported 4 aneurysms out of 20 located in the ICA terminus. The authors reported no periprocedural complications and overall good rates of aneurysm occlusion [50]. Similar results were reported by Limbucci et al. in the treatment of two aneurysms located in the ICA bifurcation [47].
Anterior Communicating Artery (ACoA) Aneurysms
Aneurysms located in the ACoA account for the most common location based on historical cohorts with rates ranging between 23% and 39% [2, 62]. Surgical clipping has demonstrated to be an effective strategy but requires surgical expertise to dissect the lesion due to the unique position of the arterial segment and arterial branches which can be difficult to find and easily injured [63, 64]. When a favorable dome-to-neck ratio is present, coiling with or without device assistance has shown satisfactory outcomes [65,66,67,68]. Occasionally, a single device is not enough to reconstruct the parent vessel, and dual-stent reconstruction is warranted such as X- or Y-configurations.
When considering Y-stenting configuration, it is paramount to identify both A1 and A2 segments since it is not uncommon to find asymmetry among vessels. As a general rule, the patient should have a “good-sized” A1 artery since the proximal edges of both stents will be placed in this segment and distal edges will be located in both of the proximal A2 segments. The ipsilateral ICA should be catheterized extending into the dominant A1. Subsequently, coils are deployed into the aneurysm sac with the goal of achieving an initial complete occlusion. Rohde et al. described the first experience using Y-stent reconstruction for the treatment of a recurrent ACoA aneurysm using two closed-cell stents (Enterprise) and proof of aneurysm occlusion at 6-month follow-up [69]. Further small case series have demonstrated technical feasibility and overall good outcomes [45, 49]. In a Turkish study, the authors treated 42 aneurysms in the ACoA with dual-stent reconstructions exclusively with closed-cell stents (Enterprise and Solitaire). For 5 out of 42 ACoA aneurysms, they opted for stenting in X configuration. Although angiographic occlusion rates were not separated per aneurysm location, the overall rate for the total sample was 97.8% [44]. In a European study, the authors analyzed dual-stent reconstructions with X- and Y-configuration for the treatment of 105 aneurysms, and among all lesions, they treated 30 located in the ACoA. Interestingly, in nine patients, the attempt to place the stent failed, and three of them had their aneurysm located in the ACoA [70]. Their results were not reported per aneurysm location, but the overall rate of aneurysm occlusion at the last available imaging follow-up (a mean of 17 months) was 85.8% with only two re-treatments during the study period.
MCA Bifurcation Aneurysms
In general, aneurysms located in the MCA represent approximately 20% of all intracranial aneurysms, and among these, the MCA bifurcation is the most common location – approximately 75% of cases [2, 71, 72]. Surgical clipping remains the gold standard to treat this type of aneurysm due to straightforward access through the Sylvian fissure. However, endovascular alternatives have been explored, and Y-stent configuration has demonstrated good clinical outcomes. The first successful case was reported in 2005 with a dual-stent reconstruction using Neuroform stents [73].
It is recommended that the distal edge of the first stent be placed in the proximal segment of the larger M2 branch and subsequently a second device be deployed from the proximal segment of the other M2 branch to the M1 segment so that the proximal ends of both stents align. The coil embolization should be performed after both stents are placed either with the jailing technique or through the stent struts. Several case series have shown overall good outcomes. Straus et al. treated ten MCA bifurcation aneurysms with no complications, and imaging follow-up was available for six lesions demonstrating complete occlusion [61]. Yavuz et al. reported the largest series of treatments for this subset of aneurysms [44]. The authors successfully intervened in 113 lesions that accounted for 58.5% of the total sample, and most of them (87.6%) were treated with closed-cell stents. Bartolini et al. reported the treatment of 105 aneurysms, of which 57 lesions were located at the MCA bifurcation. As previously mentioned, the authors reported dual-stenting failure in nine cases, and the most common locations were the MCA bifurcation in four cases and the ACoA in three [70]. More recently, Limbucci et al. reported a case series that included 20 MCA aneurysms, and similar to the Turkish study, they performed all interventions with closed-cell stents in this subset of aneurysms [47]. Although the authors did not show outcomes per aneurysm location, their study demonstrated the safety and efficacy in the total sample with two procedural complications and complete aneurysm occlusion in 93.6% at the last follow-up.
X-Stenting Configuration
The X-stenting technique is a valid alternative for the treatment of wide-necked aneurysms located in the ACoA. The clinical experience is limited to case reports or small case series with overall good outcomes [74,75,76]. The technique consists of deploying one of the stents from one of the A2 segments to the contralateral A1 and subsequently to navigate the stent system from the opposite ICA and deploy the second device in the same fashion through the interstices of the first stent placed. We prefer to coil after both devices are placed. The target is to maintain the patency of both branches while the X configuration supports the coil mass and decreases the jet flow entering the aneurysm sac (Fig. 13.2). This hemodynamic phenomenon is believed to be enhanced with closed-cell stents. Although this technique is feasible, the major limitation is the vessel size where the stent may be deployed, and we agree with Saatci et al. that a good-sized A1 segment is paramount for the success of this technique [76]. Otherwise, a Y-configuration reconstruction through the larger vessel is an alternative option, especially for patients with A1 hypoplasia on one side. Bartolini et al. reported their experience with dual-stent reconstructions and treated seven aneurysms with X-stenting configuration [70]. Although the authors did not perform any conclusive analysis per technique, they described difficulties in dual-stent reconstructions mostly for aneurysms located in the ACoA and MCA bifurcation. Undoubtedly, larger studies are required to evaluate the safety and effectiveness of this type of configuration, but outcomes to date are encouraging.
Waffle-Cone Technique
The waffle-cone technique is an uncommon alternative to the Y-stenting technique (Fig. 13.3). This procedure was first described by Horowitz et al. in 2006 and was described as the “waffle-cone” technique because of the appearance of the stent-coil combination after treatment [77]. The procedure consists of placing the distal edge of a stent in the base of the aneurysm neck and coiling through the implant resulting in a cone-shaped configuration that preserves both branches. This stent configuration is recommended when the acute angulation of branch arteries at the location of a wide-necked aneurysm make it difficult to navigate any stent system. Additional benefits include a reduction in the amount of metal and a technically easier strategy than dual-stent reconstructions. The waffle-cone technique has been used to treat aneurysms located in the basilar apex, ACoA, and MCA bifurcation. Initially, open-cell stents were used for this reconstruction, but successful cases with closed-cell stents have also been reported [78].
Waffle-cone technique for the treatment of an aneurysm located at the basilar apex. A microcatheter is navigated over a microwire into the aneurysm sac. (a) Once in place, an intracranial stent is deployed within the aneurysm, (b) and finally coils are deployed resulting in a cone-shaped configuration that preserves both branches (c)
Endovascular Experience per Aneurysm Location
Basilar Apex Aneurysms
Although the clinical experience with the waffle-cone technique is limited, it is a valid alternative for the treatment of wide-necked bifurcation aneurysms. In the treatment of lesions located at the basilar apex, accurate measurements should be obtained of the size of the aneurysm neck and the diameter of the BA in order to determine the diameter of the stents. The procedure is technically easier than other reconstructions since only one device is used. The stent system is brought within the aneurysm sac and carefully deployed to anchor the distal edge of the device inside the lesion. Once the stent is placed, a rotational cone-beam CT angiogram is recommended to evaluate stent wall apposition. After successful stenting, a microcatheter is navigated to the aneurysm dome over a microwire through the lumen reconstruction. Enough coils should be deployed to obtain a near or complete aneurysm embolization. Horowitz et al. described this technique using open-cell stents with favorable results in four wide-necked bifurcation aneurysms located at the BA with no complications and immediate near-to-complete aneurysm occlusion [77]. A similar successful case was reported by Yang et al. using a Neuroform stent. In theory, open-cell stents facilitate blood flow from the parent artery through the stent reconstruction into the branching arteries where it covers them at their origins. However, a few cases have been reported with closed-cell stents resulting in overall good outcomes [78, 79]. Compared to the Y-stenting configuration, the waffle technique involves a shorter total length of stented vessel and no stents overlapping, which may reduce the risk of stent thrombosis. The rate of periprocedural complications has been reported up to 2%, mainly thromboembolic events. Regarding aneurysm occlusion, the rates have ranged between 60% and 100% at the last follow-up available [77, 78, 80].
ACoA and MCA Aneurysms
Experience with the waffle technique in other locations other than the basilar apex is very limited in part due to the vascular anatomy which precludes this technique in certain locations. Only a few cases have been reported; Horowitz et al. successfully treated one aneurysm located at the ACoA and two at the MCA with no complications and satisfactory immediate results [77]. Similar results have been described in the treatment of three ACoA aneurysms and one MCA aneurysm [81]. In the largest series reported, to our knowledge, in these locations using the waffle technique, Liu et al. reported 6 ACoA aneurysms and 3 MCA aneurysms with encouraging results and long-term aneurysm occlusion [80]. Although the technique itself is feasible, there is no current evidence to support its recommendation for the treatment of these lesions, and other alternatives should be considered before using waffle technique for ACoA or MCA aneurysms.
T-Stent Configuration
The T-stent reconstruction is also known as the nonoverlapping Y-configuration and was first described by Cho et al. in 2012 for the treatment of six basilar apex aneurysms [82]. Their results showed a safe and feasible alternative to other traditional stent reconstructions. From a technical point of view, this is a modified Y-stent reconstruction, and the general rule of stenting the hardest branch should be followed. The first stent is navigated into the P1 segment of the PCA, and a microcatheter for coil delivery should be placed in the aneurysm sac. The first stent is deployed from the P1 segment to the basilar trunk. Later, a second stent is navigated and deployed into the contralateral PCA without overlapping the initial device. Finally, coils are deployed as compactly as possible under devices protection. This reconstruction may be performed on a staged fashion, or both stents can be placed in the same procedure. This technique shows clear advantages over the traditional Y-configuration: (1) no stent deployment through the struts of one of the devices, (2) less amount of metal inside the basilar trunk, and (3) proper apposition of the stents into the arterial wall. However, the alignment of the second stent without overlapping may be challenging, or otherwise, the stent may be placed far distal resulting in sufficient aneurysm neck coverage to protect against coil protrusion. Therefore, accurate vessel measurement should be obtained.
Publication on this technique is scant, and long-term outcomes are unknown. To date, Aydin et al. have published the largest case series [83]. They intervened 24 patients with 24 aneurysms located in the anterior circulation. T-stent reconstruction was performed using low-profile stents (Leo + Baby; Balt). The technical success rate was 95.8%, and an immediate total occlusion rate of 79.2% was achieved. The rate of periprocedural complications was 16.7% with no mortality and a permanent morbidity of 4.2%. At the last imaging follow-up, the rate of aneurysm occlusion was achieved in 81.2% of the cases.
Cross-Court Approach for Aneurysm Stenting
When dealing with complex aneurysms, contralateral approaches or posterior to anterior/posterior strategies are feasible options for stenting (Fig. 13.4). Some examples of these maneuvers include as follows: ipsilateral A1 to contralateral A1 for ACoA lesions, P1 into the posterior communicating artery (PComm) and distal ICA (reverse PComm stenting), contralateral vertebral access to posterior inferior cerebellar artery (PICA) aneurysms, contralateral carotid to A1-M1 stenting for carotid terminus lesions, contralateral carotid to distal ICA into the PComm, ICA-PComm approach for P1-P1 stenting for basilar tip aneurysms, and ICA-PComm approach for basilar and superior cerebellar artery aneurysms. Taking advantage of these anatomical connections should be taken into consideration before considering more complex stent reconstructions.
Case illustration. A 73-year-old male presented to the hospital with a subarachnoid hemorrhage, Hunter and Hess grade 4. A cerebral angiogram was performed, which revealed a right vertebral artery (VA) dissection (a) and a pseudoaneurysm (b) arising from the origin of the right posterior inferior cerebellar artery (PICA). Decision was made to secure the lesion and due to inaccessibility through the right VA, a contralateral approach was performed. A microcatheter was navigated through the left VA, and a coiling microcatheter was placed into the pseudoaneurysm sac. (c) An intracranial stent was deployed from the proximal basilar artery into the left vertebral artery (white arrows). (d) Final digital subtracted angiogram demonstrating complete occlusion of the lesion and patency of the right PICA, basilar artery, and left VA
Here, we will illustrate the utilization of the contralateral vertebral approach to PICA aneurysms (Fig. 13.5). Aneurysms located in the PICA are extremely rare with an estimated prevalence that ranges between 0.5% and 3% [62]. For lesions involving the VA/PICA origin, the goal of any therapy should be aneurysm occlusion with preservation of the PICA. With the advance of technology and the development of smaller intracranial stents, endovascular approaches may be feasible and safe. In fact, current microcatheters may easily be navigated into any of the vertebral arteries (VAs) and, subsequently, into the PICA. In some instances, due to the variability in the anatomy of the VAs and the sharp angle of the PICA origin, a straightforward access may be difficult, and a contralateral approach to the aneurysm should be performed. The technique consists as follows: a 6-F guide catheter is positioned in both VAs; then a microcatheter is navigated to the PICA, distal to the aneurysm, through the contralateral VA; and it is positioned across the aneurysm neck. Then, a microcatheter is navigated to the PICA aneurysm through the contralateral VA, and it is positioned across the aneurysm neck. Finally, the stent is slowly deployed. Once the stent is placed, a microcatheter for coiling is navigated into the guide placed in the VA ipsilateral to the lesion and navigated through the struts of the stent [84]. Recently, we published a dual-center study for the treatment of PICA aneurysms with the LVIS Jr. stent [85]. This device is an excellent alternative for the treatment of these lesions since it can be delivered through a 0.017-in microcatheter, which also allows for coiling. In our series, two patients were treated through a contralateral approach with no technical complications. Other case series using older stents have reported technical difficulties and limitations for the treatment of this type of aneurysms, especially when lesions are located in distal segments of the PICA [86,87,88]. Current data are scant to provide enough evidence to support either surgery or endovascular treatment, but a multidisciplinary team should address treatment choice for PICA aneurysms.
Case illustration. A 52-year-old female with history of a left-sided posterior cerebellar inferior artery (PICA) aneurysm that was previously coiled presented with recurrence of the lesion. Cerebral angiogram with anteroposterior (a) and lateral (b) views demonstrated a daughter sac coming from the PICA origin aneurysm. Catheterization of the PICA from the ipsilateral vertebral artery was unsuccessful, and decision was made to navigate a second microcatheter through the right vertebral artery and ultimately to catheterize the left PICA (c). A microcatheter was navigated into the aneurysm sac through the ipsilateral vertebral artery, and the second microcatheter was positioned across the aneurysm neck; once in place, coils were deployed (d), and an LVIS Jr (white arrows) was slowly deployed spanning the aneurysm (e). A final angiogram demonstrated patency of the PICA and near-complete aneurysm occlusion (f). Another valid strategy is to deploy the stent first and eventually coil the aneurysm through the struts of the device (g)
Current and Future Endovascular Alternatives for Wide-Necked Bifurcation Aneurysms
Despite the advance in neuroendovascular technology, there is no current standard of treatment for bifurcation aneurysms, and other stent-like devices have been used with encouraging results. The pCONus (Phenox, Bochum, Germany) is a self-expanding device with four distal petals and a nylon cross in the distal end of the shaft to support coils inside the aneurysm. The device is designed to optimize the waffle-stenting technique. The experience with this new technology is limited to small case series, but results have demonstrated its safety and overall good rates of aneurysm occlusion at midterm follow-up [89,90,91,92].
The PulseRider (Pulsar Vascular, San Jose, CA, USA) is a novel device with lesser metal content than intracranial stents, and it supports the aneurysm neck while maintaining coil mass inside the sac. Its deployment does not require branch vessel catheterization, and unlike y-stenting configuration, one device is sufficient. However, literature offers limited data, and larger prospective studies are needed to evaluate the safety and efficacy of this technology [93,94,95].
Recently, flow diverters have revolutionized the treatment of large side-wall aneurysms demonstrating safety and long-term efficacy in multicenter cohorts [96, 97]. However, their usage on bifurcation aneurysms remains controversial, especially because the aneurysm neck may not be completely covered by the flow diverter and aneurysm thrombosis over time is uncertain. A few case series have been published with the emphasis on bifurcation aneurysms, and the rate of periprocedural complications has been reported in up to 9.4% with rates of aneurysm occlusion ranging from 33.3% to 97.7% [98,99,100].
Based on flow diversion technology, a new concept in intrasaccular flow disruption has been developed. The Woven EndoBridge device (WEB, Sequent Medical, Palo Alto, CA, USA) is a self-expanding ovoid mesh that is placed inside the aneurysm sac, modifying the blood flow and inducing lesion thrombosis; additionally, its placement does not require dual-antiplatelet therapy, and it can be used in acutely ruptured aneurysms. Several studies have demonstrated its safety, but complete aneurysm occlusion is far from definitive with rates ranging from 53.1% to 69% [101,102,103,104].
Other devices are currently under evaluation, and preclinical studies have shown promising results. The Artisse device (formerly known as the Luna Parent Vessel Occlusion device, LUNA, Medtronic, Minneapolis, MN, USA) is a self-expanding ovoid mesh of nitinol wires. Its design intends to disrupt the flow inside the aneurysm, and a study in rabbits showed a rate of 88% complete aneurysm occlusion at 3 months; additionally, microscopic examinations demonstrated neointimal overgrowth in all cases [105]. A preliminary experience has been reported in France in the treatment of 64 aneurysms with an excellent technical profile but suboptimal rates of aneurysms occlusion: 40% at 6-month follow-up [106]. A clinical trial evaluating this device is planned to be started in the USA in July 2017.
The Reverse Barrel Vascular Reconstruction Device (VRD, Medtronic, Minneapolis, MN, USA) is a resheathable stent-like device with a cone shape in the middle of the implant that prevents coil herniation into the parent vessel. A study in canine models demonstrated excellent technical outcomes in the treatment of 32 aneurysms, and histological examinations showed neointimal formation onto the device [107]. An ongoing French registry has reported preliminary results in seven patients treated with this new technology, and initial results showed immediate complete occlusion in 71% of the cases and no technical complications [108].
The Endovascular Clip Systems (eCLIPs, Evasc Medical Systems, Vancouver, BC, USA) is a hybrid leaf-shaped device that works as a flow diverter and as an intrasaccular flow disrupter. A preclinical study in eight porcine models has shown a good technical profile and complete aneurysm occlusion at 30-day angiography in all cases with neo-endothelialization at microscopic examinations [109]. The fast evolution of new endovascular technology should be taken with cautious optimism, and pros and cons of each technology should be considered when deciding treatment management for bifurcation aneurysms.
Conclusions
The decision-making process to treat wide-necked bifurcation aneurysms relies on a thorough knowledge of the patient’s vascular anatomy as well as the neurosurgical armamentarium available. The goal of treatment should be to obtain a long-term aneurysm occlusion with patent bifurcation branches. This chapter summarized complex stent reconstructions that should be considered in the decision process based on appropriate patient selection and the neurointerventionalist experience. Certainly, the Y-stenting configuration has demonstrated reproducibility, safety, and effectiveness for aneurysms in multiple cohorts, but other reconstructions, although feasible, still lack strong evidence to use in comparison to other approaches. In addition, future devices should be designed to overcome current difficulties in covering the aneurysm neck, conformability on sharply curved vessels, and complete aneurysm obliteration.
References
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725–33.
Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr., Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC; International Study of Unruptured Intracranial Aneurysms I. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.
Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93(3):379–87.
Lee EJ, Lee HJ, Hyun MK, Choi JE, Kim JH, Lee NR, Hwang JS, Kwon JW. Rupture rate for patients with untreated unruptured intracranial aneurysms in South Korea during 2006–2009. J Neurosurg. 2012;117(1):53–9.
Investigators UJ, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–82.
Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013;44(9):2414–21.
Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41(9):1969–77.
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R; International Subarachnoid Aneurysm Trial Collaborative G. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. 2002;11(6):304–14.
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015;385(9969):691–7.
McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, Albuquerque FC. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116(1):135–44.
Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills NK, Partovi S, Nakaji P, Wallace RC. The barrow ruptured aneurysm trial: 3-year results. J Neurosurg. 2013;119(1):146–57.
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P, Wallace RC. The barrow ruptured aneurysm trial: 6-year results. J Neurosurg. 2015;123(3):609–17.
Munarriz PM, Gomez PA, Paredes I, Castano-Leon AM, Cepeda S, Lagares A. Basic principles of hemodynamics and cerebral aneurysms. World Neurosurg. 2016;88:311–9.
Gao B, Baharoglu MI, Cohen AD, Malek AM. Y-stent coiling of basilar bifurcation aneurysms induces a dynamic angular vascular remodeling with alteration of the apical wall shear stress pattern. Neurosurgery. 2013;72(4):617–29. discussion 628–619
Zhao B, Yin R, Lanzino G, Kallmes DF, Cloft HJ, Brinjikji W. Endovascular coiling of wide-neck and wide-neck bifurcation aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37(9):1700–5.
Krischek O, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg. 2011;54(1):21–8.
Nam HG, Yoo CM, Baek SM, Kim HK, Shin JH, Hwang MH, Jo GE, Kim KS, Cho JH, Lee SH, Kim HC, Lim CH, Choi H, Sun K. Enhancement of mechanical properties and testing of nitinol stents in cerebral aneurysm simulation models. Artif Organs. 2015;39(12):E213–26.
Heer T, Juenger C, Gitt AK, Bauer T, Towae F, Zahn R, Senges J, Zeymer U; Acute Coronary Syndromes Registry I. Efficacy and safety of optimized antithrombotic therapy with aspirin, clopidogrel and enoxaparin in patients with non-ST segment elevation acute coronary syndromes in clinical practice. J Thromb Thrombolysis. 2009;28(3):325–32.
Savcic M, Hauert J, Bachmann F, Wyld PJ, Geudelin B, Cariou R. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999;25(Suppl 2):15–9.
Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908–13.
Gasparyan AY. Aspirin and clopidogrel resistance: methodological challenges and opportunities. Vasc Health Risk Manag. 2010;6:109–12.
Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 2008;29(2):281–5.
Wiviott SD, Antman EM. Clopidogrel resistance: a new chapter in a fast-moving story. Circulation. 2004;109(25):3064–7.
Comin J, Kallmes DF. Platelet-function testing in patients undergoing neurovascular procedures: caught between a rock and a hard place. AJNR Am J Neuroradiol. 2013;34(4):730–4.
Fifi JT, Brockington C, Narang J, Leesch W, Ewing SL, Bennet H, Berenstein A, Chong J. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol. 2013;34(4):716–20.
Goh C, Churilov L, Mitchell P, Dowling R, Yan B. Clopidogrel hyper-response and bleeding risk in neurointerventional procedures. AJNR Am J Neuroradiol. 2013;34(4):721–6.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–85.
Hanel RA, Taussky P, Dixon T, Miller DA, Sapin M, Nordeen JD, Tawk RG, Navarro R, Johns G, Freeman WD. Safety and efficacy of ticagrelor for neuroendovascular procedures. A single center initial experience. J Neurointerv Surg. 2014;6(4):320–2.
Akbari SH, Reynolds MR, Kadkhodayan Y, Cross DT 3rd, Moran CJ. Hemorrhagic complications after prasugrel (Effient) therapy for vascular neurointerventional procedures. J Neurointerv Surg. 2013;5(4):337–43.
Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, Pollack CV Jr, Montalescot G, Mahaffey KW, Kleiman NS, Goodman SG, Amine M, Angiolillo DJ, Becker RC, Chew DP, French WJ, Leisch F, Parikh KH, Skerjanec S, Bhatt DL. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318–29.
Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimsky P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Genereux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA, Investigators CP. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–13.
Bhatt DL, Lincoff AM, Gibson CM, Stone GW, McNulty S, Montalescot G, Kleiman NS, Goodman SG, White HD, Mahaffey KW, Pollack CV Jr, Manoukian SV, Widimsky P, Chew DP, Cura F, Manukov I, Tousek F, Jafar MZ, Arneja J, Skerjanec S, Harrington RA, Investigators CP. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330–41.
Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Buttner HJ, Neumann FJ. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111(20):2560–4.
Nordeen JD, Patel AV, Darracott RM, Johns GS, Taussky P, Tawk RG, Miller DA, Freeman WD, Hanel RA. Clopidogrel resistance by P2Y12 platelet function testing in patients undergoing neuroendovascular procedures: incidence of ischemic and hemorrhagic complications. J Vasc Interv Neurol. 2013;6(1):26–34.
Cekirge HS, Yavuz K, Geyik S, Saatci I. A novel “Y” stent flow diversion technique for the endovascular treatment of bifurcation aneurysms without endosaccular coiling. AJNR Am J Neuroradiol. 2011;32(7):1262–8.
Saglam M, Kizilkilic O, Anagnostakou V, Yildiz B, Kocer N, Islak C. Geometrical characteristics after Y-stenting of the basilar bifurcation. Diagn Interv Radiol. 2015;21(6):483–7.
Melber K, Meila D, Draheim P, Grieb D, Greling B, Schlunz-Hendann M, Brassel F. Vascular angular remodeling by kissing-Y stenting in wide necked intracranial bifurcation aneurysms. J Neurointerv Surg. 2016; https://doi.org/10.1136/neurintsurg-2016-012858.
Chow MM, Woo HH, Masaryk TJ, Rasmussen PA. A novel endovascular treatment of a wide-necked basilar apex aneurysm by using a Y-configuration, double-stent technique. AJNR Am J Neuroradiol. 2004;25(3):509–12.
Ogilvy CS, Crowell RM, Heros RC. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, Heros RC, editors. Surgical management of neurovascular disease. 3rd ed. Baltimore: Williams and Wilkins; 1995. p. 269–90.
Henkes H, Fischer S, Mariushi W, Weber W, Liebig T, Miloslavski E, Brew S, Kuhne D. Angiographic and clinical results in 316 coil-treated basilar artery bifurcation aneurysms. J Neurosurg. 2005;103(6):990–9.
Spiotta AM, Gupta R, Fiorella D, Gonugunta V, Lobo B, Rasmussen PA, Moskowitz SI. Mid-term results of endovascular coiling of wide-necked aneurysms using double stents in a Y configuration. Neurosurgery. 2011;69(2):421–9.
Thorell WE, Chow MM, Woo HH, Masaryk TJ, Rasmussen PA. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56(5):1035–40; discussion 1035–1040.
Yavuz K, Geyik S, Cekirge S, Saatci I. Double stent-assisted coil embolization treatment for bifurcation aneurysms: immediate treatment results and long-term angiographic outcome. AJNR Am J Neuroradiol. 2013;34(9):1778–84.
Fargen KM, Mocco J, Neal D, Dewan MC, Reavey-Cantwell J, Woo HH, Fiorella DJ, Mokin M, Siddiqui AH, Turk AS, Turner RD, Chaudry I, Kalani MY, Albuquerque F, Hoh BL. A multicenter study of stent-assisted coiling of cerebral aneurysms with a Y configuration. Neurosurgery. 2013;73(3):466–72.
Conrad MD, Brasiliense LB, Richie AN, Hanel RA. Y stenting assisted coiling using a new low profile visible intraluminal support device for wide necked basilar tip aneurysms: a technical report. J Neurointerv Surg. 2014;6(4):296–300.
Limbucci N, Renieri L, Nappini S, Consoli A, Rosi A, Mangiafico S. Y-stent assisted coiling of bifurcation aneurysms with Enterprise stent: long-term follow-up. J Neurointerv Surg. 2016;8(2):158–62.
Jeon P, Kim BM, Kim DJ, Kim DI, Park KY. Y-configuration double-stent-assisted coiling using two closed-cell stents for wide-neck basilar tip aneurysms. Acta Neurochir. 2014;156(9):1677–86.
Zhao KJ, Yang PF, Huang QH, Li Q, Zhao WY, Liu JM, Hong B. Y-configuration stent placement (crossing and kissing) for endovascular treatment of wide-neck cerebral aneurysms located at 4 different bifurcation sites. AJNR Am J Neuroradiol. 2012;33(7):1310–6.
Heller RS, Rahal JP, Malek AM. Y-stent embolization technique for intracranial bifurcation aneurysms. J Clin Neurosci. 2014;21(8):1368–72.
Chalouhi N, Jabbour P, Gonzalez LF, Dumont AS, Rosenwasser R, Starke RM, Gordon D, Hann S, Tjoumakaris S. Safety and efficacy of endovascular treatment of basilar tip aneurysms by coiling with and without stent assistance: a review of 235 cases. Neurosurgery. 2012;71(4):785–94.
Lee WJ, Cho CS. Y-stenting endovascular treatment for ruptured intracranial aneurysms: a single-institution experience in Korea. J Korean Neurosurg Soc. 2012;52(3):187–92.
Ko JK, Han IH, Cho WH, Choi BK, Cha SH, Choi CH, Lee SW, Lee TH. Crossing Y-stent technique with dual open-cell stents for coiling of wide-necked bifurcation aneurysms. Clin Neurol Neurosurg. 2015;132:54–60.
Kyoshima K, Kobayashi S, Nitta J, Osawa M, Shigeta H, Nakagawa F. Clinical analysis of internal carotid artery aneurysms with reference to classification and clipping techniques. Acta Neurochir. 1998;140(9):933–42.
Miyazawa N, Nukui H, Horikoshi T, Yagishita T, Sugita M, Kanemaru K. Surgical management of aneurysms of the bifurcation of the internal carotid artery. Clin Neurol Neurosurg. 2002;104(2):103–14.
Sakamoto S, Ohba S, Shibukawa M, Kiura Y, Okazaki T, Arita K, Kurisu K. Characteristics of aneurysms of the internal carotid artery bifurcation. Acta Neurochir. 2006;148(2):139–43. discussion 143
Gupta SK, Khosla VK, Chhabra R, Mohindra S, Bapuraj JR, Khandelwal N, Mukherjee KK, Tewari MK, Pathak A, Mathuriya SN. Internal carotid artery bifurcation aneurysms: surgical experience. Neurol Med Chir (Tokyo). 2007;47(4):153–7; discussion 157–158.
Lehecka M, Dashti R, Romani R, Celik O, Navratil O, Kivipelto L, Kivisaari R, Shen H, Ishii K, Karatas A, Lehto H, Kokuzawa J, Niemela M, Rinne J, Ronkainen A, Koivisto T, Jaaskelainen JE, Hernesniemi J. Microneurosurgical management of internal carotid artery bifurcation aneurysms. Surg Neurol. 2009;71(6):649–67.
van Rooij WJ, Sluzewski M, Beute GN. Internal carotid bifurcation aneurysms: frequency, angiographic anatomy and results of coiling in 50 aneurysms. Neuroradiology. 2008;50(7):583–7.
Oishi H, Yamamoto M, Nonaka S, Arai H. Endovascular therapy of internal carotid artery bifurcation aneurysms. J Neurointerv Surg. 2013;5(5):400–4. https://doi.org/10.1136/neurintsurg-2012-010414.
Straus D, Johnson AK, Lopes DK. Overlapping stents in “Y” configuration for anterior circulation aneurysms. EJMINT Original Article. 2013:1304000095.
Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25(2):219–39.
Sekhar LN, Natarajan SK, Britz GW, Ghodke B. Microsurgical management of anterior communicating artery aneurysms. Neurosurgery. 2007;61(5 Suppl 2):273–90; discussion 290-272.
Hernesniemi J, Dashti R, Lehecka M, Niemela M, Rinne J, Lehto H, Ronkainen A, Koivisto T, Jaaskelainen JE. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol. 2008;70(1):8–28; discussion 29.
Proust F, Debono B, Hannequin D, Gerardin E, Clavier E, Langlois O, Freger P. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99(1):3–14.
Elias T, Ogungbo B, Connolly D, Gregson B, Mendelow AD, Gholkar A. Endovascular treatment of anterior communicating artery aneurysms: results of clinical and radiological outcome in Newcastle. Br J Neurosurg. 2003;17(3):278–86.
Raslan AM, Oztaskin M, Thompson EM, Dogan A, Petersen B, Nesbit G, Lee DS, Barnwell SL. Neuroform stent-assisted embolization of incidental anterior communicating artery aneurysms: long-term clinical and angiographic follow-up. Neurosurgery. 2011;69(1):27–37; discussion 37.
Brasiliense LB, Yoon JW, Orina JN, Miller DA, Tawk RG, Hanel RA. A reappraisal of anterior communicating artery aneurysms: a case for stent-assisted embolization. Neurosurgery. 2016;78(2):200–7.
Rohde S, Bendszus M, Hartmann M, Hahnel S. Treatment of a wide-necked aneurysm of the anterior cerebral artery using two Enterprise stents in “Y”-configuration stenting technique and coil embolization: a technical note. Neuroradiology. 2010;52(3):231–5.
Bartolini B, Blanc R, Pistocchi S, Redjem H, Piotin M. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol. 2014;35(11):2153–8.
Dashti R, Hernesniemi J, Niemela M, Rinne J, Porras M, Lehecka M, Shen H, Albayrak BS, Lehto H, Koroknay-Pal P, de Oliveira RS, Perra G, Ronkainen A, Koivisto T, Jaaskelainen JE. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007;67(5):441–56.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P; International Subarachnoid Aneurysm Trial Collaborative G. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17.
Sani S, Lopes DK. Treatment of a middle cerebral artery bifurcation aneurysm using a double neuroform stent “Y” configuration and coil embolization: technical case report. Neurosurgery. 2005;57(1 Suppl):E209; discussion E209.
Lazzaro MA, Zaidat OO. X-configuration intersecting Enterprise stents for vascular remodeling and assisted coil embolization of a wide neck anterior communicating artery aneurysm. J Neurointerv Surg. 2011;3(4):348–51.
Zelenak K, Zelenakova J, DeRiggo J, Kurca E, Boudny J, Polacek H. Flow changes after endovascular treatment of a wide-neck anterior communicating artery aneurysm by using X-configured kissing stents (cross-kissing stents) technique. Cardiovasc Intervent Radiol. 2011;34(6):1308–11.
Saatci I, Geyik S, Yavuz K, Cekirge S. X-configured stent-assisted coiling in the endovascular treatment of complex anterior communicating artery aneurysms: a novel reconstructive technique. AJNR Am J Neuroradiol. 2011;32(6):E113–7.
Horowitz M, Levy E, Sauvageau E, Genevro J, Guterman LR, Hanel R, Wehman C, Gupta R, Jovin T. Intra/extra-aneurysmal stent placement for management of complex and wide-necked- bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 2006;58(4 Suppl 2):ONS-258–262; discussion ONS-262.
Sychra V, Klisch J, Werner M, Dettenborn C, Petrovitch A, Strasilla C, Gerlach R, Rosahl S, Holtmannspotter M. Waffle-cone technique with Solitaire AB remodeling device: endovascular treatment of highly selected complex cerebral aneurysms. Neuroradiology. 2011;53(12):961–72.
Padalino DJ, Singla A, Jacobsen W, Deshaies EM. Enterprise stent for waffle-cone stent-assisted coil embolization of large wide-necked arterial bifurcation aneurysms. Surg Neurol Int. 2013;4:9.
Liu W, Kung DK, Policeni B, Rossen JD, Jabbour PM, Hasan DM. Stent-assisted coil embolization of complex wide-necked bifurcation cerebral aneurysms using the “waffle cone” technique. A review of ten consecutive cases. Interv Neuroradiol. 2012;18(1):20–8.
Guo X, Chen Z, Wang Z, Guan S. Preliminary experiences of “waffle cone” technique for the treatment of intracranial aneurysm. Zhonghua Yi Xue Za Zhi. 2014;94(17):1346–8.
Cho YD, Park SW, Lee JY, Seo JH, Kang HS, Kim JE, Han MH. Nonoverlapping Y-configuration stenting technique with dual closed-cell stents in wide-neck basilar tip aneurysms. Neurosurgery. 2012;70(2 Suppl Operative):244–9.
Aydin K, Sencer S, Barburoglu M, Berdikhojayev M, Aras Y, Sencer A, Izgi N. Midterm results of T-stent-assisted coiling of wide-necked and complex intracranial bifurcation aneurysms using low-profile stents. J Neurosurg. 2017;127(6):1288–96. https://doi.org/10.3171/2016.9.JNS161909.
Zaidat OO, Szeder V, Alexander MJ. Transbrachial stent-assisted coil embolization of right posterior inferior cerebellar artery aneurysm: technical case report. J Neuroimaging. 2007;17(4):344–7.
Samaniego EA, Abdo G, Hanel RA, Lima A, Ortega-Gutierrez S, Dabus G. Endovascular treatment of PICA aneurysms with a Low-profile Visualized Intraluminal Support (LVIS Jr) device. J Neurointerv Surg. 2016;8(10):1030–3.
Chalouhi N, Jabbour P, Starke RM, Tjoumakaris SI, Gonzalez LF, Witte S, Rosenwasser RH, Dumont AS. Endovascular treatment of proximal and distal posterior inferior cerebellar artery aneurysms. J Neurosurg. 2013;118(5):991–9.
Mericle RA, Reig AS, Burry MV, Eskioglu E, Firment CS, Santra S. Endovascular surgery for proximal posterior inferior cerebellar artery aneurysms: an analysis of Glasgow Outcome Score by Hunt-Hess grades. Neurosurgery. 2006;58(4):619–25. discussion 619–625
Peluso JP, van Rooij WJ, Sluzewski M, Beute GN, Majoie CB. Posterior inferior cerebellar artery aneurysms: incidence, clinical presentation, and outcome of endovascular treatment. AJNR Am J Neuroradiol. 2008;29(1):86–90.
Aguilar-Perez M, Kurre W, Fischer S, Bazner H, Henkes H. Coil occlusion of wide-neck bifurcation aneurysms assisted by a novel intra- to extra-aneurysmatic neck-bridging device (pCONus): initial experience. AJNR Am J Neuroradiol. 2014;35(5):965–71.
Gory B, Aguilar-Perez M, Pomero E, Turjman F, Weber W, Fischer S, Henkes H, Biondi A. pCONus device for the endovascular treatment of wide-neck middle cerebral artery aneurysms. AJNR Am J Neuroradiol. 2015;36(9):1735–40.
Lubicz B, Morais R, Alghamdi F, Mine B, Collignon L, Eker OF. The pCONus device for the endovascular treatment of wide neck bifurcation aneurysms. J Neurointerv Surg. 2016;8(9):940–4.
Ulfert C, Pfaff J, Schonenberger S, Bosel J, Herweh C, Pham M, Bendszus M, Mohlenbruch M. The pCONus Device in Treatment of Wide-necked Aneurysms : Technical and Midterm Clinical and Angiographic Results. Clin Neuroradiol. 2018;28(1):47–54. https://doi.org/10.1007/s00062-016-0542-z.
Gory B, Spiotta AM, Mangiafico S, Consoli A, Biondi A, Pomero E, Killer-Oberpfalzer M, Weber W, Riva R, Labeyrie PE, Turjman F. PulseRider stent-assisted coiling of wide-neck bifurcation aneurysms: periprocedural results in an international series. AJNR Am J Neuroradiol. 2016;37(1):130–5.
Mukherjee S, Chandran A, Gopinathan A, Putharan M, Goddard T, Eldridge PR, Patankar T, Nahser HC. PulseRider-assisted treatment of wide-necked intracranial bifurcation aneurysms: safety and feasibility study. J Neurosurg. 2017;127(1):61–8. https://doi.org/10.3171/2016.2.JNS152334.
Spiotta AM, Chaudry MI, Turk AS, Turner RD. Initial experience with the PulseRider for the treatment of bifurcation aneurysms: report of first three cases in the USA. J Neurointerv Surg. 2016;8(2):186–9.
Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafe A, Cekirge S, Fiorella D, Jabbour P, Levy E, McDougall C, Siddiqui A, Szikora I, Woo H, Albuquerque F, Bozorgchami H, Dashti SR, Delgado Almandoz JE, Kelly ME, Turner R, Woodward BK, Brinjikji W, Lanzino G, Lylyk P. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015;36(1):108–15.
Kallmes DF, Brinjikji W, Boccardi E, Ciceri E, Diaz O, Tawk R, Woo H, Jabbour P, Albuquerque F, Chapot R, Bonafe A, Dashti SR, Delgado Almandoz JE, Given C 2nd, Kelly ME, Cross DT 3rd, Duckwiler G, Razack N, Powers CJ, Fischer S, Lopes D, Harrigan MR, Huddle D, Turner R, Zaidat OO, Defreyne L, Pereira VM, Cekirge S, Fiorella D, Hanel RA, Lylyk P, McDougall C, Siddiqui A, Szikora I, Levy E. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol. 2016;5(1–2):89–99.
Saleme S, Iosif C, Ponomarjova S, Mendes G, Camilleri Y, Caire F, Boncoeur MP, Mounayer C. Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery. 2014;75(6):623–31. quiz 631
Gawlitza M, Januel AC, Tall P, Bonneville F, Cognard C. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. J Neurointerv Surg. 2016;8(5):481–7.
Dabus G, Grossberg JA, Cawley CM, Dion JE, Puri AS, Wakhloo AK, Gonsales D, Aguilar-Salinas P, Sauvageau E, Linfante I, Hanel RA. Treatment of complex anterior cerebral artery aneurysms with Pipeline flow diversion: mid-term results. J Neurointerv Surg. 2017;9(2):147–51. https://doi.org/10.1136/neurintsurg-2016-012519.
Muskens IS, Senders JT, Dasenbrock HH, Smith TR, Broekman ML. The Woven Endobridge (WEB) device for treatment of intracranial aneurysms: a systematic review. World Neurosurg. 2016; https://doi.org/10.1016/j.wneu.2016.11.020.
Pierot L, Costalat V, Moret J, Szikora I, Klisch J, Herbreteau D, Holtmannspotter M, Weber W, Januel AC, Liebig T, Sychra V, Strasilla C, Cognard C, Bonafe A, Molyneux A, Byrne JV, Spelle L. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg. 2016;124(5):1250–6.
Pierot L, Moret J, Turjman F, Herbreteau D, Raoult H, Barreau X, Velasco S, Desal H, Januel AC, Courtheoux P, Gauvrit JY, Cognard C, Molyneux A, Byrne J, Spelle L. WEB treatment of intracranial aneurysms: clinical and anatomic results in the French observatory. AJNR Am J Neuroradiol. 2016;37(4):655–9.
Pierot L, Spelle L, Molyneux A, Byrne J, Webcast, French Observatory I. Clinical and anatomical follow-up in patients with aneurysms treated with the WEB device: 1-year follow-up report in the cumulated population of 2 prospective, multicenter series (WEBCAST and French observatory). Neurosurgery. 2016;78(1):133–41.
Kwon SC, Ding YH, Dai D, Kadirvel R, Lewis DA, Kallmes DF. Preliminary results of the luna aneurysm embolization system in a rabbit model: a new intrasaccular aneurysm occlusion device. AJNR Am J Neuroradiol. 2011;32(3):602–6.
Piotin M, Biondi A, Sourour N, Blanc R. O–036 treatment of intracranial aneurysms with the LUNA AES: midterm clinical and angiographic follow-up. J Neurointerv Surg. 2014;6(Suppl 1):A19–20.
Tateshima S, Niemann D, Moskowitz S, Baxter B, Frei D. O–017 preliminary experience with a new barrel shaped bifurcation aneurysm bridging device. J Neurointerv Surg. 2013;5(Suppl 2):A10.
Piotin M, Blanc R, Berge J, Turjman F. O–030 preliminary French registry clinical experience with the barrel bifurcation vascular reconstruction device. J Neurointerv Surg. 2014;6(Suppl 1):A15–6.
Marotta TR, Gunnarsson T, Penn I, Ricci DR, McDougall I, Marko A, Bourne G, Da Costa L. A novel endovascular clip system for the treatment of intracranial aneurysms: technology, concept, and initial experimental results. Laboratory investigation. J Neurosurg. 2008;108(6):1230–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aguilar-Salinas, P., Brasiliense, L.B.C., Lima, J., Aghaebrahim, A., Sauvageau, E., Hanel, R.A. (2019). Complex Stent Reconstruction for the Treatment of Intracranial Aneurysms. In: Spiotta, A., Turner, R., Chaudry, M., Turk, A. (eds) Management of Cerebrovascular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-99016-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-99016-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99015-6
Online ISBN: 978-3-319-99016-3
eBook Packages: MedicineMedicine (R0)