Abstract
Stent-assisted coil embolization is undoubtedly a valuable tool for the treatment of cerebral aneurysms. While it is clearly not a technique that should be applied to every aneurysm, the continued development and refinement of intracranial stents have allowed for safe and effective endovascular treatment of an increasing number of aneurysms. It is important to be cognizant that, as is the case with many surgical techniques, one approach does not fit all. Just as some aneurysms may be better suited for balloon-assisted or single-catheter coil embolization, the same stent-assisted technique is not useful across all aneurysms that are deemed to warrant stent assistance. It therefore behooves the treating physician to be familiar with all of these techniques, including single-stent placement with or without jailing of the microcatheter, X- or Y-stenting, waffle-cone stenting, and rescue-stent placement including the placement of overlapping stents. By gaining familiarity with these variations and technical nuances, treatment can be tailored to find the optimal option for each individual aneurysm.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stent-assisted coil embolization
- Coil embolization
- Embolization with stent-assisted coils
- Cerebral aneurysm treatment
- Intracranial aneurysm
- Aneurysm treatment with embolization
The endovascular treatment of cerebral aneurysms has become progressively more frequent over the last decade. While endovascular techniques were traditionally reserved for narrow-necked, saccular aneurysms, improvements in endovascular techniques and devices have enabled neuroendovascular surgeons to consider treatment for more complex lesions. Perhaps, no development has had as much impact on the ability to treat an increasing number of aneurysms as the intracranial stent. Wide-necked aneurysms that were once deemed amenable only to surgical clipping now may warrant serious endovascular consideration, due in large part to the advent and refinement of intracranial stents that allow for coil embolization while protecting parent artery patency. In this chapter we will review the indications, techniques, perioperative management, and results of this stent-assisted coil embolization.
Brief Historical Overview
Catheterization of the intracranial vessels was first described in 1964 by Luessenhop and Velasquez [1]. Ten years later, Serbinenko reported the endovascular treatment of over 300 patients using both detachable and nondetachable balloons to treat direct carotid-cavernous fistulae and cerebral aneurysms [2]. Unfortunately, the first generation of balloons lacked compliance and frequently deflated over time.
Given the failure and morbidity associated with these early balloons, Guglielmi introduced soft, controllable, retrievable, and detachable platinum coils for as means of endosaccular obliteration of cerebral aneurysms [3, 4]. While detachable coils became increasingly used for aneurysm treatment, the primary treatment method for wide-necked and irregularly shaped aneurysms remained microsurgical clipping. Attempts to treat these aneurysms with coiling alone frequently resulted in either incomplete coiling or coil migration or prolapse.
The first report of stent-assisted coil embolization was described by Higashida in 1997 for the treatment of a ruptured vertebral artery aneurysm [5]. In this case, a balloon-expandable coronary stent was deployed, and coiling was performed via catheterization of the aneurysm through the stent tines. Since that time, with the development of intracranial stents, stent-assisted coiling has become an integral part of the armamentarium of the neurointerventionalist. Improvements in catheters, microcatheters, coils, and stents have improved the safety and efficacy of stent-assisted coil embolization and paved the way for their routine use.
Indications and Contraindications
Clearly not every intracranial aneurysm requires stent-assisted coiling for treatment. Many aneurysms can be treated effectively with primary coiling alone. Aneurysms that are considered for stent-assisted coil embolization are typically wide-necked aneurysms or those that incorporate branch arteries into the aneurysm neck. A number of aneurysms that may not be ideal for primary coiling, and would generally be candidates for stent assistance, may also be reasonable candidates for other adjuvant strategies such as balloon remodeling or dual catheter embolization. It is therefore important to consider all of these techniques when devising a treatment plan.
Traditional definitions of wide aneurysm neck include a neck diameter of larger than 4 mm and a dome-to-neck ratio of less than 2. In these situations, coiling alone is associated with an increased risk of coil migration and compromise of parent vessel patency. Stent assistance provides a permanent buttress for the coil mass, thereby preventing herniation into the parent artery. Stent assistance may also permit increased packing density, which has been associated with higher rates of aneurysm occlusion [6, 7].
A significant consideration for utilizing stent-assisted coiling techniques is the requirement for dual antiplatelet therapy. Patients with histories of significant gastrointestinal hemorrhage, bleeding diatheses, or significant coagulation disorders may be at increased risk of hemorrhagic complications related to dual antiplatelet therapy. Similarly, assessment of patient compliance with dual antiplatelet therapy is critical. Patients with medical, social, and/or psychiatric conditions that may preclude compliance with dual antiplatelet therapy may be better suited with other techniques.
The use of stent-assisted coiling in the acute period following aneurysm rupture remains a controversial topic primarily due to the need for dual antiplatelet therapy. While there is increasing data supporting its use, particular consideration should be given to avoiding stent placement in patients who may be at increased risk for developing hydrocephalus and, therefore, require CSF diversion procedures. The risk associated with performing these procedures in a patient with platelet inhibition must be considered.
Preoperative Preparation/Evaluation
Preparation for stent-assisted coiling begins well before the patient is in the angiography suite. Critical evaluation of noninvasive imaging (e.g., CTA, MRA) should suggest the possibility of the need for stent assistance. It can also warn the surgeon of potential obstacles that may be encountered during the procedure (e.g., tortuous anatomy necessitating the need for an intermediate support catheter, the need for “Y” or “X” stenting techniques, etc.).
The importance of a preoperative, baseline neurologic exam prior to endovascular aneurysm treatment cannot be understated. This is particularly important with stent-assisted techniques in which in-stent stenosis or compromise of small branch vessels may result in subtle postoperative deficits. For these reasons, frequent and precise postoperative neurologic assessments are of equal importance.
When the situation allows, such as in planned, elective cases, preoperative antiplatelet therapy is recommended. Traditional dosing schemes include aspirin 325 mg daily and clopidogrel 75 mg daily for at least 5 days prior to the scheduled procedure. Alternatively, loading doses of aspirin 325–650 mg and clopidogrel 300–600 mg can be administered the evening before the scheduled procedure. We prefer a loading dose of aspirin 325 mg and clopidogrel 600 mg. Confirmation of adequate platelet inhibition with point-of-care testing has become standard in many centers. Adequate platelet inhibition has been shown to be associated with a decreased risk of thromboembolic complications; conversely, insufficient platelet inhibition has been shown to be associated with increased frequency of complications in some studies [8,9,10].
Resistance to aspirin is relatively rare, occurring in 2–5% [11, 12]. However, resistance to clopidogrel has been reported in up to 50% [13]. Given the high incidence of clopidogrel resistance, alternative antiplatelet agents are sometimes utilized. Identifying resistance to either agent with point-of-care testing allows surgeons to adjust antiplatelet therapy, theoretically ensuring platelet inhibition and decreasing thromboembolic complications. Their increased reliability is due primarily to their administration in the active form.
Review of Equipment
The critical decision involved in stent-assisted coiling is which stent to use. Until recently, the only stents designed for intracranial use were the Neuroform (Stryker, Fremont, CA) and the Enterprise (Codman Neuro, Raynham, MA). Within the last several years, however, a number of variations of the low-profile visualized intraluminal support device (LVIS) (Microvention, Tustin, CA) have become available. A summary of the available intracranial stents used in stent-assisted coiling is provided in Table 12.1. Each of the stents has unique features that differentiate them and therefore may allow neuroendovascular surgeons the opportunity to more individually tailor treatment.
The Neuroform stent is an open-cell intracranial stent used in stent-assisted coil embolization. This design makes the stent easily conformable to both the vessel wall and the aneurysm neck. This may provide wider neck coverage for bifurcation aneurysms and excellent wall apposition, which may decrease the risk of stent-related thromboemboli. Another benefit of the open-cell design is that it makes it easier for a coiling microcatheter to pass through the stent into the aneurysm. Of course there are potential downsides to the stent. Most notably, it requires a 0.027″ microcatheter, which may limit the ability to place the stent in smaller vessels or in branch vessels that are particularly tortuous or arise at acute angles. In addition, the open-cell design is theoretically more penetrable to coils. Just as the stent allows for easier passage of coiling microcatheters, the coils themselves are more likely to find their way through an open cell into the parent vessel. This should be taken into consideration when coiling an aneurysm following placement of a Neuroform stent. In other words, one should not coil indiscriminately, but rather careful attention should be paid to ensuring parent artery patency. While much of the Neuroform experience in the United States is with the classic device, at the time of this publication the Neuroform Atlas (Stryker, Fremont, CA) was just recently approved by the FDA. This is essentially a smaller Neuroform that is delivered through an 0.017″ microcatheter.
The Enterprise stent is a closed-cell intracranial stent used for stent-assisted coil embolization. The closed-cell design provides a more reliable buttress to subsequent coils but is less conformable to the parent artery, particularly at curves or angles, such as those seen with bifurcation aneurysms. Criticism of the Enterprise includes its propensity to ovalize in such curves, something that has reportedly been improved upon in the second generation Enterprise 2. The stent is deliverable through a 0.021″ microcatheter, which has often led to it being the stent of choice as the second stent in a Y- or X-stent construct when compared to the 0.027″ catheter needed for the original Neuroform, as the smaller catheter is more likely to pass through a cell of an existing stent.
The LVIS stents are also now available for use in the stent-assisted coil embolization. The LVIS is deliverable through a 0.021″ microcatheter, with available sizes up to 5.5 mm. LVIS Blue is the newest modification of the original LVIS design. The angle of the braid is smaller, resulting in better wall apposition and increased flow diversion, although this change came with an associated increase in difficulty passing a coiling catheter through the cells of the stent. While a microcatheter can be passed through its cells with significant effort, the difficulty in achieving this suggests that it should be used almost exclusively with the jailing technique, discussed below. The LVIS Jr. stent was the first stent available in the USA that can be delivered through a 0.017″ microcatheter, a significant advance in the treatment of intracranial aneurysms. This is significant not only for the improved ability to access smaller, more tortuous vessels, but it also allows for the deployment of the stent through dual-lumen balloon catheters such as the Scepter (Microvention, Tustin, CA) or the Eclipse (Balt, Montmorency, France). This enables the neuroendovascular surgeon to first attempt treatment without a stent using balloon remodeling, and if this technique fails, a stent can be placed through the balloon catheter.
In addition to the widely available stents, a number of devices exist that are currently in clinical trials or are available out of the USA. The LEO Baby (Balt, Montmorency, France) stent is a low-profile braided stent that is also deliverable through a 0.017″ microcatheter. It is not yet available in the USA, but there is a considerable international experience with it, and many people who have access to it prefer it for small vessel aneurysms. Other devices that are designed for stent assistance of bifurcation devices include the Barrel device (ev3/Covidien), which is a stent with an enlarged mid-segment, and the pCONus bifurcation aneurysm implant (Phenox, Bochum, Germany), which aims to produce the waffle-cone effect described below and is not currently available in the USA. Lastly, the PulseRider (Codman Neuro, Raynham, MA) is an FDA-approved device that is not technically a stent but is a luminal device designed to support coil placement by maintaining luminal patency.
Stent-assisted coiling can be performed using a variety of coils. A review of coil characteristics is beyond the scope of this chapter but can be found in a review by White et al. [14]. It is advisable that the surgeon uses the type of coils with which he/she is most comfortable.
Stenting Techniques
“Traditional” Stent-Assisted Coiling
Stent-assisted coiling traditionally was considered aneurysm coiling via a microcatheter that was navigated into the aneurysm through the struts of a stent that had been completely deployed across the aneurysm neck (Figs. 12.1 and 12.2). The inherent difficulty in this technique lies in having to catheterize through the very narrow stent struts, which is made even more difficult when a closed-cell stent is used. When this technique is used, maintaining microcatheter access within the aneurysm is particularly important, since reaccess through stent struts is even more challenging when a coil mass is present within the aneurysm. Although this technique may be used to treat an aneurysm that has not been previously treated with a stent, it is obviously the only option for the re-treatment of recurrent or residual aneurysms where a stent has already been placed.
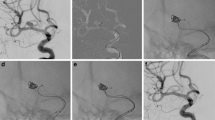
Basilar apex aneurysm in which both posterior cerebral arteries (PCA) arise from the left side of the aneurysm. It was treated with a single LVIS Jr. stent and subsequent coil embolization. The coiling microcatheter was advanced into the aneurysm after placement of the stent. The unusual configuration of the aneurysm allowed a single-stent to cover both PCAs and the left superior cerebellar artery by placing the stent from the right PCA to the basilar artery. The second and third images demonstrate that the stent caused mild straightening of the right PCA
Illustration depicting techniques for stent-assisted coil embolization. The left image shows traditional stent-assisted coil embolization, where the coiling microcatheter is passed into the aneurysm through a cell of the already-placed stent. The right image shows the jailing technique, in which the coiling microcatheter is passed into the aneurysm prior to stent deployment. This results in the coiling catheter being located outside of the stent in a parallel fashion
Jailing Technique
In this technique, the microcatheter is navigated into the aneurysm before deployment of the stent. Once the microcatheter is within the aneurysm, the stent is deployed through a second parallel microcatheter, “jailing” the coiling microcatheter between the stent and the parent vessel wall, while the microcatheter tip remains in the aneurysm (Fig. 12.2). The aneurysm is then coiled and the microcatheter removed. While the presence of the coiling microcatheter outside of the stent may initially prevent complete wall apposition of the stent, the radial force of the stent typically results in adequate opening once the microcatheter is removed. However, if wall apposition remains insufficient, balloon angioplasty can be performed to further expand the stent.
This technique may be considered technically easier since it does not require passing a microcatheter through the stent struts. Jailing the microcatheter gives it stability and may make it less susceptible to kickback. However, this can also be a shortcoming of this technique, as the stent limits the ability of the microcatheter to move back and forth with coil deployment. This movement can be useful, as it may enable the microcatheter to move to pockets of decreased resistance, filling portions of the aneurysm that otherwise may not have filled. Perhaps more importantly, kickback of the microcatheter can prevent undue forward pressure that can cause intraoperative aneurysmal rupture. It is therefore critical to be mindful of this lack of catheter feedback that normally exists during coil embolization.
Y-Stenting
Stent-assisted coil embolization of bifurcation aneurysms presents a unique challenge since there are potentially three parent arteries (the main trunk and the two branches of the bifurcation) that require protection by a stent. For the Y-stenting technique, a stent is deployed from one of the branch vessels into the main trunk. A microcatheter is then navigated through the struts of the stent into the other branch vessel, and a second stent is deployed from there into the main trunk. This results in single-stent coverage in each branch vessel and overlapping, double-stent coverage in the main trunk, forming a “Y.” A coiling microcatheter is then passed through the stents into the aneurysm, and the aneurysm is coiled, with the stents providing protection to both of the branch vessels and the main trunk (Fig. 12.3). As is the case with single-stent placement, jailing the coiling catheter is also an option. A variation of the standard Y-stent technique is the double-barrel technique, in which the two stents are deployed alongside each other without having one pass through the other. This, however, results in a stent-stent interface within the center of the artery lumen, rather than along the arterial wall, and therefore is not generally the preferred Y-stent technique.
Illustrative schematic depicting Y-stent-assisted coil embolization of a basilar apex aneurysm. The first microcatheter is placed in the left PCA, followed by stent deployment centered over the aneurysm neck. Next, a second microcatheter is advanced through the first stent into the right PCA, and again a stent is placed from that point into the basilar artery. Following deployment of both stents, a coiling microcatheter is advanced through the stents into the aneurysm, and coiling performed
Other variations are the X-stent configuration, or the H-stent configuration, which are occasionally needed at the anterior communicating artery complex. Unlike basilar or middle cerebral artery bifurcation aneurysms, these aneurysms usually have two main trunks and two branch vessels that require protection from the stents. For the X-stent technique, one stent is placed from an A1 segment across the anterior communicating artery to the contralateral A2, before the second stent is placed from the other A1 segment, through the existing stent, to the last remaining A2 segment. The H-stent entails deploying the two stents from each A1 to A2 without crossing over the AComm, a maneuver that generally sacrifices the anterior communicating artery.
Though various stent constructs have been successfully used for Y- or X-stent configurations, there is no consensus on the type of stents to use. Many advocate for an open-cell stent as the first stent that is deployed. The argument is that this permits easier catheterization through the stent struts into the second branch vessels, a phenomenon that is becoming less of a concern with the development of stents that are deliverable through 0.017″ microcatheters. Another argument favoring placement of an open-cell stent first is that it reduces the constraint placed on the second stent that is seen when a closed-cell stent is placed through another closed-cell stent, although some argue that this constraint can be a good thing, as it may result in mild flow-diverting effect away from the aneurysm.
One additional possible variation that treating physicians should consider is the timing of the treatment steps of a Y-stent-assisted coil embolization. While it may seem optimal to place both stents and coil the aneurysm in one setting, in some situations it may be beneficial to stage the treatments. First, a newly placed stent is more likely to be displaced with manipulation (Fig. 12.4), particularly if reasonable force is required to pass the second microcatheter through a cell for placement of the second stent. Again, this may be less of a concern with stents that are deliverable through 0.017″ microcatheters; however, if the first stent is in a tenuous position, for instance, if there is not a reasonable amount of length of stent proximal to the aneurysm neck, staging the first and second stents should be strongly considered. The other reason to consider staging is to reduce the amount of radiation in one procedure. This may not be an issue for straightforward cases; however, for cases that require substantial time and effort to catheterize one or both of the branch vessels, procedural times can be excessive, and again staging should be considered.
(a, b) Large basilar apex aneurysm treated with Y-stent-assisted coil embolization. (a) Unsubtracted images are shown demonstrating placement of the stents. Panel 1 shows placement of the initial Neuroform stent from the left PCA to the basilar artery, with white arrows indicating the proximal and distal tines of the stent. Panel 2 shows that the proximal tines of the Neuroform, indicated by the white arrow, have migrated distally with passage of a 0.021″ microcatheter through the stent cells into the right PCA. The second stent, an Enterprise stent, was then placed from the right PCA into the basilar. The black arrows indicate the proximal and distal tines of the Enterprise stent. Following successful deployment of both stents, coil embolization was performed after passing the coiling microcatheter through the stents into the aneurysm. (b) Pre- and posttreatment images are shown
Finally, it is important to recognize that some aneurysms that are felt to require treatment with a Y-stent may, in fact, receive sufficient parent artery protection with the placement of a single stent in an L-shaped or half T configuration (Fig. 12.5). This can generally be achieved by pushing the stent out of the microcatheter while crossing the aneurysm neck rather than unsheathing the stent by retracting the microcatheter. While this does not provide the complete parent artery protection you would expect from the Y-stent, it may in essence turn the wide-necked aneurysm into a narrow-necked one. This result is essentially what the Barrel device is designed to achieve. The obvious benefit of eliminating the need for a second stent is that it limits the amount of metal placed within the artery. This may decrease the risk of thrombus formation or perforator occlusion, which clearly can have devastating results in the basilar artery or the M1 segment of the middle cerebral artery.
(a, b) Large basilar apex aneurysm in a patient who presented with subarachnoid hemorrhage. Initial treatment was performed with single-catheter coil embolization, with pre- and posttreatment images shown (a). Several months later, a substantial recurrence was noted (b). Stent-assisted coil embolization was performed using an Atlas stent in an L configuration, which obviated the need for a second stent. Coil embolization was then performed after passing the coiling catheter through the stent into the aneurysm
Waffle-Cone Technique
The waffle-cone technique, named for the appearance of the stent and coil mass after treatment, is a technique in which the distal aspect of a stent is deployed within the aneurysm fundus and extends into the parent artery. The flared distal stent tines help maintain stent position, reconstruct the neck, and support the coil mass within the aneurysm. The microcatheter then passes into the aneurysm along the axis of the stent (rather than through the stent struts as in Y-stenting), and coiling is completed in the usual fashion.
This technique theoretically offers some advantages over Y-stenting [15]. First, it is arguably technically easier, as there is no need to pass a microcatheter through a cell of the existing stent for placement of a second stent or aneurysm coils. It also does not require catheterizing the branch vessels, which may come off the aneurysm neck at very acute angles. Secondly, because only one stent is utilized, there is less metal surface area within the vessel, possibly reducing the risk of thromboembolic complications. One downside of this technique is that while it does theoretically provide excellent protection of the branch vessels arising from the neck of the aneurysm, it may provide very little protection of the parent artery that the stent comes from. Therefore, coiling cannot be performed without careful attention paid to coil prolapse into the parent artery. It also is obviously not advisable with stents that have a distal tip, as the tip would need to be within the aneurysm sac. One additional limitation is that the neck of the aneurysm needs to be smaller than the diameter of the stent, which is generally no bigger than 4.5 mm.
Of note, the pCONus is designed to act in this fashion; however, unlike the standard intracranial stents that have been described for the technique, it also incorporates a nylon net at the distal aspect. This theoretically keeps coils in the aneurysm and prevents herniation into the parent vessel. The PulseRider also allows for a similar effect when its leaflets are deployed within the aneurysm dome rather than in the associated branch vessels.
Rescue Stenting
While most stent-assisted coil embolizations are planned ahead of time, the technique is quite versatile and may be used as a rescue strategy when other techniques fail. For example, if treatment with single-catheter or balloon-assisted techniques results in coil loop herniation into, or impingement of, the parent vessel, a stent may be required to preserve parent vessel patency. This is a particular advantage of dual-lumen balloons such as the Scepter, which allow for balloon-assisted coil embolization and then stent placement through the inner lumen if coil prolapse is seen once the balloon is deflated. When a dual-lumen balloon is not already in place, a new microcatheter must be advanced past the prolapsed coil mass in order to place the stent. In these instances, special attention should be paid to ensure that the catheter does not disturb or go through the coil mass, which could result in coil migration or incomplete opening of the stent. It is also occasionally necessary to place additional overlapping stents for rare instances when a coil tail or loop herniates through an existing stent.
Of course, a major consideration for stent placement is the antiplatelet therapy. In preparation for the possibility of rescue or unanticipated stent placement, some centers initiate dual antiplatelet therapy prior to elective aneurysm treatment, even if a stent is not planned. However, this is probably not the norm at most centers, and therefore patients will often require intraoperative administration of antiplatelet medications once a stent is deemed necessary.
Clearly, oral administration of aspirin or clopidogrel will take time to act before adequate antiplatelet activity is present. Therefore, an intravenous antiplatelet medication is often administered intraoperatively. Abciximab, a GPIIb/IIIa inhibitor with a time of onset of 10 min, is probably the most commonly described medication for this indication. A bolus of 0.125–0.25 mg/kg is administered, which allows for antiplatelet coverage until traditional antiplatelet agents are administered orally or rectally. If, for some reason, it is anticipated that long-acting antiplatelet agents will not be able to be given for a particularly long time, abciximab can be given as an infusion, typically for an additional 12 h. While intraoperative administration of abciximab may give some neuroendovascular surgeons a pause, evidence suggests that there is no difference in complications associated with stenting intracranial aneurysms when pretreated with antiplatelets or if given abciximab intraoperatively [16].
Results and Literature Review
Coil embolization remains the workhorse of endovascular cerebral aneurysm treatment. For “routine” aneurysms with favorable anatomic characteristics, long-term occlusion rates are good, with an excellent procedural safety profile. However, additional techniques are often necessary for the treatment of wide-necked aneurysms. The addition of a stent to standard endovascular coiling results in better occlusion rates. However, it does require antiplatelet medication, and therefore it is important to consider more than just aneurysmal configuration when deciding whether to employ stent-assisted coil embolization.
Two meta-analyses published in 2016 compared stent-assisted coiling and primary coiling alone [17, 18]. Both studies demonstrated no statistically significant difference in immediate occlusion rates. However, progressive thrombosis was significantly more likely in aneurysms that underwent stent-assisted coiling (range 29.9–40.9% vs. 17.5–22.7%). Additionally, the rate of recurrence was found to be lower in aneurysms treated with stent assistance (range 12.7–13.3% vs. 27.9–29.1%). These studies found no difference in the periprocedural morbidity, which ranged from 11.8% to 12.2% in the stent-assisted coiling group and 8.0–12.0% in the coiling-only group. In one of these meta-analyses, the rate of ischemic/thrombotic complications was found to be the same [18]; however, in the other study, perioperative ischemic strokes occurred more frequently in the stent-assisted coiling group (4.7% vs. 2.0%) [17].
Long-term results of stent-assisted coiling have been encouraging. Johnson et al. found an annual recurrence rate of 0.89% in 262 aneurysms with a mean follow-up of 3.63 years [19]. Most aneurysm recurrences (9.7%) were diagnosed at the 6-month follow-up, with only 1.7% being diagnosed subsequently. They found large (10–25 mm) and giant aneurysms required re-treatment more frequently. Overlapping or Y-configurations were also found to require re-treatment at a higher rate, though a higher proportion of large/giant aneurysms were treated using these configurations.
In contrast to primary coiling, the success of stent-assisted coiling depends not only on coil embolization but also on stent deployment. In an analysis of 1510 aneurysms treated in 1457 patients, Shapiro et al. found the technical stent-related failure rate to be 9% [20], with stent migration or premature/misplaced deployment occurred in 5% and the inability to deploy the stent in 4%. The rates of overall stent problems and delivery failure were found to significantly decrease with physician experience.
The two most studied stents used in stent-assisted coiling are Neuroform and Enterprise. A comparative analysis including 47 studies and 2111 aneurysms treated with Neuroform stent and 2127 treated with Enterprise demonstrated no difference in initial occlusion rates (52.7% in Neuroform group vs. 52.8% in the Enterprise group) [21]. However, there was a statistically significant difference in the rate of complete occlusion at the last follow-up, which occurred in 61.1% of patients treated with Neuroform and in 74.7% of patients treated with Enterprise. Similarly, the rates of recanalization were significantly more in patients treated with Neuroform (13.9% vs. 10.6%). There was no difference in the rates of permanent morbidity and mortality.
More recently, the low-profile visualized intraluminal support (LVIS) device has been introduced. A systematic review of 384 patients with 390 aneurysms treated with the LVIS for stent-assisted coiling found the device to be safe and effective [22]. The overall technical success rate was 96.8%. Raymond class I or II occlusion was observed in 87.2% on immediate control angiogram and progressed to 93.1% at follow-up (mean 6 months). Aneurysm recanalization occurred in 2.5%. The overall procedure-related complication rate was 6.5%, with symptomatic thromboembolic events occurring in 2.4%. These data compare favorably with those of the Neuroform and Enterprise stents and demonstrated the LVIS device’s safety and efficacy for stent-assisted coiling of cerebral aneurysms.
Small Aneurysms
Stent-assisted coiling may be a particularly useful strategy for the endovascular treatment of small aneurysms. Though the decision whether or not to treat small aneurysms remains controversial, some argue that they cause more extensive subarachnoid hemorrhage when they rupture [23]. Due to their small dome size, these aneurysms usually have a much lower dome-to-neck ratio and therefore may be less likely to support coils alone without a stent. The use of a stent in the treatment of these aneurysms provides support to the coil mass but also to the microcatheter used for coiling.
Small case series have been reported supporting the safety and efficacy of stent-assisted coiling in the treatment of small aneurysms [24,25,26]. In these series, the rate of periprocedural complications was between 0% and 12%. Complete occlusion was reported to occur between 77% and 100%.
Y- and X-Configured Stent-Assisted Coiling
The use of multiple stents in Y- or X-configurations introduces a layer of complexity to the procedure that necessitates an experienced operator. The Y-stenting procedure has many potential variations consisting of many constructs: open-cell stent + closed-cell stent, two open-cell stents, two closed-cell stents, overlapping limb, and kissing stents. While it is predominantly employed at the basilar apex, it is applicable for wide-necked bifurcation aneurysms at other locations [27].
A multicenter study of cerebral aneurysms undergoing Y-stent-assisted coiling demonstrated the safety and efficacy of this technique [27]. Acceptable initial aneurysm occlusion (Raymond class I or II) occurred in 84%, which progressed to 93% at follow-up (mean 9.8 months). In aneurysms that initially were Raymond III, 83% progressed to better occlusion grades at follow-up. Technical complications related to stent deployment occurred in 6.7%, and intraoperative rupture was observed in 4.4%.
Given the relatively small number of indications for Y-stenting, comparison of the various aforementioned stent constructs is difficult. In one study evaluating the use of two closed-cell stents, there were no deployment failures [28]. Perioperative morbidity was 12% and due to thromboembolic complications. Initial complete occlusion occurred in 36%, with an additional 36% progressing from incomplete occlusion to complete occlusion at follow-up (mean 16 months). Y-stenting with two open-cell stents has been equally achievable, with one group demonstrating no deployment failures and 5% perioperative morbidity [29]. Initial complete occlusion was observed in 85%, with an additional 5% progressing from incomplete occlusion to complete occlusion at follow-up. Comparison of various stent constructs across multiple centers revealed no difference in initial aneurysm occlusion, occlusion at follow-up, need for re-treatment, in-stent stenosis, or clinical outcome [27].
Regarding the X-stent configuration, although reports of large cohorts for this technique are lacking in the literature, small series have demonstrated safety and efficacy of X-stent-assisted coiling when performed by experienced practitioners [30,31,32].
Waffle-Cone Technique
Several small series utilizing the waffle-cone technique are reported in the literature [33,34,35,36]. These studies report high rates (>95%) of technical success, with perioperative morbidity occurring in 0–10%. Raymond class I or II occlusion also occurred in >90% of cases. These data suggest that the waffle-cone technique may be an acceptable alternative to Y-stenting for wide-necked, bifurcation aneurysms.
More recently, the pCONus bifurcation aneurysm implant (Phenox, Bochum, Germany) was designed for use in a waffle-cone-like fashion for bifurcation aneurysms. Early experience with this device has demonstrated acceptable clinical and radiographic outcomes, with perioperative morbidity ranging from 5% to 12% and Raymond class I or II occlusion occurring in 75–82% [37,38,39,40].
Stent-Assisted Coiling in the Acute Rupture Period
Stent-assisted coiling in the setting of acute subarachnoid hemorrhage is highly controversial, largely due to the necessity for antiplatelet therapy. This can be very problematic in patients with recent bleeds, particularly those with ventriculostomies or at high risk for one. Therefore, balloon-assisted coiling is almost certainly preferable in the setting of subarachnoid hemorrhage for the practitioner who is experienced with this technique. However, stent-assisted coiling may be a safe, reasonable option, with several studies demonstrating its efficacy and safety. A comparison of stent-assisted coiling and balloon-assisted coiling for ruptured wide-necked aneurysms demonstrated no statistically significant difference in periprocedural complications (coil protrusion, symptomatic ischemic events, and hemorrhage related to antiplatelet medication) or clinical outcome [41]. Favorable clinical outcome (modified Rankin Scale score 0–2) occurred in 91.8% of patients treated with stent-assisted coiling and in 90.6% of those undergoing balloon-assisted coiling. Adequate aneurysm occlusion (Raymond class 1 or 2) occurred in 84.4% of patients undergoing stent-assisted coiling and in 83.3% of patients undergoing balloon-assisted coiling.
A systematic review of 17 studies including 339 patients similarly demonstrated safety and efficacy of stent-assisted coiling in acutely ruptured cerebral aneurysms [42]. Raymond class 1 or 2 occlusion was achieved in 82% of patients. In 96% of the cases reviewed, dual antiplatelet therapy was administered only after the procedure (i.e., no preoperative loading doses). The rate of thromboembolic complications was 5.6%, while hemorrhagic complications occurred in 8%. Of note, the rate of EVD-related hemorrhagic complications was 10%; however, approximately half of these occurred at one center.
Another endovascular option for neuroendovascular surgeons who are looking to avoid stent placement for wide-necked acutely ruptured aneurysms is to postpone stent placement until at least several days after the ictus. For some patients it may be feasible to partially coil the aneurysm dome in the acute phase, and return for more definitive stent-assisted coiling once a ventriculostomy tube is removed, a shunt placed, or when the concern for hydrocephalus has lessened. However, one recent series suggests that stent-assisted coiling at any time during the acute rupture period may be safe [43]. Thromboembolic events occurred in 5.7% of patients treated between post-bleed days 0 and 3 and in 5.4% of those treated between post-bleed days 4 and 10. Similarly, hemorrhagic complications occurred in 2.9% of patients in the early cohort and in 5.4% of those in the late cohort. Comparable results were reported in a series of 59 patients treated with stent-assisted (with Enterprise) coiling within 48 h of aneurysm rupture [44]. Procedure-related complications in this series occurred in 6.8% of patients.
Conclusion
Stent-assisted coil embolization is undoubtedly a valuable tool for the treatment of cerebral aneurysms. While it is clearly not a technique that should be applied to every aneurysm, the continued development and refinement of intracranial stents have allowed for safe and effective endovascular treatment of an increasing number of aneurysms. It is important to be cognizant that, as is the case with many surgical techniques, one approach does not fit all. Just as some aneurysms may be better suited for balloon-assisted or single-catheter coil embolization, the same stent-assisted technique is not useful across all aneurysms that are deemed to warrant stent assistance. It therefore behooves the treating physician to be familiar with all of these techniques, including single-stent placement with or without jailing of the microcatheter, X- or Y-stenting, waffle-cone stenting, and rescue-stent placement including the placement of overlapping stents. By gaining familiarity with these variations and technical nuances, treatment can be tailored to find the optimal option for each individual aneurysm.
References
Luessenhop AJ, Velasquez AC. Observations on the tolerance of the intracranial arteries to catheterization. J Neurosurg. 1964;21:85–91.
Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125–45.
Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75:8–14.
Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7.
Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–9.
Linzey JR, Griauzde J, Guan Z, Bentley N, Gemmete JJ, Chaudhary N, et al. Stent-assisted coiling of cerebrovascular aneurysms: experience at a large tertiary care center with a focus on predictors of recurrence. J Neurointerv Surg. 2017;9(11):1081–5.
Sadato A, Adachi K, Hayakawa M, Kato Y, Hirose Y. Effects of anatomic characteristics of aneurysms on packing density in endovascular coil embolization: analysis of a single center’s experience. Neurosurg Rev. 2016;39:109–14. discussion 114
Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, et al. Last-recorded p2y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. AJNR Am J Neuroradiol. 2014;35:128–35.
Heller RS, Dandamudi V, Lanfranchi M, Malek AM. Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. J Neurosurg. 2013;119:1603–10.
Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK. Thromboembolic complications with pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure p2y12 reaction unit (pru) value. J Neurointerv Surg. 2015;7:217–21.
Harrison P, Segal H, Blasbery K, Furtado C, Silver L, Rothwell PM. Screening for aspirin responsiveness after transient ischemic attack and stroke: comparison of 2 point-of-care platelet function tests with optical aggregometry. Stroke. 2005;36:1001–5.
Mansour K, Taher AT, Musallam KM, Alam S. Aspirin resistance. Adv Hematol. 2009;2009:937352.
Mallouk N, Labruyere C, Reny JL, Chapelle C, Piot M, Fontana P, et al. Prevalence of poor biological response to clopidogrel: a systematic review. Thromb Haemost. 2012;107:494–506.
White JB, Ken CG, Cloft HJ, Kallmes DF. Coils in a nutshell: a review of coil physical properties. AJNR Am J Neuroradiol. 2008;29:1242–6.
Horowitz M, Levy E, Sauvageau E, Genevro J, Guterman LR, Hanel R, et al. Intra/extra-aneurysmal stent placement for management of complex and wide-necked- bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 2006;58:ONS-258–262; discussion ONS-262.
Levitt MR, Moon K, Albuquerque FC, Mulholland CB, Kalani MY, McDougall CG. Intraprocedural abciximab bolus versus pretreatment oral dual antiplatelet medication for endovascular stenting of unruptured intracranial aneurysms. J Neurointerv Surg. 2016;8:909–12.
Feng M, Wen W, Feng Z, Fang Y, Liu J, Huang Q. Endovascular embolization of intracranial aneurysms: to use stent(s) or not? Systematic review and meta-analysis. World Neurosurg. 2016;93:271–8.
Phan K, Huo YR, Jia F, Phan S, Rao PJ, Mobbs RJ, Mortimer AM. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracanial aneurysms. J Clin Neurosci. 2016;31:15–22.
Lopes DK, Johnson AK, Kellogg RG, Heiferman DM, Keigher KM. Long-term radiographic results of stent-assisted embolization of cerebral aneurysms. Neurosurgery. 2014;74:286–91.
Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012;33:159–63.
King B, Vaziri S, Singla A, Fargen KM, Mocco J. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with enterprise and neuroform stents: a comparative analysis of the literature. J Neurointerv Surg. 2015;7:905–9.
Zhang X, Zhong J, Gao H, Xu F, Bambakidis NC. Endovascular treatment of intracranial aneurysms with the lvis device: a systematic review. J Neurointerv Surg. 2017;9(6):553–7.
Russell SM, Lin K, Hahn SA, Jafar JJ. Smaller cerebral aneurysms producing more extensive subarachnoid hemorrhage following rupture: a radiological investigation and discussion of theoretical determinants. J Neurosurg. 2003;99:248–53.
Fang CLM, Zhang PL, Wang W, Tan HQ, Xu HW, Zhou B. Endovascular treatment for very small supraclinoid aneurysms with stent-assisted coiling. Long-term follow-up in six cases. Interv Neuroradiol. 2009;15:37–44.
Li CH, Su XH, Zhang B, Han YF, Zhang EW, Yang L, et al. The stent-assisted coil-jailing technique facilitates efficient embolization of tiny cerebral aneurysms. Korean J Radiol. 2014;15:850–7.
Zhao R, Shen J, Huang QH, Nie JH, Xu Y, Hong B, et al. Endovascular treatment of ruptured tiny, wide-necked posterior communicating artery aneurysms using a modified stent-assisted coiling technique. J Clin Neurosci. 2013;20:1377–81.
Fargen KM, Mocco J, Neal D, Dewan MC, Reavey-Cantwell J, Woo HH, et al. A multicenter study of stent-assisted coiling of cerebral aneurysms with a y configuration. Neurosurgery. 2013;73:466–72.
Jeon P, Kim BM, Kim DJ, Kim DI, Park KY. Y-configuration double-stent-assisted coiling using two closed-cell stents for wide-neck basilar tip aneurysms. Acta Neurochir (Wien). 2014;156:1677–86.
Ko JK, Han IH, Cho WH, Choi BK, Cha SH, Choi CH, et al. Crossing y-stent technique with dual open-cell stents for coiling of wide-necked bifurcation aneurysms. Clin Neurol Neurosurg. 2015;132:54–60.
Saatci I, Geyik S, Yavuz K, Cekirge S. X-configured stent-assisted coiling in the endovascular treatment of complex anterior communicating artery aneurysms: a novel reconstructive technique. AJNR Am J Neuroradiol. 2011;32:E113–7.
Cohen JE, Melamed I, Itshayek E. X-microstenting and transmesh coiling in the management of wide-necked tent-like anterior communicating artery aneurysms. J Clin Neurosci. 2014;21:664–7.
Bartolini B, Blanc R, Pistocchi S, Redjem H, Piotin M. “Y” and “x” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol. 2014;35:2153–8.
Lee SM, Kim YJ, Ho KJ. The effectiveness of the waffle-cone technique in treating complex intracranial aneurysms. Interv Neuroradiol. 2015;21:470–8.
Padalino DJ, Singla A, Jacobsen W, Deshaies EM. Enterprise stent for waffle-cone stent-assisted coil embolization of large wide-necked arterial bifurcation aneurysms. Surg Neurol Int. 2013;4:9.
Liu W, Kung DK, Policeni B, Rossen JD, Jabbour PM, Hasan DM. Stent-assisted coil embolization of complex wide-necked bifurcation cerebral aneurysms using the “waffle cone” technique. A review of ten consecutive cases. Interv Neuroradiol. 2012;18:20–8.
Xu F, Qin X, Tian Y, Gu Y, Leng B, Song D. Endovascular treatment of complex intracranial aneurysms using intra/extra-aneurysmal stent. Acta Neurochir. 2011;153:923–30.
Lubicz B, Morais R, Alghamdi F, Mine B, Collignon L, Eker OF. The pconus device for the endovascular treatment of wide neck bifurcation aneurysms. J Neurointerv Surg. 2016;8:940–4.
Perez MA, Bhogal P, Moreno RM, Wendl C, Bazner H, Ganslandt O, et al. Use of the pCONus as an adjunct to coil embolization of acutely ruptured aneurysms. J Neurointerv Surg. 2017;9(1):39–44.
Ulfert C, Pfaff J, Schonenberger S, Bosel J, Herweh C, Pham M, et al. The pCONus device in treatment of wide-necked aneurysms: technical and midterm clinical and angiographic results. Clin Neuroradiol. 2018;28(1):47–54.
Fischer S, Weber A, Titschert A, Brenke C, Kowoll A, Weber W. Single-center experience in the endovascular treatment of wide-necked intracranial aneurysms with a bridging intra−/extra-aneurysm implant (pconus). J Neurointerv Surg. 2016;8:1186–91.
Cai K, Zhang Y, Shen L, Ni Y, Ji Q. Comparison of stent-assisted coiling and balloon-assisted coiling in the treatment of ruptured wide-necked intracranial aneurysms in the acute period. World Neurosurg. 2016;96:316–21.
Bodily KD, Cloft HJ, Lanzino G, Fiorella DJ, White PM, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol. 2011;32:1232–6.
Qian Z, Feng X, Kang H, Wen X, Xu W, Zhao F, et al. Ruptured wide-necked aneurysms: is stent-assisted coiling during post-hemorrhage days 4–10 safe and efficient? World Neurosurg. 2017;101:137–43.
Liu A, Peng T, Qian Z, Li Y, Jiang C, Wu Z, et al. Enterprise stent-assisted coiling for wide-necked intracranial aneurysms during ultra-early (48hours) subarachnoid hemorrhage: a single-center experience in 59 consecutive patients. J Neuroradiol. 2015;42:298–303.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Munich, S.A., Lopes, D.K., Crowley, R.W. (2019). Stent-Assisted Coil Embolization. In: Spiotta, A., Turner, R., Chaudry, M., Turk, A. (eds) Management of Cerebrovascular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-99016-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-99016-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99015-6
Online ISBN: 978-3-319-99016-3
eBook Packages: MedicineMedicine (R0)