Abstract
Recently, it has been proposed, that nanodevices can be injected in the human body and perform non-invasive medical diagnostics. However, due to their small size, a plethora of such nanodevices need to be used in practical applications, thus, creating a nanonetwork, which collects the information and communicates with out-of-body nodes. For these networks, several models have been proposed for the THz channel in the in-vivo scenario. Most of them are based on well-defined theories and physical laws, while some of them are based on experiments. In this paper, we review the state-of-the-art of channel modeling of in-vivo communications in the THz band and discuss future trends and research challenges.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
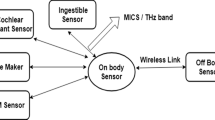
In recent years, advancements in nanotechnology allowed to envision the use of nanomachines in everyday human life. Nanodevices can be utilised for a plethora of different applications, ranging from flexible electronics to medical technologies [1]. Coordinating and information sharing among them can lead to the creation of nanonetworks that cover different areas simultaneously and can perform several tasks in a non-invasive way [2]. As a result, nanosensing has emerged as a very promising field. Nanosensors are not just tiny sensors, but complete nanomachines that can identify and measure events in the nanoscale. Especially for medical applications, it provides a novel way of diagnosis, since the nanosensors can –for example– detect chemical compounds in concentrations as low as one part per billion [3]. However, in order to achieve such sensing accuracy, the range of sensing must be limited to the nano-environment –just a few micrometers in most cases– of the nanomachine. Also, the need of a mechanism to transmit the collected information outside the body is a challenging task of the nanonetworks scientific field.
As mentioned above, nanonetworks can cover an area of interest in the human body and perform nanosensing to detect irregularities in the tissues or even perform standard medical exams, in a non-invasive way. It must be noted, that nanonetworks can also be used as drug delivery systems. With the use, recently, of nanomaterials, such as graphene and its derivatives, communication in a nanonetwork can be made feasible. It has been found that the operational frequency of nanoantennas, constructed from such materials, belongs in the band of THz (0.1 THz–10 THz), otherwise known as the terahertz gap. Note that THz radiation is non-ionizing and, as such, it is thought to be safe for the human body.
In this paper, we review the state-of-the-art of channel modeling of in-vivo communications in the THz band and discuss future trends and research challenges.
2 Noise Models
Noise in THz systems can originate from multiple sources. The main contribution comes from the molecular absorption noise, which is generated during the transmission process. Other sources include the noise created by surrounding nanomachines or the same device, termed as background noise. However, while there is no accurate noise model for graphene-based electronic devices, the electronic noise temperature is considered to be very low [4]. As such, the research mostly focuses on the molecular absorption noise.
2.1 Background Noise
Kokkoniemi et al. [5] described the background noise with the help of the sky noise model. Sky noise is created by the temperature of the absorbing medium, causing the medium to be an effective black body radiator and, as such, it is independent of the transmitted signals. Sky noise is producing a noise temperature \(T_{mol}\), which can be evaluated as
where \(T_0\) is the reference temperature and a(f) is the absorption coefficient of the medium in terms of frequency f.
In the past, this model was used to describe the atmosphere as a medium for transmission [4]. In [6] it was proposed that this model is not sufficient to describe the body radiation noise, since it is not reliable to use the molecular temperature as a parameter in order to evaluate the radiation from the absorbing medium in human tissues. Kokkoniemi et al. [5] and Zhang et al. [6] used Planck’s law to evaluate the background radiation noise as
where \(k_B\) is the Boltzmann and h is the Planck constants, correspondingly. Planck’s function is multiplied with \(\pi \) to transform the unit from W/Hz/cm\({}^2\)/sr to W/Hz/cm\({}^2\).
Assuming that the human tissue can be approximated as an isothermal and homogeneous layer with thickness r and that this radiation is created only from the original energy state of the molecules (black body radiation) the noise power can be evaluated as
Taking into account the antenna aperture term, due to the isotropic radiation at the receiver, the power spectral density (psd) of the body radiation noise with the unit W/Hz is given by
2.2 Molecular Absorption Noise
This subsection is devoted to the molecular absorption noise, which contributes the most in the noise of the THz channel. What makes this noise different than usual is that it is self-induced, which means that it is induced by the transmissions of the users sharing the medium. A part of the radiation that is transmitted is absorbed by the molecules of the medium and becomes kinetic energy, i.e. heat. The other part is re-emitted back in random directions and, as such, it is considered as noise to the receiver. In the pioneering work of Jornet et al. [3], they derived a model for the psd of the molecular absorption noise for the atmosphere. The parameter that describes this phenomenon is the emissivity of the channel, \(\epsilon \), which is defined as
where f is the frequency of the electromagnetic propagating wave, r stands for the total path length and \(\tau \) is the transmissivity of the medium given by the Beer-Lambert Law
with \(P_i\) and \(P_0\) being the incident and radiated power, respectively, and a(f) is the absorption coefficient of the medium. Then, the equivalent noise temperature due to molecular absorption can be written as
where \(T_0\) is the reference temperature and \(\epsilon \) is given by (5). For a given bandwidth, B, the molecular absorption noise power at the receiver can be evaluated as
This model can be adjusted to fit a different medium. As such, Yang et al. [1] and Piro et al. [7] proposed the use of the same model for in-vivo communications. The only difference is that they use the extinction coefficient, \(\kappa \), instead of the absorption coefficient. The noise power spectral density is, then, given by
and the absorption coefficient is derived from the extinction coefficient by using the following formula
Kokkoniemi et al. [5] considered that, since it is a self-induced noise, the source term (Planck’s function) in the sky noise model should be replaced by an appropriate transmit energy function. Furthermore, they noticed that the molecular absorption noise energy at point r depends on the derivative of the complement of the transmittance. Then, accounting for the spreading loss, \(1/(4\pi r^2)\), as well, as the transmit signal psd, \(S_{Tx}(f)\), they get the existing molecular absorption noise model for the receiver at distance r from the transmitter as
Based on [5], Zhang et al. [6] proposed the following formula
where \(S_{Tx}(f)\) is the transmitted signal psd and the term \((c/(4\pi nfr))^2\) accounts for the spreading loss and the antenna aperture.
It is worth mentioning here, that this formula was derived with the assumption that all the absorbed energy from the transmitted signal received at the receiver would turn into molecular absorption noise psd at point r.
2.3 Total Noise
In the previous subsections, we have presented the main noise sources for the THz in-vivo channel. The total channel noise psd for the in-vivo nanonetwork can, then, be derived as the sum of the two
It was shown [4], that the molecular absorption noise tends to be significantly higher than the background radiation noise. As such, in many cases it is considered the dominant noise source in THz in-vivo nanonetworks, Fig. 1.
It is worth noting here that these results are based on graphene-based receivers, which have yet to be developed. The available nanodevices today, are not based on these new materials and thus, the Johnson-Nyquist thermal noise should be taken into account. In Sect. 4, we discuss in more detail the use of new materials and the future challenges on this field.
3 Path Loss Models
As discussed in the previous section, in THz nanonetworks the molecular absorption of the medium deteriorates the propagation of the EM waves. Also, path loss is present, due to the spreading of the signal [8]. Most of the research focuses on spread and absorption losses for the in-vivo THz channel.
3.1 Spreading Loss
Spreading loss is a well known power attenuation contributor in wireless communications. The proposed model, even for in-vivo THz communications, assumes an isotropic radiation and as such, the spreading loss can be defined in dB as
where r is the propagation distance of the wave and \(\lambda _g\) is the wavelength in the medium.
3.2 Absorption Loss
We have pointed out in Sect. 2, that part of the transmitted energy is absorbed by the medium. Details on the absorption coefficient, a(f), of human tissues was provided in [1] and is frequency dependent. The main issue with the molecular absorption is that there are not any analytic functions of the absorption coefficient and, the numerical data are limited to frequencies in the range of 1 THz or frequencies closer to the optical spectrum.
The behavior of the absorption of each tissue varies with the kind of tissue; more specifically, it seems to depend on the percentage of water, since water molecules create peaks in the absorption-frequency relation, due to resonance phenomena. The transmittance of the medium, \(\tau \), can be evaluated using the Beer-Lambert Law, as in (6), which is the fraction of incident EM radiation at a given frequency, that is able to pass through the medium [3]. Obviously, since we need the fraction of the radiation that is absorbed by the medium we need the inverse of \(\tau \).
or in dB
3.3 Total Path Loss
In order to evaluate the total path loss, it is evident that both the spreading path loss and the molecular absorption path loss must be taken into account.
Recently, some research was devoted to obtain alternative, simpler and more accurate formulas, by using experimental data. More specifically, Javed et al. [9] proposed an approximation to the analytical path loss model, named SimpleNano, that is described by the following equation,
where \(K_0\) is the path-loss at a reference distance \(r_0\), which is chosen to be 0.01 m, n is the path loss exponent and \(X_\sigma \) is a log-normal random variable that takes into account the shadowing. In another work of Abbasi et al. [10], they used an artificially synthesized collagen layer from QMUL, which was used to model the human skin layer, so that they could perform actual measurements for the path loss in THz frequencies. After intensive experiments they proposed the model
where the regression technique is used to find the functions A(N), B(N), C(N), which represent the constant offset and the coefficients for distance and frequency respectively, as a function of the number of sweat ducts. The reason that sweat ducts play such an important role is because they are approximately consisted of \(99\%\) water and as we mentioned above, water molecules appear to be highly absorbent in THz frequencies. So, variations in the number of sweat ducts can drastically change the channel.
In Fig. 2 we compare the above models through (17) and (20). The equation we used is [10, Eq. 16]
where r is expressed in mm, and f is expressed in THz. The two models do not match, although in both cases, path loss seems to increase with the frequency. We can justify the difference in the fact that in [10] a synthesized skin model was used out of collagen. However, by tuning the parameters of (20), it is possible to get a variety of results that accommodate the difference between the models.
4 Research Challenges
It is evident from the previous sections, that there is space for research both for noise and path loss models. Some research challenges are as follow:
-
In-vivo electromagnetic propagation is a critical topic in nanonetworks. Reflection, diffraction and scattering need to be modeled properly for highly dispersive mediums, such as the blood.
In addition to that, the channel characteristics changes from person to person, depending on health conditions. A complete model needs to capture that behavior and be robust enough to adjust to all scenarios, regarding the human body condition.
-
The biggest research challenge is the development of nanodevices, based on new materials. The development of graphene-based nanodevices will provide the opportunity to validate the theoretical models. Furthermore, it important to note here that these nanomachines allow only low complexity coding schemes, due to their limited processing capacity.
-
Medical research challenges are also present. The THz band is a part of the EM spectrum, between far infrared light and microwaves, and, as such, it is a non-ionizing radiation that is considered safe for the human body. Despite that, there is still a need to investigate the way that THz radiation interacts with human cells.
-
Finally, the effects of the use of carbon in these nanodevices, that are injected into the human body, specifically graphene, need to be investigated.
References
Yang, K., Pellegrini, A., Munoz, M.O., Brizzi, A., Alomainy, A., Hao, Y.: Numerical analysis and characterization of Thz propagation channel for body-centric nano-communications. IEEE Trans. Terahertz Sci. Technol. 5, 419–426 (2015)
Akyildiz, I.F., Jornet, J.M.: The Internet of nano-things. IEEE Wirel. Commun. 17, 58–63 (2010)
Jornet, J.M., Akyildiz, I.F.: Channel modeling and capacity analysis for electromagnetic wireless nanonetworks in the terahertz band. IEEE Trans. Wirel. Commun. 10, 3211–3221 (2011)
Jornet, J.M.: Fundamentals of electromagnetic nanonetworks in the terahertz band. Ph.D. thesis, School of Electrical and Computer Engineering, Georgia Institute of Technology (2013)
Kokkoniemi, J., Lehtomäki, J., Juntti, M.: A discussion on molecular absorption noise in the terahertz band. Nano Commun. Netw. 8, 35–45 (2016)
Zhang, R., Yang, K., Alomainy, A., Abbasi, Q.H., Qaraqe, K., Shubair, R.M.: Modelling of the terahertz communication channel for in-vivo nano-networks in the presence of noise. In: 2016 16th Mediterranean Microwave Symposium (MMS), pp. 1–4. IEEE (2016)
Piro, G., Yang, K., Boggia, G., Chopra, N., Grieco, L.A., Alomainy, A.: Terahertz communications in human tissues at the nanoscale for healthcare applications. IEEE Trans. Nanotechnol. 14, 404–406 (2015)
Zogas, D.A., Karagiannidis, G.K.: Infinite-series representations associated with the bivariate rician distribution and their applications. IEEE Trans. Commun. 53, 1790–1794 (2005)
Javed, I.T., Naqvi, I.H.: Frequency band selection and channel modeling for WNSN applications using simplenano. In: Proceedings of IEEE International Conference on Communications, ICC 2013, Budapest, Hungary, 9–13 June 2013, pp. 5732–5736. IEEE (2013)
Abbasi, Q.H., El Sallabi, H., Chopra, N., Yang, K., Qaraqe, K.A., Alomainy, A.: Terahertz channel characterization inside the human skin for nano-scale body-centric networks. IEEE Trans. Terahertz Sci. Technol. 6, 427–434 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 ICST Institute for Computer Sciences, Social Informatics and Telecommunications Engineering
About this paper
Cite this paper
Papanikolaou, V.K., Karagiannidis, G.K. (2018). Channel Modeling of In-Vivo THz Nanonetworks: State-of-the-Art and Research Challenges. In: Perego, P., Rahmani, A., TaheriNejad, N. (eds) Wireless Mobile Communication and Healthcare. MobiHealth 2017. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering, vol 247. Springer, Cham. https://doi.org/10.1007/978-3-319-98551-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-98551-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98550-3
Online ISBN: 978-3-319-98551-0
eBook Packages: Computer ScienceComputer Science (R0)