Abstract
Information derived from polysomnography (PSG) provides essential elements for the diagnosis of multiple sleep disorders and therefore is performed commonly in the clinical practice of pediatric sleep medicine. In this chapter, we review the basics of PSG setup, scoring of sleep and its events, standard indications for testing, and interpretation of results. In addition, we provide a more focused discussion for selected neurodevelopmental disorders, as well as practical advice on preparing patients for the night of their sleep study. Beyond standard overnight PSG, we consider the role of specialized testing such as the multiple sleep latency test, maintenance of wakefulness test, and extended PSG in the clinical evaluation of patients. Throughout, areas of uncertainty and possible future directions are highlighted, such as alternative measures of work of breathing and sleep disturbance. Sleep testing remains in flux and an exciting arena for developing improved tools and metrics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Polysomnogram
- Sleep architecture
- Work of breathing
- REM sleep
- Non-REM sleep
- PSG montage
- Infant sleep
- Limb movements
- MSLT
- Extended sleep monitoring
Roberto, a 13-year-old child with a history of epilepsy, underwent a diagnostic polysomnogram (PSG) because of abnormal respirations observed during sleep by the parents. In addition, he had a history of difficulty awakening in the morning and was falling asleep in school during the day. His PSG demonstrated numerous respiratory events, with a representative event depicted in Fig. 3.1. His overall obstructive apnea-hypopnea index was 12 events per hour. Working with the child’s neurologist, following the sleep study, the patient’s vagus nerve stimulator settings were adjusted by lowering the frequency and increasing the cycle time. These adjustments decreased the frequency of respiratory events and improved his clinical symptoms.
Introduction
The polysomnogram , which is often referred to as an overnight sleep study, represents one diagnostic tool available to the sleep clinician and serves an important purpose in the clinical evaluation of many patients evaluated in the sleep clinic. However, like any diagnostic test, its results need to be interpreted with the particular patient and clinical context in mind. The case presented above is a good example of this. Although this patient does have frequent respiratory events such that he could have been diagnosed with obstructive sleep apnea (OSA), the knowledge that the patient has a seizure disorder clues the practitioner into the fact that the etiology of these respiratory events is primarily the patient’s vagus nerve stimulator. This clinical context aids in interpretation of the PSG and therefore subsequent clinical management of the patient.
In this chapter, we will review some basics of polysomnography, including selected technical aspects, scoring of important parameters, and interpretation of derived metrics in a clinical context. We will also discuss preparing a child to undergo a sleep study, which is of special importance for children with neurodevelopmental disorders. The current guidelines for indications for performing a PSG will be reviewed, and we will discuss other modes of sleep diagnostic testing, including multiple sleep latency test (MSLT). Finally, we will discuss selected sleep metrics and modalities that are currently experimental but hold some promise for future clinical application.
Indications for Polysomnography
Standard Practice Parameters
Practice parameters for indications for PSG in children, both respiratory and non-respiratory, were recently published by the American Academy of Sleep Medicine (AASM) [1, 2]. These practice parameters were developed by the AASM Standards of Practice Committee, who combined the best scientific evidence available at the time with expert opinion within a framework of an evidence grading system. Indications are outlined in Tables 3.1 and 3.2. For reference, standard recommendations are felt to be based on a high level of evidence, guideline recommendations have a moderate evidence base, and option recommendations reflect areas of uncertainty with conflicting evidence or expert opinion. While Tables 3.1 and 3.2 outline the standard indications for the use of PSG in children, a more focused discussion on the use of PSG in selected special populations follows below.
Down Syndrome
Obstructive sleep apnea is substantially more prevalent in children with Down syndrome as compared to the general population, with estimates in the 50% range [3]. This increased prevalence is likely related to several factors associated with Down syndrome, including craniofacial features, macroglossia, hypotonia, and obesity. Sleep apnea in this population may result in worsening neurocognitive impairment as well as cardiovascular disease.
Due to its high prevalence and potential associated morbidity, sleep apnea in children with Down syndrome has received special attention. Guidelines published by the American Academy of Pediatrics (AAP) recommend that starting at 6 months of life, providers should discuss symptoms of OSA with parents and that all children with Down syndrome should undergo PSG to evaluate for OSA by age 4 years [4]. Formal assessment for OSA with PSG is important especially in this population given the poor correlation between reported symptoms and sleep study abnormalities [5]. A recent study suggests that PSG should perhaps be considered earlier than 4 years, especially in children with Down syndrome living at altitudes >1500 m [6].
Furthermore, while its lower cost and decreased need for manpower are attractive, overnight nocturnal oximetry as a screening tool has not been shown to be sufficiently sensitive to detect OSA in children, either healthy or with Down syndrome, compared to formal PSG [7, 8]. Finally, PSG assessment for residual OSA in children with Down syndrome following adenotonsillectomy or other sleep surgery is important given the observed lower rate of surgical cure compared to typically developing children [9, 10].
Prader-Willi Syndrome
Children with Prader-Willi syndrome (PWS), which has a prevalence of approximately 1 in 10,000 to 1 in 25,000 live births, have a high prevalence of OSA, estimated to be about 80% [11,12,13,14]. This increased risk for OSA is likely related to several features of the disorder including craniofacial abnormalities, hypotonia, and increased prevalence of obesity. Similar to children with Down syndrome, children with Prader-Willi syndrome also have a lower cure rate with adenotonsillectomy for OSA [11], so a postoperative PSG to evaluate for residual disease should be considered. Drug-induced sleep endoscopy may play a role in identifying additional surgical sites of obstruction, such as the tongue base [15].
Of special significance is the role for PSG prior to initiation of growth hormone therapy in this population. The impetus for this testing prior to initiation of therapy came about in the early 2000s when several fatalities were noted worldwide that appeared to coincide with the use of growth hormone treatment in children with Prader-Willi syndrome [16, 17]. Therefore, although it is unknown what exact role growth hormone may have played in those deaths, one hypothesis is that growth hormone may have caused or worsened existing OSA by stimulating adenotonsillar hypertrophy; therefore, current guidelines recommend evaluation with PSG prior to, as well as 6–10 weeks after, initiation of growth hormone therapy, regardless of age [18]. In children who are found to have OSA on their study, treatment of OSA should be initiated, and the provider should consider delaying or stopping growth hormone therapy until adequate control is achieved [18, 19]. Besides growth hormone initiation, increasing body mass index (BMI) percentile also confers additional risk of OSA among children with PWS [11].
Achondroplasia
Achondroplasia is a disorder related to a mutation in fibroblast growth factor receptor 3 that results in short-limb dwarfism and affects more than 250,000 individuals worldwide [20]. Children with achondroplasia are at risk for several different sleep-related breathing disorders (SRBD): craniofacial changes including midface hypoplasia may predispose to OSA; a restrictive lung physiology may lead to hypoventilation and nocturnal hypoxemia without sleep apnea; and increased risk for compression of the medulla from foramen magnum stenosis may lead to central sleep apnea [21]. In fact, compression of the foramen magnum resulting in central apneas is postulated to contribute to the 2–5% rate of unexpected infant deaths in this population [22]. Current AAP guidelines recommend that infants who are not diagnosed in the newborn period should undergo PSG at the time of diagnosis [23].
Epilepsy
Epilepsy occurs in approximately 1% of the population and has recently been explored in relation to OSA and children. The prevalence of sleep-disordered breathing, estimated with a questionnaire, was found to be higher in children with epilepsy compared to those without [24] and even higher in the subset of children with severe epilepsy compared to those with mild epilepsy [25]. Subsequent research has revealed that poorly controlled epilepsy is a risk factor for OSA and that more severe sleep apnea is associated with use of multiple antiepileptic drugs [26]. It has been hypothesized that the increased prevalence of OSA in children with epilepsy may be related to altered neuronal control of breathing resulting in respiratory events, or, conversely, OSA may exacerbate pre-existing epilepsy by causing increased sleep fragmentation and time in stages N1 and N2 when seizures most commonly occur [26]. Importantly, treatment of OSA in children with epilepsy has been found to be associated with decreased seizure frequency [27]. Taken together, the above evidence suggests that clinicians should strongly consider assessing for OSA with PSG in children with poorly controlled epilepsy.
Chiari 1 Malformation
Chiari malformations, abnormalities affecting the anatomy of the brainstem, come in several different forms. People with Chiari type 1 malformations are typically asymptomatic until adulthood, at which time they most commonly develop headaches [28]. That said, Chiari 1 malformations can cause headaches, neck pain, behavioral difficulties, and cognitive impairment in children [28]. Other symptoms may include abnormal eye movements, hoarse voice, stridor, and cyanotic breath holding spells. Children with Chiari 1 malformations are at increased risk for both obstructive and central respiratory events, with the hypothesized mechanisms being compression of cranial nerves innervating pharyngeal/laryngeal muscles and compression of the medullary respiratory centers, respectively [28]. Among children with Chiari 1 malformation, having a SRBD may be an indication for decompression [28]. That said, at this time there are no guidelines for when children with Chiari 1 malformation should undergo PSG. Because studies have found that relying on symptoms alone to detect SRBD among patients with Chiari 1 malformation will miss a substantial portion of those with a sleep related breathing disorder (SRBD), providers caring for these patients should consider routine evaluation with PSG [28]. While symptoms of Chiari 1 malformation in children may include headaches, neck pain, hypersomnia, and developmental delay, this condition may also be asymptomatic.
Preparing Children for Polysomnography
Once it had been determined that a PSG is indicated for a patient, it is important to prepare for a successful study. Simply ordering a PSG does not guarantee clinically useful study results. While technical limitations, such as unreliable equipment or poor electrode placement, can contribute to diminished PSG results, common reasons that pediatric PSG studies “fail” also include insufficient hours of sleep due to the patient being too upset to sleep and/or intolerance of essential data collection equipment. Failed PSG studies result in increased family frustration and a significant revenue reduction. Therefore, preparation for a successful study is well worth pre-PSG resource allocation.
The first step to prepare for a successful PSG is to assess the likelihood that the child will cooperate with the study procedures and adequately tolerate the study’s physical sensations. This assessment utilizes clinical judgment based on communication with the child’s caregivers, history gathering, and direct observation. After hearing an adequate description of the PSG, the caregiver may indicate that the child will probably struggle with the study. Clinical history review may reveal that the child has not done well with previous medical procedures or has poor adherence with past medical interventions. Perhaps the child is observed reacting negatively to descriptions of the PSG or not responding appropriately to typical expectations and limits in the medical setting. General groups of children who often benefit from PSG preparation include children with the following conditions: mental health concerns (autism spectrum disorder, anxiety disorders, attention-deficit/hyperactivity disorder, trauma histories, oppositional and aggressive behaviors), negative experiences with past medical procedures and hospitals, developmental delays, overwhelmed caregivers, and “sensory issues.” These can all be indications that additional preparation for a PSG is warranted.
If it appears that a child may require minimal training for a PSG, a “tour” of the sleep lab can be scheduled to see how the child reacts to the setting and the placement of some of the PSG sensory items. If the child does well, they can be given instructions regarding home practice and a sleep study training kit (described below). If the child responds poorly to the sleep lab tour, or if it is clear from the clinic appointment that more extensive training is likely necessary, they are scheduled for a “desensitization” appointment. One working model for desensitization for a diagnostic PSG is a 1 h clinic visit with two trained staff members followed by one to three follow-up phone calls. A two-staff member model decreases the chaos in the room and increases the probability of a successful appointment by allowing one staff member to focus on managing the child, while the other staff member teaches the caregiver(s) the key training points. These roles can be exchanged as needed throughout the appointment. A second set of trained hands is often advantageous when it is time to place items on the child. It is ideal if one staff member has behavioral expertise (e.g., psychologist, social worker, licensed counselor, child life specialist), while the other has specific medical training with the sleep equipment (e.g., sleep technician, respiratory therapist, sleep-trained nurse, nurse practitioner, physician).
The first task in the desensitization clinic visit is to build rapport with the caregiver(s) and child. The potential benefits of the PSG are discussed to help increase the family’s motivation to prepare for the study. When skepticism is observed, families may be reminded that with consistent practice, virtually all children, even with special needs, have been successfully prepared to complete PSG studies. An overview of what to expect during the PSG study can be provided by showing a short video demonstration of a pediatric sleep study. A number of these videos are available on YouTube, such as this video (under 5 min), “What to Expect from Your Sleep Test at Children’s Colorado” (https://www.youtube.com/watch?v=rrUr4cyEHEc). A color step-by-step photograph book is typically reviewed and given to the family to continue to use at home for cognitive training and normalization. Caregivers are then taught to use some key behavioral interventions (i.e., positive reinforcement (a reward is chosen which can be earned by following directions during the appointment); graduated exposure (PSG sensations are gradually introduced); counter conditioning (PSG sensations are paired with enjoyable distractions such as movies, video games, tactile toys, and music); selective attention/differential positive reinforcement (the child is given attention and praised when exhibiting calm, cooperative behaviors, and negative or oppositional behaviors are ignored); and escape/response prevention (negative vocalizations are ignored, the child’s hands are redirected when attempting to remove the PSG practice sensations, if the items are removed they are quickly replaced, the items are only removed by the caregivers and staff and are only removed when the child is being calm and cooperative).
Oftentimes it is important to also discuss limit management issues with caregivers. By observation and/or by family report, it may become clear that the caregivers have not experienced success in setting and enforcing developmentally appropriate limits with the child. Simply stated, the child “runs the show.” To be successful in preparing for a PSG, this dynamic will need to shift, at least in this one area. It may be necessary to explore what prevents the caregiver from successfully setting and enforcing limits. A “reframe” can be provided by suggesting that good parenting results in children being angry and upset with their caregivers at times. Learning to tolerate not getting their way and having to do things they don’t want to do actually help children be more successful in life. Caregivers may need to be given suggestions on how they can tolerate their emotions when their child is upset with them (e.g., rather than giving in, remain calm in the moment, and then scream into a towel, or debrief with another adult later). Caregivers need to be prepared that their child will likely escalate their resistance to limits, especially when historically they are used to controlling the situation and getting their way. Caregivers should know that “it will get worse before it gets better,” but if they consistently follow through, their child can learn to comply.
During the appointment initially, one provider demonstrates the skills, while the other provider narrates or explains to the caregiver what is being done and what it accomplishes. Eventually the parent is coached to perform the hands-on training so that it can be replicated in the home setting prior to the PSG. The parent’s progress in using the skills is pointed out and praised throughout the meeting. Goals for in-home practice are to gradually work up to the point that all of the sleep study sensors are placed on the child, and the child can fall asleep with the sensors on several nights in a row. The family is given a home practice “kit,” which includes paper tape to imitate the pulse oximeter, thermistor/flow sensor, and PSG “stickers” (leads on the legs, chest, neck, and face), rolls of gauze to serve as the abdominal and chest effort belts, net mask or gauze to wrap the head, nasal cannula, and detailed written practice instructions and tips. After the family’s appointment, the desensitization staff initiates telephone calls to the family every 2–3 weeks to make sure they are progressing on their goals. If needed, another face-to-face meeting can be scheduled to provide greater encouragement and troubleshooting. The staff also helps to ensure that a PSG is scheduled to occur around the time when it is anticipated the child will be prepared for a successful study.
Increasing the likelihood of a successful PSG can better utilize health-care expenditures, increase family satisfaction with services, and, ultimately, optimize treatment efficacy. This topic has also been reviewed elsewhere [29].
Polysomnogram Setup and Measurements
The typical PSG montage utilizes several sensors and measures. A limited electroencephalogram (EEG) montage, electrooculogram (EOG), and submental electromyogram (EMG) are recorded in order to accurately detect and stage sleep. Respiratory inductance plethysmography belts (RIP) are one of several possible techniques to measure chest and abdominal wall motion. Pulse oximeter and end-tidal capnography sensors detect oxygen saturation and expired carbon dioxide levels, respectively. Oral and nasal airflow are measured with thermistor and nasal pressure transducer. Limb muscle activity is recorded with surface EMG leads. Cardiac rhythm is monitored with limited electrocardiogram (ECG).
The EEG setup utilizes a smaller number of electrodes compared to standard EEG recording, which follows the International 10–20 system [30]. In this system, an imaginary line is drawn from the bridge of the nose to the prominence at the base of the occiput from anterior to posterior and then from the preauricular points on the left and right in the lateral dimension. The 10 and 20 numbers refer to fractions of distance of head circumference from those landmarks to the placement of individual EEG electrodes. Individual electrodes are abbreviated either F, C, or O depending on the region of the brain that they cover, corresponding to frontal, central, and occipital regions. A subscript is assigned as well in order to describe the lateral position of the electrode with Z referring to midline, odd numbers referring to the left side, and even numbers referring to the right side. Right and left mastoid electrodes are also applied.
Eye movements and chin tone are monitored. Specific eye movement patterns can be recognized, including blinking, reading eye movements, slow eye movements, and rapid eye movements. Slow eye movements are important for identifying the onset of sleep, and rapid eye movements are important for identifying rapid eye movement (REM) stage sleep. Measuring chin tone is of particular importance as reduction of muscle tone is characteristic of REM sleep. Abnormally maintained or increased chin tone during REM sleep may be seen in REM behavior disorder.
Given that the majority of sleep studies are performed in order to evaluate for SRBD, measurement of airflow and breathing effort is of paramount importance. The use of an oronasal thermistor is recommended for scoring apneic respiratory events [31]. The advantage of thermal sensors is that they can detect both nasal and oral airflow based on temperature change from exhaled air [30]. In contrast, measurement of nasal pressure provides an approximation of nasal airflow and is recommended for scoring of hypopneas (partial decreases in airflow associated with oxygen desaturation or arousal) [31]. Importantly, the nasal pressure signal can underestimate airflow at low flow rates and overestimate at high flow rates [30]. The thermistor is used to score apneas rather than the nasal pressure sensor because in some patients with mouth breathing, a hypopnea might be misclassified as an apnea if the nasal pressure signal is used alone, as any oral airflow might not be captured [30]. One important characteristic to note on the nasal pressure signal is the shape of the waveform; a drop in amplitude of the signal with flattening of the waveform may indicate an obstructive etiology, while a drop in amplitude with preserved waveform shape suggests a reduced inspiratory effort [30]. Finally, determination of respiratory effort by measuring abdominal and chest wall movement is required in order to classify respiratory events properly: obstructive events are characterized by preserved (albeit sometimes altered) attempts at movements, while these are absent in central events. Most commonly, chest and abdominal movements are sensed with respiratory impedance plethysmography. Other modalities include esophageal manometry (gold standard but uncomfortable), respiratory muscle EMG, and piezoelectric sensors.
Measurement of oxygenation and ventilation is essential for characterizing respiration during sleep. During a sleep study, a pulse oximeter is used in order to estimate oxygen saturation. It is important to note that this measurement can be influenced by factors including abnormal hemoglobin and acid base abnormalities. It is also important to be cognizant of your particular pulse oximeter sampling rate, which may be faster and more sensitive to intermittent desaturations than those used on the typical inpatient ward unit; clinically, this may become important when explaining to parents why a sleep study performed on their child has detected frequent oxygen desaturations when continuous overnight oximetry performed as a part of routine care during previous hospitalizations did not reveal the same finding. Hypoventilation is assessed with measurement of carbon dioxide, either estimated from end-tidal carbon dioxide (ETCO2) or transcutaneously (TcpCO2) . Values from both of these tools need to be interpreted with caution. Transcutaneous CO2 sensors need to be calibrated, the temperature of the probes should be sufficient, and values obtained from this device during the study may lag behind actual changes and arterial PCO2 by a short time [31]. Similarly, ETCO2 values may be falsely low in patients with nasal obstruction or in mouth breathers; ensuring that a good end-tidal CO2 waveform is obtained is essential to ensure accurate values [31].
Limb muscle activity is recorded with EMG in order to assess for leg movements and is part of the standard PSG montage. Sensors are typically placed over the right and left anterior tibialis muscles, and, in patients with suspected REM behavior disorder, arm muscle EMG is also monitored [30]. The limb movements and periodic limb movements of sleep derived from these measures are discussed later in this chapter.
A single-channel recording of ECG is useful for characterizing cardiac rhythms and arrhythmias. Normal sinus rhythm is characterized by an upright P wave, R wave, and T wave in lead II [30]. For children 6 years and older, bradycardia during sleep is defined as sustained (>30 s) heart rate less than 40 bpm [31]. Clinically, children who have congenital heart disease and have previously undergone cardiac surgery are at increased risk for heart block. The ECG can sometimes cause an artifact in the EEG channel, which can mimic spikes.
The snore channel is customarily a part of the pediatric PSG montage. Clinically, this can be helpful, as snoring is a sign of upper airway resistance and work of breathing. In addition, the presence or absence of snoring often helps to distinguish central from obstructive respiratory events in ambiguous cases.
Finally, body position is a standard part of PSG. While this is a simple measurement, it is clinically important as many times obstructive respiratory events are more common in the supine position. If this is the case, positional therapy may play a role in the overall management of an individual child.
Information Derived from the Polysomnogram
Sleep Staging and Architecture
Of course, at its most basic level, a sleep study measures sleep. This is readily apparent in clinic, as many times the first question that parents will ask a sleep provider after their child has had a PSG is, “so, how did my child sleep?” Parents are often interested in exactly how much “light sleep,” “deep sleep,” or “dream sleep” the child had the night of the study and have attitudes and beliefs regarding the meaning of those measures for their child’s health and well-being. Therefore, it is imperative that sleep providers have an expert understanding of how these measures are derived, what normal and abnormal is, and what the implications for those findings are for their individual patient.
Sleep staging is achieved through the use of EEG, EOG, and EMG measures. The AASM has published detailed rules for the scoring of sleep and its associated events for infants as well as children [31], and the following discussion is meant to highlight important differences from adult rules and is based on the AASM scoring manual unless otherwise specified.
Unlike in adults and older children, sleep in infants is staged into wakefulness, non-REM, REM, and transitional [32]. Using previous terminology, REM sleep may also be referred to as active sleep, and non-REM sleep may be referred to as quiet sleep [33]. In addition, a major difference in infants compared to older children and adults is that sleep is scored based not only on EEG/EOG/EMG but also on behavioral and respiratory characteristics. Wake is associated with the infant having their eyes open, crying, or feeding; irregular respirations; low-voltage irregular or mixed EEG; rapid eye movements, blinks, or scanning eye movements; and chin tone on EMG. During non-REM sleep, body movements are reduced compared to wake; respirations are regular; the EEG may demonstrate tracé alternant (three or more alternating runs of symmetrical synchronous high-voltage bursts of delta activity and low-amplitude theta activity), high-voltage slow frequency, sleep spindles, or mixed; the eyes are closed; and the chin EMG may be present or low. REM sleep is characterized by having the eyes closed with small body movements, irregular respirations, low-voltage irregular or mixed EEG, rapid eye movements on EOG, and low or transient muscle activity on chin EMG. If there are characteristics of both non-REM and REM sleep stages in a single epoch, it should be scored as transitional sleep. Periodic breathing is common during REM sleep in infants, and the regularity of respirations may be one of the most reliable characteristics differentiating REM and non-REM sleep in infants, with non-REM sleep being characterized by a more regular respiratory pattern. In terms of overall sleep architecture, newborns typically have sleep periods lasting 3–4 h, with awakenings for feeding; they typically begin their sleep going right into REM sleep; and REM sleep comprises approximately 50% of the total sleep time, which begins to decrease at approximately 3 months of age and reaches adult levels by adolescence [30].
After about 2 months of age, sleep may be able to be staged according to the pediatric, rather than infant, rules. This is made possible by the fact that sleep spindles characteristic of stage N2 sleep appear around 2–3 months of age, K complexes (also characteristic of N2) around 4–6 months, and slow wave activity (frequent during stage N3 sleep) by 4–5 months. Because of the variable appearance of these characteristics, which allow one to differentiate stages of non-REM sleep, there will be inter-individual differences in the exact age when N1, N2, and N3 can be scored, but this is usually possible by age 6 months. Examples of sleep stages are presented in Fig. 3.2. A posterior dominant rhythm, which is observed in the occipital region during wakefulness and eyes closed, first appears in infants around 3–4 months of age and is slower in frequency. Hypnagogic hypersynchrony, consisting of bursts of high-amplitude low-frequency (3–4 Hz) sinusoidal waves in the frontal and central regions, usually begins around 3 months of age, disappears by age 12 years, and is associated with stages N1 and N2. Hypnagogic hypersynchrony is important for identifying the onset of sleep in those children who do not generate a posterior dominant rhythm. The scoring of stages N2, N3, and REM sleep is the same for children as they are for adults.
(a) Rapid eye movements , increased chin tone, and posterior dominant rhythm denote wake. (b) Slow eye movements and vertex sharp waves signal the onset of N1 sleep. (c) K complexes are associated with N2 sleep. (d) Delta waves are characteristic of N3 sleep. (e) Rapid eye movements in association with low chin tone identify REM sleep
As noted above, sleep architecture changes with age, so when discussing this with parents, one must have an understanding of what is normal for that child in the context of their development. In infants less than 3 months of age, sleep onset is usually characterized by rapid entry into REM stage sleep, which constitutes approximately 50% of total sleep time, and sleep cycles that last for 45–60 min. Typically starting around 3 months of age, the percentage of REM sleep decreases, children start to enter sleep via non-REM rather than REM sleep, and sleep cycles began to increase in duration. Normative data regarding sleep architecture for children aged 1–18 years have been previously published [34] and are presented in Table 3.3. These ranges for sleep architecture should be viewed as possible typical values for reference rather than strict cut points for normal versus abnormal, and the authors of this chapter strongly urge sleep providers to interpret any one individual patient’s sleep architecture within the clinical context rather than as a stand-alone metric on which diagnoses or treatment decisions are based. In addition, it should be noted that sleep architecture may be different in the sleep laboratory than in the home environment, as a “first night effect” has been observed in non-infant children who are naïve to the laboratory environment, characterized by an increased sleep latency and decreased sleep efficiency on the first night of study [35]. With those caveats in mind, alterations in sleep architecture may provide clues to individual sleep disorders. For example, a shortened REM latency (<15 min) and/or substantial sleep fragmentation would provide supportive evidence for the diagnosis of narcolepsy in the appropriate context.
Breathing
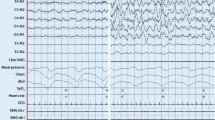
The scoring of respiratory events forms the basis upon which SRBD are diagnosed. The cutoff age for which pediatric, rather than adult, respiratory rules are used is anywhere from 13 to 18 years, at the discretion of the sleep practitioner; note that this distinction is not as critical as it used to be, because hypopneas in adults may now include events associated with arousal rather than only desaturation. Apnea refers to a drop in amplitude of thermistor signal by at least 90% for at least two breaths. If there is continued respiratory effort for the duration of the event, it is scored as obstructive. In contrast, if there is no respiratory effort, it is scored as central; of importance, if the central apnea is not associated with arousal or desaturation in a child, it must be either 20 s in duration or be associated with bradycardia. Hypopnea refers to a decrease in nasal pressure signal by at least 30% associated with desaturation or arousal, as demonstrated in Fig. 3.3. While differentiating obstructive from central hypopneas can be challenging and is optional by AASM standards, it may be of clinical utility, especially in infants and children with neurodevelopmental disorders and at high altitude. A hypopnea is likely of central origin if there is no associated snoring, flattening of the nasal pressure waveform, or paradoxical breathing. Scoring respiratory effort-related arousals (RERAs) is optional; these events represent increasing respiratory effort or flattening of nasal pressure waveform that does not meet criteria for hypopnea but is associated with the consequence of arousal. If >25% of the total sleep time is spent with carbon dioxide as measured by ETCO2 or TcpCO2 above 50 mmHg, then hypoventilation is said to be present. Periodic breathing may be scored if there are three or more episodes of absent airflow and respiratory effort lasting at least 3 s and separated by less than 20 s of normal breathing; note that individual central apneas nested within periodic breathing sequences should also be individually scored.
A decrease of 30% in the nasal pressure signal accompanied by oxygen desaturation defines this respiratory event as a hypopnea. Note that the desaturation occurs following, rather than concomitant with, the decrease in airflow and that snoring identifies this as obstructive rather than central in nature
Once individual respiratory events are scored, they must be combined into summary indices that are clinically useful and carry diagnostic significance. It is useful to combine obstructive and mixed respiratory events into a single obstructive apnea-hypopnea index (OAHI). There exists controversy regarding the appropriate cutoff for OAHI, but in the most recent ICSD-3, an OAHI ≥ 1 event per hour is considered as part of the diagnostic criteria for pediatric OSA. In contrast, values of 1.5 events per hour or greater have been proposed elsewhere [35], and values of 2 events per hour or greater for OAHI were used in the only randomized controlled trial of adenotonsillectomy in children with OSA [36]. It should be noted that many of the original studies establishing normative data for the OAHI used varying definitions of hypopnea and utilized nasal thermistor rather than the now standard nasal pressure transducer (Table 3.4). Some practitioners view an OAHI from 1 to 5 events per hour as a diagnostic gray zone in children, with management directed by the presence or absence of clinical symptoms (e.g., daytime sleepiness or neurobehavioral symptoms) and OSA-associated morbidity (e.g., pulmonary hypertension). Central respiratory events may be combined into a central AHI (CAHI), with a threshold of ≥5 events per hour (plus CAHI > OAHI) as consistent with central sleep apnea. This number is much higher in infants living at high altitude [37]. Furthermore, periodic breathing that composes 5% or more of the total sleep time is considered abnormal and supportive of central sleep apnea of infancy/prematurity (again, altered by altitude). If significant periodic breathing is present in the older child, consider neurologic, medication, and cardiac etiologies. Hypoventilation may be associated with OSA or neuromuscular disease, but one should also be aware of its association with a condition known as congenital central hypoventilation syndrome; worsening respiratory status during NREM sleep, compared to REM sleep, may help clue one into this diagnosis as opposed to other SRBD. The oxygen saturation does not typically decrease below 91% in normal children at sea level, and when there are 5 min or greater of saturations ≤90% during sleep without hypoventilation or OSA, one may diagnose sleep-related hypoxemia.
Limb Movements
The scoring of single-limb movements and combining them to score periodic limb movements of sleep (PLMS) are detailed and covered thoroughly in the AASM scoring manual [31]. Having five or more PLMS per hour is considered abnormal [43] and may be a sign of periodic limb movement disorder, restless leg syndrome, REM behavior disorder, or narcolepsy. Elevated PLMS have also been associated with iron deficiency, OSA, ADHD, migraines, seizures, and autism spectrum disorders in children [44, 45]. The clinical significance of PLMS, with or without arousals, remains controversial, with some studies in children demonstrating no substantial clinical impact at all [46]. Normative data for periodic and single-limb movements and their associations with arousals have also been reported for children aged 1–18 years [47].
Given a previously observed relationship between PLMS and autism as well as PLMS and iron deficiency, many practitioners screen for and treat ferritin levels <50 mcg/L in children with autism spectrum disorders and elevated PLMS. Indeed, in an open-label trial in children with ASD, subjective sleep disturbance and restless sleep improved, and ferritin levels increased with supplemental oral iron therapy [48]. That being said, studies that have examined the relationship between PLMS and iron deficiency within ASD have not found a significant association [45, 49]. Therefore, the relationship between iron status, PLMS, and autism remains to be fully elucidated.
Artifacts
There are several artifacts that clinicians will encounter on a frequent basis while interpreting PSGs, so familiarity with them is imperative to avoid misinterpretation. Popping refers to an electrode pulling away from the skin, usually associated with breathing, and results in high-amplitude deflections [30]. When there is electrical contamination from power lines during the sleep study, this can cause a 60-cycle artifact, characterized by 60 Hz contamination in the channels as well as a “rope-like” artifact in the chin EMG; this can sometimes be alleviated by the use of a 60 Hz notch filter [30]. As discussed above with respect to the ECG, this can sometimes contaminate the EEG channel and mimic spikes [30]. In addition, vagus nerve stimulator activity can distort multiple channels and was illustrated in the introductory case. When patients sweat during a study, this can sometimes result in slow-frequency artifact and can mimic stage N3. Sweat artifact is thought to be due to sweat changing the electrode potential [30] and is more frequently seen during NREM sleep, when sweating is more common. Excellent illustrations of these and other common artifacts are available elsewhere [30].
Experimental Measures of Sleep Disturbance and Respiratory Effort
The current gold standard metric to diagnose and grade the severity of OSA is the obstructive apnea-hypopnea index (OAHI) [50]. While this does provide a standardized measure for classification purposes, it is an imperfect measure. For example, a similar degree of neurocognitive deficits has been observed in children with primary snoring compared to those who meet formal diagnostic criteria for OSA [43, 44]. In addition, adenotonsillectomy in children with primary snoring results in significant improvement in symptoms compared to observation alone [51]. Finally, data from the Childhood Adenotonsillectomy Trial (CHAT), which was the first large randomized controlled trial of adenotonsillectomy for OSA in children, demonstrate minimal, if any, relationship between OSA severity as assessed by the OAHI and either baseline morbidity or response to adenotonsillectomy [52, 53]. As a result, alternative metrics to characterize sleep disturbance and respiratory effort are actively being developed.
Cyclic Alternating Patterns
Cyclic alternating pattern (CAP) is a finding on EEG that is characterized by transient electrocortical events during non-REM sleep and is a measure of sleep microstructure [54]. CAP is thought to reflect the brain’s effort to preserve the normal structure of sleep and is therefore taken as a metric of sleep instability and quality [54]. Altered CAP measures have been associated with selected sleep disorders in children, as reviewed by Parrino and colleagues [55]. Namely, abnormal CAP has been associated with sleepwalking, sleep terrors, and narcolepsy. There have been contradictory results with respect to OSA. In addition, CAP has been shown to be altered in several neurodevelopmental disorders, including fragile X syndrome, Down syndrome, autism spectrum disorders, Prader-Willi syndrome, ADHD, and dyslexia [55]. Therefore, studies to date suggest that the CAP represents a clinically important metric related to sleep disturbance and daytime functioning, but more research into appropriate reference ranges and scoring is needed.
Duty Cycle
Duty cycle refers to the inspiratory time relative to the duration of the respiratory cycle (length of time required to breathe in divided by length of entire respiratory cycle, including both inspiration and expiration) [56]. Experimental manipulations of the inspiratory resistance of upper airways, by abruptly lowering applied nasal pressure, have demonstrated that duty cycle increases substantially compared to unobstructed breathing [57]. It is hypothesized that an increase in duty cycle represents a compensatory response to upper airway obstruction. It has also been demonstrated that children with OSA treated with a high-flow nasal cannula system have decreases in duty cycle [56]. However, a more recent analysis comparing children with and without sleep-disordered breathing did not find any between-group difference in duty cycle [58].
Pulse Transit Time
Pulse transit time (PTT) is calculated as the time between ECG peak and the photoplethysmography waveform measured by the finger pulse oximeter (illustration is available at [59]) and is thought to be related to arterial stiffness, blood pressure, and autonomic state. An acute decrease in PTT is thought to represent an autonomic, or subcortical, arousal. Indeed, changes in PTT have been shown to be correlated with changes in systolic blood pressure during PSG [60]. During acute respiratory events (apneas and hypopneas), the heart rate increases and the PTT decreases, which may represent changes associated with the development of hypertension [61]. Interestingly, PTT may help identify more respiratory events and microarousals compared to standard EEG [62, 63]. The PTT arousal index may be able to distinguish between primary snoring and upper airway resistance syndrome, as assessed with esophageal manometry [63]. Finally, changes in PTT may help differentiate obstructive and central respiratory events (large PTT oscillations with obstructive versus reduced oscillations with central events), which can sometimes be challenging without the use of esophageal manometry [64].
Biomarkers
An alternative approach to identifying individual patients at risk for end-organ disease associated with OSA who should be targeted for therapy involves the use of biomarkers. A vast array of biomarkers has been examined over the last decade, and a recent review and synthesis of the literature have been published [65]. In all, the reviewers found 31 biomarkers that were evaluated in the literature, both blood and urine. One potential biomarker identified was high-sensitivity C-reactive protein (hsCRP), which was found to be elevated in children with neurocognitive deficits associated with either OSA or primary snoring [66]. Other potential biomarkers with positive results in children included IL-6, MRP 8/14, and urinary neurotransmitters [65].
Other Modes of Sleep Testing
Home Sleep Testing
To date, in-lab PSG remains the lone validated and standardized method for diagnosing OSA in children. However, the high cost and limited access to laboratory-based PSG can cause large financial and travel burdens on patients and families as well as delays in diagnosis and treatment. Additionally, environmental differences between the sleep laboratory and home may reduce the applicability of laboratory findings to the home setting. These limitations make home sleep testing (HST) an attractive alternative, and this method has already been adopted and is mainstream in the adult population.
In contrast to the adult literature, there are a limited number of studies examining HST in children. In a 1995 Canadian study, Jacob and colleagues [67] compared in-laboratory PSG to a limited home setup consisting of cardiorespiratory monitor, video, and inductive plethysmography in diagnosing OSA in pediatric subjects with adenotonsillar hypertrophy. The authors observed higher sleep efficiency and fewer environment-related arousals during the home studies and no differences in other measured sleep indices. Similarly, Brockmann and colleagues performed a feasibility study and found that 95% of children who underwent HST had technically acceptable recordings [68]. A more recent feasibility study performed by Marcus and colleagues demonstrated technically satisfactory HST in 91% of cases in school-aged children [69]. This is an area of active development and with recent progress and future directions elucidated by Tan and colleagues [70].
Actigraphy
Actigraphy is covered separately in detail elsewhere in this book. It should be noted, however, that this is still a developing area and may hold potential beyond the simple measurement of total sleep time and circadian phase shifts, such as being able to identify and differentiate disorders of hypersomnia [71].
Multiple Sleep Latency Test
The multiple sleep latency test (MSLT) is a daytime test intended to measure an individual’s propensity for sleep. It typically immediately follows overnight PSG in patients being evaluated for hypersomnia, consists of five nap opportunities, and is validated in children 5 years and older [1]. While the MSLT is considered the gold standard for measuring sleep propensity and is part of the diagnostic criteria for idiopathic hypersomnia and narcolepsy, it is not without substantial limitations. For example, a mean sleep latency of ≤8 min is taken as the cut point for hypersomnia diagnoses. However, in patients with a clear-cut clinical diagnosis of idiopathic hypersomnia but sleep latency >8 min, there are no clinical or PSG differences compared to those with sleep latency less than 8 min; furthermore, a normal MSLT does not predict less severe symptoms or response to treatment [72]. The MSLT has a poor test-retest reliability among patients with narcolepsy without cataplexy and those with idiopathic hypersomnia, with a change in diagnosis in over half of patients on retest [73].
Maintenance of Wakefulness Test
While the MSLT is designed to measure an individual’s propensity to fall asleep, the maintenance of wakefulness test (MWT) is meant to measure one’s ability to stay awake. Specifically, during the MSLT, patients are instructed to “try to fall asleep,” while at the start of each nap opportunity during MWT, patients are instructed to “sit still and remain awake for as long as possible.” Results of the MWT may be clinically useful to assess wakefulness when public safety is a concern or to evaluate response to treatment [74]. There are currently no normative data available for the pediatric population. The use of MSLT and MWT is covered elsewhere in this book in greater detail.
Extended Polysomnography
Due to the significant limitations of MSLT outlined above, the development of extended PSG protocols has been pursued in the research arena. Vernet and Arnulf had patients with idiopathic hypersomnia undergo a habituation night, next day MSLT, and then 24 h spontaneous sleep monitoring; 71% of those with hypersomnia with long sleep time (>10 h total sleep time) had MSLT sleep latency >8 min [75]. In a separate protocol by Fabio Pizza and colleagues, patients underwent 48 h of continuous PSG (first 24 h adaptation) with spontaneous sleep followed by MSLT [76]. Certainly, while these procedures are still in the research phase, they may hold promise to better diagnose and characterize sleep in patients with hypersomnia.
Areas of Uncertainty and Future Directions
In terms of its overall development, sleep testing in its current form is likely in its toddler years. While the AASM has made great strides by formally codifying PSG scoring in its manual [31] and defining the role of derived metrics in the diagnosis of specific sleep disorders in its recent ICSD-3 [50], many areas of uncertainty remain. For instance, the role of preoperative PSG in the context of adenotonsillectomy for OSA in the otherwise generally healthy child remains controversial. While the AAP guidelines recommend routine preoperative PSG for proper diagnosis of OSA when available (leaving open an option for referral to an otolaryngologist or sleep specialist) [50, 77], the American Academy of Otolaryngology-Head and Neck Surgery recommends that preoperative PSG should be reserved for patients with selected conditions or those with discrepancy between tonsil size on examination and reported history [78]. Other important areas of uncertainty have already been discussed above in context and include improved measures of respiratory effort and sleep disturbance, reliability, and validity of the MSLT in hypersomnias, the meaning of the OAHI in terms of measuring the presence of sleep apnea, the use of MWT for driving clearance, and home sleep testing.
Conclusions
Polysomnography is an integral part of pediatric sleep medicine. In this chapter we have reviewed common indications, scoring procedures, and interpretation of results with a focus on selected neurodevelopmental disorders. From a practical standpoint, we also discussed how to prepare children for their sleep study, identified common artifacts, and highlighted areas of uncertainly along the way. To reiterate an important theme, PSG should be viewed similarly to any other diagnostic test, in that the results always need to be interpreted in the clinical context, and testing should only be performed when the results will change patient management.
References
Aurora RN, Lamm CI, Zak RS, Kristo DA, Bista SR, Rowley JA, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35:1467–73.
Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379–88.
Lal C, White DR, Joseph JE, van Bakergem K, LaRosa A. Sleep-disordered breathing in Down syndrome. Chest. 2015;147:570–9.
Bull MJ, Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406.
Ng DK, Chan C-H, Cheung JM. Children with Down syndrome and OSA do not necessarily snore. Arch Dis Child. 2007;92:1047–8.
Jensen KM, Sevick CJ, Seewald LAS, Halbower AC, Davis MM, McCabe ERB, et al. Greater risk of hospitalization in children with Down syndrome and OSA at higher elevation. Chest. 2015;147:1344–51.
Jheeta S, McGowan M, Hadjikoumi I. Is oximetry an effective screening tool for obstructive sleep apnoea in children with Down syndrome? Arch Dis Child. 2013;98:164.
Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–12.
Thottam PJ, Trivedi S, Siegel B, Williams K, Mehta D. Comparative outcomes of severe obstructive sleep apnea in pediatric patients with Trisomy 21. Int J Pediatr Otorhinolaryngol. 2015. https://doi.org/10.1016/j.ijporl.2015.04.015.
Shete MM, Stocks RMS, Sebelik ME, Schoumacher RA. Effects of adeno-tonsillectomy on polysomnography patterns in Down syndrome children with obstructive sleep apnea: a comparative study with children without Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74:241–4.
Sedky K, Bennett DS, Pumariega A. Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med. 2014;10:403–9.
Vandeleur M, Davey MJ, Nixon GM. Are sleep studies helpful in children with Prader-Willi syndrome prior to commencement of growth hormone therapy? J Paediatr Child Health. 2013;49:238–41.
O’Donoghue FJ, Camfferman D, Kennedy JD, Martin AJ, Couper T, Lack LD, et al. Sleep-disordered breathing in Prader-Willi syndrome and its association with neurobehavioral abnormalities. J Pediatr. 2005;147:823–9.
Lin H-Y, Lin S-P, Lin C-C, Tsai L-P, Chen M-R, Chuang C-K, et al. Polysomnographic characteristics in patients with Prader-Willi syndrome. Pediatr Pulmonol. 2007;42:881–7.
Lan M-C, Hsu Y-B, Lan M-Y, Chiu T-J, Huang T-T, Wong S-B, et al. Drug-induced sleep endoscopy in children with Prader-Willi syndrome. Sleep Breath. 2016. https://doi.org/10.1007/s11325-016-1338-8.
Van Vliet G, Deal CL, Crock PA, Robitaille Y, Oligny LL. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr. 2004;144:129–31.
Nagai T, Obata K, Tonoki H, Temma S, Murakami N, Katada Y, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A. 2005;136:45–8.
The Committee on Genetics. Health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127:195–204.
Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS, et al. Growth Hormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072–87.
Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–72.
DelRosso LM, Gonzalez-Toledo E, Hoque R. A three-month-old achondroplastic baby with both obstructive apneas and central apneas. J Clin Sleep Med. 2013;9:287–9.
Pauli RM, Scott CI, Wassman ER Jr, Gilbert EF, Leavitt LA, Ver Hoeve J, et al. Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr. 1984;104:342–8.
Trotter TL, Hall JG, American Academy of Pediatrics Committee on Genetics. Health supervision for children with achondroplasia. Pediatrics. 2005;116:771–83.
Maganti R, Hausman N, Koehn M, Sandok E, Glurich I, Mukesh BN. Excessive daytime sleepiness and sleep complaints among children with epilepsy. Epilepsy Behav. 2006;8:272–7.
Jain SV, Simakajornboon S, Shapiro SM, Morton LD, Leszczyszyn DJ, Simakajornboon N. Obstructive sleep apnea in children with epilepsy: prospective pilot trial. Acta Neurol Scand. 2012;125:e3–6.
Jain SV, Horn PS, Simakajornboon N, Glauser TA. Obstructive sleep apnea and primary snoring in children with epilepsy. J Child Neurol. 2013;28:77–82.
Segal E, Vendrame M, Gregas M, Loddenkemper T, Kothare SV. Effect of treatment of obstructive sleep apnea on seizure outcomes in children with epilepsy. Pediatr Neurol. 2012;46:359–62.
Leu RM. Sleep related breathing disorders and the Chiari 1 malformation. Chest. 2015. https://doi.org/10.1378/chest.14-3090.
Paasch V, Hoosier TM, Accardo J, Ewen JB, Slifer KJ. Technical tips: performing EEGs and polysomnograms on children with neurodevelopmental disabilities. Neurodiagn J. 2012;52:333–48.
Berry RB. Fundamentals of sleep medicine. Philadelphia: Elsevier Saunders; 2012.
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV, for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Darien: American Academy of Sleep Medicine; 2012.
Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J Clin Sleep Med. 2016;12:429–45.
Anders TF, Emde RN, Parmelee AH. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. Los Angeles: UCLA Brain Information Service/BRI Publications Office, NINDS Neurological Information Network; 1971.
Scholle S, Beyer U, Bernhard M, Eichholz S, Erler T, Graness P, et al. Normative values of polysomnographic parameters in childhood and adolescence: quantitative sleep parameters. Sleep Med. 2011;12:542–9.
Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4:393–406.
Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76.
Duenas-Meza E, Bazurto-Zapata MA, Gozal D, González-García M, Durán-Cantolla J, Torres-Duque CA. Overnight polysomnographic characteristics and oxygen saturation of healthy infants, 1 to 18 months of age, born and residing at high altitude (2,640 meters). Chest. 2015;148:120–7.
Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22–30.
Wong TK, Galster P, Lau TS, Lutz JM, Marcus CL. Reliability of scoring arousals in normal children and children with obstructive sleep apnea syndrome. Sleep. 2004;27(6):1139–45.
Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol. 2007;42(4):159–67.
Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168(12):1540.
Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–53.
Marcus CL, Traylor J, Gallagher PR, Brooks LJ, Huang J, Koren D, et al. Prevalence of periodic limb movements during sleep in normal children. Sleep. 2014;37:1349–52.
Bokkala S, Napalinga K, Pinninti N, Carvalho KS, Valencia I, Legido A, et al. Correlates of periodic limb movements of sleep in the pediatric population. Pediatr Neurol. 2008;39:33–9.
Youssef J, Singh K, Huntington N, Becker R, Kothare SV. Relationship of serum ferritin levels to sleep fragmentation and periodic limb movements of sleep on polysomnography in autism spectrum disorders. Pediatr Neurol. 2013;49:274–8.
Chervin RD, Chung S, O’Brien LM, Hoban TF, Garetz SL, Ruzicka DL, et al. Periodic leg movements during sleep in children scheduled for adenotonsillectomy: frequency, persistence, and impact. Sleep Med. 2014;15:1362–9.
Scholle S, Scholle HC. Leg movements and periodic leg movements during sleep in the development across childhood and adolescence from 1 to 18 years. Sleep Med. 2014;15:1068–74.
Dosman CF, Brian JA, Drmic IE, Senthilselvan A, Harford MM, Smith RW, et al. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol. 2007;36:152–8.
Lane R, Kessler R, Buckley AW, Rodriguez A, Farmer C, Thurm A, et al. Evaluation of periodic limb movements in sleep and iron status in children with autism. Pediatr Neurol. 2015;53(4):343–9.
American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien: American Academy of Sleep Medicine; 2014.
Goldstein NA, Pugazhendhi V, Rao SM, Weedon J, Campbell TF, Goldman AC, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics. 2004;114:33–43.
Rosen CL, Wang R, Taylor HG, Marcus CL, Katz ES, Paruthi S, et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome. Pediatrics. 2015;135:e662–71.
Garetz SL, Mitchell RB, Parker PD, Moore RH, Rosen CL, Giordani B, et al. Quality of life and obstructive sleep apnea symptoms after pediatric adenotonsillectomy. Pediatrics. 2015;135:e477–86.
Parrino L, Grassi A, Milioli G. Cyclic alternating pattern in polysomnography: what is it and what does it mean? Curr Opin Pulm Med. 2014;20:533–41.
Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16:27–45.
McGinley B, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics. 2009;124:179–88.
Schneider H, Patil SP, Canisius S, Gladmon EA, Schwartz AR, O’Donnell CP, et al. Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. J Appl Physiol. 2003;95:11–9.
Immanuel SA, Pamula Y, Kohler M, Martin J, Kennedy D, Kabir MM, et al. Respiratory timing and variability during sleep in children with sleep-disordered breathing. J Appl Physiol. 2012;113:1635–42.
Pagani J, Villa MP, Calcagnini G, Alterio A, Ambrosio R, Censi F, et al. Pulse transit time as a measure of inspiratory effort in children. Chest. 2003;124:1487–93.
Vlahandonis A, Biggs SN, Nixon GM, Davey MJ, Walter LM, Horne RSC. Pulse transit time as a surrogate measure of changes in systolic arterial pressure in children during sleep. J Sleep Res. 2014;23:406–13.
Nisbet LC, Yiallourou SR, Nixon GM, Biggs SN, Davey MJ, Trinder J, et al. Characterization of the acute pulse transit time response to obstructive apneas and hypopneas in preschool children with sleep-disordered breathing. Sleep Med. 2013;14:1123–31.
Pépin J-L, Delavie N, Pin I, Deschaux C, Argod J, Bost M, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–30.
Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–8.
Argod J, Pépin JL, Lévy P. Differentiating obstructive and central sleep respiratory events through pulse transit time. Am J Respir Crit Care Med. 1998;158:1778–83.
De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Biomarkers associated with obstructive sleep apnea and morbidities: a scoping review. Sleep Med. 2015;16:347–57.
Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–93.
Jacob SV, Morielli A, Mograss MA, Ducharme FM, Schloss MD, Brouillette RT. Home testing for pediatric obstructive sleep apnea syndrome secondary to adenotonsillar hypertrophy. Pediatr Pulmonol. 1995;20:241–52.
Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013;77:1960–4.
Marcus CL, Traylor J, Biggs SN, Roberts RS, Nixon GM, Narang I, et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10:913–8.
Tan H-L, Kheirandish-Gozal L, Gozal D. Pediatric home sleep apnea testing: slowly getting there! Chest. 2015. https://doi.org/10.1378/chest.15-1365.
Filardi M, Pizza F, Martoni M, Vandi S, Plazzi G, Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 2015;16:126–30.
Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–81.
Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9:789–95.
Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21.
Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–9.
Pizza F, Moghadam KK, Vandi S, Detto S, Poli F, Mignot E, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22:32–40.
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84.
Roland PS, Rosenfeld RM, Brooks LJ, Friedman NR, Jones J, Kim TW, et al. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145:S1–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ingram, D.G., Crane, S.C.M., Halbower, A.C. (2019). Polysomnography. In: Accardo, J. (eds) Sleep in Children with Neurodevelopmental Disabilities. Springer, Cham. https://doi.org/10.1007/978-3-319-98414-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-98414-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-98412-4
Online ISBN: 978-3-319-98414-8
eBook Packages: MedicineMedicine (R0)