Abstract
Liver fibrosis, known as cirrhosis in advanced stages, is a dynamic process in which aberrant extracellular matrix accumulates in the liver parenchyma in response to chronic injury. Recent data has shown that liver fibrosis, even in advanced stages, may regress with the cessation of liver injury. In the past two decades, there has been remarkable progress in the understanding of hepatic fibrosis and the identification of therapeutic targets. Here, we review the epidemiology, etiology, pathophysiology, current therapeutic options, and future research directions for the management of cirrhosis. We discuss the molecular mechanisms involved in hepatic fibrogenesis, the role of liver matrix stiffening in disease diagnosis and pathogenesis, and the association between liver fibrosis and tumorigenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Liver fibrosis
- Nonalcoholic fatty liver disease
- Extracellular matrix
- Matrix stiffness
- Elastography
- Hepatic stellate cells
- Hepatocellular carcinoma
- Liver metastases
Epidemiology and Burden of Disease
Cirrhosis is the 5th leading cause of death in the United States and the 13th leading cause of death worldwide [1, 2], resulting in one million deaths per year worldwide and 33,000 deaths per year in the United States [3]. In addition, an estimated 19,500 deaths per year are attributed to hepatocellular carcinoma, which occurs more frequently among patients with cirrhosis [4]. In the United States, cirrhosis ranks eighth in economic cost burden [2] with annual direct costs estimated at greater than $2 billion and indirect costs exceeding $10 billion [5]. The incidence and prevalence of cirrhosis are difficult to estimate because the majority of patients are asymptomatic during the early stages of disease.
Patients with cirrhosis are classified into two main prognostic stages: compensated or decompensated disease. Patients who develop conditions such as variceal hemorrhage, ascites, or hepatic encephalopathy are classified to have decompensated cirrhosis; those without clinical complications are classified as having compensated cirrhosis. The Child-Turcotte-Pugh classification is used clinically to stratify patients with cirrhosis. Those that belong to Child’s class A are compensated, whereas those in Child’s classes B and C are decompensated. The average life expectancy from the time of diagnosis varies from 13 years, for patients with compensated disease, to only 2 years in patients with decompensated cirrhosis [6]. The risk of death in patients with compensated versus decompensated cirrhosis is 4.7 versus 9.7 times higher than the general population, respectively [7].
Etiologies of Liver Fibrosis
Development of liver fibrosis and, ultimately, cirrhosis is the final common pathway of any chronic liver disease. The most common etiologies of chronic liver disease in the United States are alcoholic liver disease, chronic hepatitis C infection, and nonalcoholic fatty liver disease. Together, these three diseases account for approximately 80% of the disease etiologies in individuals with end-stage liver failure awaiting liver transplantation between 2004 and 2013 [8].
Nonalcoholic fatty liver disease (NAFLD), defined as the presence of steatosis in ≥5% of hepatocytes in individuals who do not consume excessive alcohol, affects 80–100 million people in the United States and is the most common cause of chronic liver dysfunction [9]. The rising prevalence of NAFLD, currently 20–30% worldwide, is linked to the obesity epidemic that has engulfed the United States and the world. More than a third of the US population is now obese, and trends indicate that prevalence of obesity will continue to increase [10]. NAFLD is a manifestation of the metabolic syndrome and coexists with obesity, type 2 diabetes, insulin resistance, dyslipidemia, and cardiovascular disease. About 10% of people with NAFLD will develop a progressive form of the disease termed nonalcoholic steatohepatitis (NASH), characterized by ballooning hepatocyte degeneration and inflammation. NASH can progress to fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma [11]. Although a minority of people with fatty liver disease develop NASH, it is the only indication for liver transplantation that is rapidly increasing in frequency and thus predicted to become the most common cause of end-stage liver disease requiring transplantation in the United States [12, 13].
Other etiologies of liver fibrosis include chronic viral hepatitis B infection, hemochromatosis, autoimmune hepatitis, primary and secondary biliary cirrhosis, primary sclerosing cholangitis, Wilson disease, alpha-1 antitrypsin deficiency, celiac disease, polycystic liver disease, idiopathic portal fibrosis, idiopathic adulthood ductopenia, granulomatous liver disease, veno-occlusive disease, hereditary hemorrhagic telangiectasia, and right-sided heart failure. In addition, medications (e.g., methotrexate) and infection (e.g., echinococcosis) can result in cirrhosis. In approximately 85–90% of patients, a specific etiology for liver fibrosis is identified [14]. Common laboratory tests ordered to identify potential causes of chronic liver disease are displayed in Table 15.1.
Histopathological Changes in the Fibrotic Liver
The liver receives a dual blood supply from the hepatic artery and the portal vein. The hepatic artery delivers highly oxygenated blood from the celiac trunk of the aorta. The portal venous system carries blood from the esophagus, stomach, small and large intestine, pancreas, gallbladder, and spleen to the liver and constitutes 75% of total hepatic blood flow. Hepatic artery and portal vein derived blood mix in the hepatic sinusoids that are permeable vascular channels lined by fenestrated endothelial cells, allowing transport of macromolecules to hepatocytes. Within the sinusoid, there exists the space of Disse, which is located extraluminal to the endothelial cell and adjacent to the hepatocyte. Hepatic stellate cells and Kupffer cells lie within the space of Disse and play important roles in fibrogenesis (Fig. 15.1).
Microarchitecture and cellular components of hepatic sinusoids and the location of the space of Disse. (Reprinted with permission from [15])
The extracellular matrix of the normal liver is unique in structure and composition in several ways. The space of Disse separates epithelial hepatocytes from the fenestrated sinusoidal endothelium and contains a basement membrane-like matrix that lacks the typical electron-dense structure of basement membranes in other tissues [16,17,18]. The low density of the liver basement membrane-like structure is critical for allowing easy bidirectional macromolecular diffusion between blood and liver cells and for maintaining the differentiated function of hepatocytes [18, 19]. Collagen IV, a non-fibrillar collagen that is a major component of most tissue basement membranes, is present in the normal space of Disse in the form of α1α1α2(IV) heterotrimers [20]. It is controversial whether laminin, another extracellular matrix protein that is typically found in basement membranes, is present in the normal liver. Some reports suggest that the space of Disse is devoid of laminin [21], while others report that both collagen IV and laminin are present [19]. The absence of an identifiable basement membrane structure, despite the presence of both collagen IV and laminin, may be explained by the absence of nidogen, a glycoprotein that typically functions to bridge collagen IV and laminin networks in basement membranes [19]. On the other hand, the space of Disse contains collagen type I and fibronectin, which are not typical constituents of basement membranes [18, 22,23,24]. Another isoform of collagen, collagen XVIII, has also been localized to the perisinusoidal zones in the liver [25,26,27]. Due to the high amounts of collagen XVIII, the liver is the major source of the collagen XVIII in the body. Additionally, in the normal liver, the heparan sulfate proteoglycans perlecan and agrin are found in small amounts.
Although the normal liver space of Disse consists of a low-density extracellular matrix, during fibrosis the sparse matrix is progressively replaced by a continuous interstitial-like matrix with the accumulation of fibrillar collagens, particularly collagens I and III [20, 28,29,30]. This process, called capillarization of the sinusoids, is accompanied by a loss of fenestration of the sinusoidal endothelium and physical changes in the hepatocytes that lose their microvilli [28, 31]. Fibrosis is also associated with markedly elevated amounts of perlecan and agrin [32,33,34]. These changes are hypothesized to adversely affect hepatocyte viability during progression toward liver cirrhosis [35].
When fibrosis advances to cirrhosis, the normal architecture of the liver is significantly disrupted by the formation of increasingly dense fibrous septa of fibronectin and interstitial collagens I, III, V, and VI. Histologically, cirrhosis is characterized by the formation of fibrotic septa, distortion of hepatic vascular architecture, and development of regenerative nodules (Fig. 15.2). The formation of a continuous basement in the space of Disse is characteristic of cirrhosis [18]. In the cirrhotic liver, sinusoidal cells and hepatocytes synthesize and secrete laminin [37, 38], which leads to a marked increase in laminin deposition in the space of Disse, eventually forming a continuous basement membrane with fibrillar collagens and perlecan [20, 21]. The increased extracellular matrix deposition during cirrhosis impairs the normal exchange of soluble proteins and fluids between sinusoidal blood and adjacent liver cells, which is a major contributor to hepatic failure [39].
Gross appearance and histological features of the cirrhotic liver. (a) Gross specimen showing the cut surface of a cirrhotic liver with nodular appearance. (b) Hematoxylin and eosin stain of a liver biopsy from a cirrhotic patient showing a regenerative nodule (thin arrow), fibrotic septa (thick arrow), and inflammation. (c) Reticulin stain of a cirrhotic liver showing regenerative nodules outlined by fibrotic septa. (Reprinted with permission from [36])
Clinical Pathology Classification Systems
There are several clinical histologic scoring systems used to characterize chronic liver disease progression that differ according to etiology. The Ishak and METAVIR scores are used to assess chronic hepatitis, and they include descriptions of degree of fibrosis and necro-inflammatory activity. The Ishak score includes several stages for describing fibrosis, which allows for documentation of small changes in fibrosis progression. The nonalcoholic fatty liver activity score (NAS) is utilized to evaluate patients with NAFLD/NASH, and the components of the score include steatosis, lobular inflammation, and ballooning. The NAS ranges from 0 to 8, with scores of 5–8 considered diagnostic of NASH [40].
Clinical Diagnosis of Liver Fibrosis
Clinicians still consider liver biopsy, performed by percutaneous, laparoscopic, or transjugular approaches, to be the gold standard for the diagnosis of liver fibrosis. However, liver biopsy is invasive and subject to sampling error [41]. As such, development of accurate and noninvasive diagnostic tools for liver fibrosis is an active area of laboratory and clinical investigation. A liver biopsy may be unnecessary to confirm diagnosis, if clinical, laboratory, and radiographic data support the diagnosis of cirrhosis.
Serum Biomarkers
Numerous serum biomarker panels were developed for the diagnosis of liver fibrosis, but there remains no consensus as to which indices are most clinically useful [42,43,44]. Direct biomarkers are measures of serum proteins indicative of the accumulation and turnover of the liver extracellular matrix and are used to estimate the extent of fibrogenesis. An example of a direct biomarker panel is the enhanced liver fibrosis (ELF) test, which measures hyaluronic acid, tissue inhibitor of metalloproteinase 1 (TIMP-1), and amino-terminal pro-peptide of procollagen type III (PIIINP) [45]. Indirect biomarkers of liver fibrosis are serum markers that change as a result of functional alterations of the liver, hepatocellular damage, and inflammation. An example of an indirect measure of liver fibrosis is the aspartate aminotransferase (AST)/platelet ratio index (APRI), which can be calculated from two commonly obtained laboratory tests [46]. The FibroTest, also known as BioPredictive in the EU and FibroSURE in the United States, is an extensively studied proprietary indirect biomarker panel that is composed of alpha-2-macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, and gamma-glutamyl transferase [47, 48]. In general, serum biomarkers are better at predicting advanced stages of fibrosis and less accurate in distinguishing earlier stages of fibrosis from no fibrosis (Table 15.2) [42,43,44].
Conventional Imaging Modalities
Conventional imaging techniques are only able to detect advanced stages of liver fibrosis. Ultrasonography is well-tolerated, and diagnostic features of cirrhosis include a small nodular liver, right lobe atrophy, and hypertrophy of the caudate or left lobes. Ultrasonography has been reported to have a sensitivity of 91% and a specificity of 94% for the diagnosis of cirrhosis [49]. Ultrasonography can also provide information regarding portal hypertension, such as flow and diameter within the portal vein. Computed tomography (CT) can also be used to identify findings suggestive of cirrhosis, including liver nodularity and caudate lobe hypertrophy. Magnetic resonance imaging (MRI) can provide information regarding fat content for diagnosis of steatosis [50] and hepatic iron concentration for determining iron overload [51], in addition to information regarding liver size. Other diagnostic features obtained by these imaging modalities are indicative of advanced fibrosis and represent sequelae of decompensated cirrhosis, including ascites, varices, splenomegaly, hepatic or portal vein thrombosis, the presence of porto-collateral circulation, or the finding of reversal of flow within the portal system. Splenomegaly is a sensitive but nonspecific sign of portal hypertension [52].
Novel Technologies: Elastography and Liver Stiffness
Increased liver tissue stiffness is a hallmark of cirrhosis. Accordingly, noninvasive imaging modalities have been developed to quantify liver stiffness as a surrogate marker of fibrosis. Transient elastography (TE) , also known as FibroScan, utilizes pulse-echo ultrasound to measure liver stiffness, and a threshold of greater than 20 kilopascals (kPa) is associated with a diagnostic accuracy of over 90% [53]. Magnetic resonance elastography (MRE) provides data of larger areas of the liver compared to TE and has been shown to be more accurate than TE [54].
Transient elastography and MRE are increasingly being used clinically to determine a patient’s liver stiffness as a proxy for fibrosis and to aid in clinical decision-making. The relative liver stiffness determined by these techniques correlates well with the histological severity grade of fibrosis. TE, expressing stiffness in elastic modulus (E), estimates normal human liver stiffness to be around 5 kPa and grade 4 cirrhotic livers to be 15–20 kPa [55]. MRE, expressing stiffness in shear storage modulus (G’), estimates normal human liver stiffness to be 2 kPa and places the matrix stiffness cutoff for grade 4 cirrhotic livers at >5 kPa [56, 57]. The advantage of these noninvasive techniques is that they evaluate the rigidity of large regions of the liver, thereby avoiding sampling error that is inherent in assessing fibrosis by liver biopsy. However, stiffness values obtained are extrapolated from the response of the liver to shear waves and, therefore, represent relative values and do not accurately reflect the absolute mechanical tissue stiffness. Direct mechanical testing of liver tissue by rheometry suggests that normal liver stiffness is around 400 Pa and fibrotic liver stiffness ranges around 2 kPa [58, 59]. Atomic force microscopy (AFM) , which measures microscale matrix rigidity, shows that increased matrix stiffness in fibrotic livers is a local phenomenon near fibrotic tracts and regions distant from the deposition of aligned collagen approaches the stiffness of normal liver. AFM determined the absolute rigidity of normal mouse liver matrix to be approximately 150 Pa and the stiffness of areas near fibrotic tracts in fibrotic livers to range between 1 and 6 kPa [60].
Determining the absolute matrix stiffness of a fibrotic liver may be clinically important as there is evidence that increased matrix rigidity directly inhibits hepatocyte functions. Therefore, increased matrix stiffness may be a key mechanism by which fibrosis causes liver dysfunction [60]. The relative tissue stiffness reported by elastography does not reflect the absolute matrix stiffness hepatocytes experience at a cellular level. Therefore, current elastography techniques may not be sensitive enough to determine the changes in liver stiffness that will impact hepatic function and clinical outcomes. In addition, elastography does not distinguish between tissue stiffness due to perfusion pressure produced by portal hypertension versus matrix rigidity associated with increased collagen deposition. It is currently unknown whether different physiological inducers of tissue rigidity have differing effects on hepatocyte function. Likewise, the relative contribution of each source of stiffness to the overall rigidity of fibrotic livers is also unknown. Moreover, liver fibrosis is not a homogenous process at the tissue level; even in advanced fibrosis, where there are bridging fibrotic bands, areas of relatively less collagen deposition remain, suggesting that a hepatocyte’s response to matrix rigidity is likely specific to its immediate local microenvironment. If hepatocytes exhibit suppressed functions at a threshold level of matrix stiffness, the overall averaged stiffness of the liver may be less clinically important to predicting hepatic functional outcomes than the proportion of liver volume that has reached a certain threshold stiffness. The experience with incorporating TE and MRE into clinical practice is evolving. There remain confounding factors whether changes in elastography correlate with changes in fibrosis and uncertainty whether liver stiffness measurements are predictive of clinical outcome [61]. Additional research is required to investigate the relationship between fibrotic liver stiffness and hepatocyte function and determine whether elastography may be used to prognosticate liver function.
Clinical Presentation
Early-stage fibrosis is largely asymptomatic. Patients with advanced liver fibrosis present with liver metabolic dysfunction and clinical sequelae of portal hypertension.
Pathophysiology of Portal Hypertension
In patients with cirrhosis, portal hypertension results from alterations in portal vascular pressure as described by Ohm’s law (∆P = F × R), where the pressure gradient in the portal circulation (∆P) is a function of portal flow (F) and resistance (R). In cirrhosis, increased intrahepatic resistance and hyperdynamic portal circulation result in portal hypertension and its clinical sequelae. The increased resistance occurs in the setting of structural mechanisms, including collagen deposition, vascular distortion, and microthrombi. In addition, increased intrahepatic vascular tone, which occurs as a result of reduced nitric oxide availability and endothelial cell dysfunction, contributes to the increased resistance [62]. Hyperdynamic portal circulation, or increase in portal venous inflow, is induced by multiple factors, including peripheral and splanchnic vasodilation, increased cardiac output, and reduced mean arterial pressure. Splanchnic vasodilation leads to activation of neurohumoral and vasoconstrictive systems mediated by norepinephrine, angiotensin II, and antidiuretic hormone, resulting in sodium and water retention and increased blood volume. Therapies to reduce portal hypertension include medications that cause splanchnic vasoconstriction, such as nonselective beta-blockers, vasopressin, and somatostatin.
Measurement of Portal Pressure
Portal hypertension is measured by obtaining a hepatic venous pressure gradient (HVPG) through catheterization of the hepatic vein. The HVPG is defined as the difference between the wedged hepatic vein pressure and the free hepatic vein pressure. Normal portal pressure is 5 mmHg or less. In patients with cirrhosis, HVPG >10 mmHg predicts the development of varices, HVPG >12 mmHg predicts variceal bleeding, and HVPG >16 mm Hg indicates a higher risk of death [63]. Patients who attain a reduction in HVPG to less than 12 mmHg or an overall reduction of 20% with medical therapy are less likely to develop complications of portal hypertension, including variceal bleeding, ascites, and encephalopathy [52].
Variceal Bleeding
Variceal bleeding contributes to the morbidity and mortality in patients with cirrhosis. Variceal formation results from the development of portosystemic collateral pathways that shunt the blood away from the liver to reduce portal venous pressure. The esophagus, stomach, and rectum are common areas in the gastrointestinal tract in which portal hypertension manifests as varices (Fig. 15.3). Gastroesophageal varices are present in approximately 30–40% of patients with compensated disease and up to 85% of those with decompensated disease [64]. Esophageal varices develop at a rate of 5–8% per year [65], and progression from small to large varices occurs at a rate of 10–12% per year [66]. The 6-week mortality associated with an acute variceal hemorrhage ranges between 15% and 25% [67].
Endoscopic manifestations of portal hypertension in the gastrointestinal tract. (a) Large varices in the distal esophagus with a “nipple sign” (arrow) showing evidence of recent bleeding. (b) Hyperemic edematous mucosa in the stomach with a “fish scale” appearance characteristic of portal gastropathy. (c) Dilated internal hemorrhoidal veins in the rectum that are varices (arrow). (Reprinted with permission from [36])
At the time of diagnosis of cirrhosis, patients usually undergo endoscopy to determine the presence and size of varices. Recent studies show that patients with a liver stiffness <20 kPa, as determined by TE, and a platelet count >150,000/mm3 have a low risk (<5%) of having high-risk varices, suggesting that endoscopy may be avoided in this subset of patients [68]. In patients undergoing endoscopy who have medium or large esophageal varices, the treatment options include endoscopic variceal ligation or medical therapy with nonselective beta-blockers. Endoscopic variceal ligation consists of placement of rubber bands on variceal columns that leads to localized mucosal and submucosal necrosis. Esophageal ulcerations may occur at the site of band ligation, and these ulcers have the potential to bleed. Ligation is repeated until all varices are obliterated. Studies show that the rates of gastrointestinal bleeding and mortality do not differ between endoscopic variceal ligation and medical therapy [69, 70].
Patients who present with variceal bleeding are treated with intravenous vasoactive drugs to reduce portal pressure, such as octreotide or terlipressin, as well as endoscopic therapy. The type of endoscopic therapy indicated depends on the location of the varix. Esophageal varices are treated with endoscopic variceal ligation. Gastric varices have been reported in up to 20% of patients with cirrhosis [52] and are classified according to their location. Gastroesophageal varices type 1 (GOV1) are esophageal varices extending into the lesser curvature, and gastroesophageal varices type 2 (GOV2) are those extending into the fundus. Isolated gastric varices type 1 (IGV1) are located in the fundus, and isolated gastric varices type 2 (IGV2) are located elsewhere in the stomach. In patients with bleeding GOV1, treatment options include endoscopic variceal ligation or injection with cyanoacrylate glue. Fundal varices (GOV2 and IGV1) have a higher rebleeding rate, and the recommended treatment is transjugular intrahepatic portosystemic shunt (TIPS) in which a stent is placed between the hepatic and portal veins. TIPS decreases portal pressure and is indicated for patients who experience recurrent variceal hemorrhage despite endoscopic variceal ligation. In addition, early TIPS within 72 h of hospital admission may be beneficial for patients with advanced cirrhosis who present with acute variceal bleeding [71].
Patients with cirrhosis presenting with variceal bleeding are at high risk of developing bacterial infections. The use of antibiotic prophylaxis is associated with a decrease in the development of infections, recurrent hemorrhage, and death [72]. Patients with acute bleeding should be transfused with packed red blood cells when the hemoglobin falls below 7 g/dL, with the goal of maintaining hemoglobin between 7 and 9 g/dL [52]. A randomized clinical study showed that this transfusion strategy is associated with increased survival in patients with Child’s class A or B cirrhosis, compared with a more liberal transfusion strategy that aimed to maintain hemoglobin above 9 g/dL [73]. Moreover, patients who recover from acute variceal hemorrhage are at a high risk for rebleeding, which is associated with a mortality rate of up to 33% [52]. Endoscopic variceal ligation and medical therapy (propranolol or nadolol) combination therapy was shown to be more effective than ligation alone in preventing recurrent bleeding [74].
Ascites
Ascites, the accumulation of fluid within the peritoneal cavity, is the most common complication of cirrhosis. The onset of ascites is associated with a 1-year mortality rate of 20% [6]. Treatment involves restriction of salt from the diet and use of diuretic medications, furosemide and spironolactone, which increase salt and water excretion into the urine. In refractory cases of ascites, treatment options include the addition of midodrine, which was shown to reduce ascites and improve systemic hemodynamics [75]. Additional approaches for the treatment of refractory ascites include TIPS placement. Patients with refractory ascites should also be considered for liver transplantation.
Spontaneous bacterial peritonitis is an infection of ascitic fluid without evidence for a secondary source. Development of spontaneous bacterial peritonitis is associated with a poor prognosis, with 30% of patients dying within a month and an additional 30% within 1 year. Clinical symptoms include fever, abdominal pain, and altered mental status. Spontaneous bacterial peritonitis is diagnosed by an absolute polymorphonuclear leukocyte count of 250 cells or greater or a positive bacterial culture from the ascitic fluid. Intravenous antibiotic and albumin therapy has been shown to reduce the risk of renal failure and death in patients with spontaneous bacterial peritonitis [76]. All patients with a prior episode of spontaneous bacterial peritonitis should be maintained on prophylactic oral antibiotics as secondary prevention.
Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a neuropsychiatric disorder in patients with cirrhosis. HE is characterized by changes in personality, motor function, level of consciousness, and cognition. The development of HE is associated with a 1-year mortality rate as high as 64% [77]. Patients who develop encephalopathy in the setting of preserved liver function should undergo imaging to evaluate for the presence of portosystemic shunts, because embolization of large shunts has been shown to be effective in a subset of patients [78]. The pathogenesis of HE is not completely understood, but several neurotoxins have been implicated. The best characterized neurotoxin is ammonia. Produced by the colon, ammonia enters the portal circulation and is converted into glutamine by the liver, preventing ammonia from entering the systemic circulation. In the setting of advanced liver disease, decreased hepatocyte function and portosystemic shunting lead to increased systemic circulating ammonia, which interferes with brain function in several ways. Studies suggest that hyperammonemia may induce astrocyte swelling, impair blood to brain transport of amino acids, and alter neuronal electrical activity. Arterial hyperammonemia is observed in 90% of patients with HE, but the serum levels are not sensitive or specific for the diagnosis of HE. Additional toxins implicated in the pathogenesis of HE include short-chain fatty acids, mercaptans, aromatic amino acids, and manganese. The recommended treatment for the prevention of recurrent encephalopathy is lactulose, a synthetic disaccharide metabolized by the colon to inhibit ammonia production and trap fecal ammonia. Lactulose was associated with a reduced risk of recurrent encephalopathy compared to placebo (20% vs 47%) [79]. When added to lactulose therapy, rifaximin, a poorly absorbed antibiotic, has been shown to reduce the risk of recurrence from 46% to 21% [80].
Treatment of Liver Fibrosis
Current treatment for liver fibrosis consists of supportive care and management of the sequelae of decompensated cirrhosis as discussed above. Until recently, liver fibrosis was thought to be a chronically progressive disease that was irreversible [81]. Effective treatments of chronic hepatitis B and C infection demonstrated that removal of agents causing injury to the liver can lead to the reversal of fibrosis [82,83,84]. Several novel drugs directly targeting various aspects of the fibrogenesis pathway are now in clinical trials (Table 15.3).
For patients who progress to end-stage liver failure as a consequence of cirrhosis, liver transplantation is the only treatment available. Donor organs are allocated according to the Model for End-stage Liver Disease (MELD) score. For patients with hepatocellular carcinoma, there are several systems for liver transplant listing, including the Milan criteria [85] and the UCSF criteria [86]. Although the clinical outcomes for patient who undergoes liver transplant are good, the effectiveness of transplantation as a treatment for cirrhosis is limited by the critical shortage of donor organs [87]. Development of therapeutic adjuncts or alternatives to liver transplantation through regenerative medicine and tissue engineering approaches are active areas of laboratory investigation.
Hepatic Stellate Cells: Central Regulators of Liver Fibrogenesis
Originally identified by von Kupffer in 1876, hepatic stellate cells (HSCs) are located in the space of Disse and represent approximately 15% of the total number of resident cells in normal liver [88]. In their quiescent state, HSCs store retinyl esters in cytoplasmic lipid droplets [89]. Following liver injury, HSCs become activated and transdifferentiate into fibrogenic myofibroblasts. This change is characterized by the loss of lipid droplets; accumulation of contractile filaments, such as α-smooth muscle actin; and proliferation. Although additional cell types have been identified that contribute to hepatic fibrogenesis, including portal fibroblasts and sinusoidal endothelial cells, fate-tracing studies have shown that activated HSCs are the major source of extracellular matrix in parenchymal and cholestatic liver injury [90, 91].
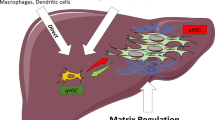
HSC activation consists of two phases, initiation and perpetuation (Fig. 15.4). During the initiation phase, paracrine stimulation from neighboring cell types, including platelets, endothelial cells, Kupffer cells, and hepatocytes, results in early changes in gene expression and phenotype that render HSCs more responsive to other stimuli. Platelets produce several fibrogenic cytokines and growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGFβ), and epidermal growth factor (EGF). In addition, injury-induced hepatocyte apoptosis promotes HSC activation [93, 94]. During the perpetuation phase, autocrine and paracrine stimulations maintain the activated HSC phenotype. HSCs primed by stimuli become responsive to growth factors and cytokines, leading to HSC retinoid loss, proliferation, chemotaxis, contractility, altered matrix degradation, inflammatory signaling, and fibrogenesis. The cumulative effect is deposition of fibrotic extracellular matrix.
Initiation, perpetuation, and resolution of hepatic stellate cell activation. (Reprinted with permission from [92])
HSCs can be distinguished from other liver cell types by several markers, including platelet-derived growth factor receptor-β (PDGFRβ), desmin, glial fibrillary acidic protein (GFAP), and lecithin retinol acyltransferase (LRAT). Conditional deletion of these genes was utilized to target HSCs in mouse models of liver disease [95].
Cytokines and Chemokines
TGFβ, an important pro-fibrotic cytokine , is produced in its latent form by several cell types in the liver, including HSCs, platelets, and Kupffer cells. When bound to its receptor, SMAD proteins are phosphorylated and activated, leading to transcription of collagen types I and III [92]. TGFβ activates several pathways that promote HSC activation, including the mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways [96, 97]. Several factors were identified that regulate TGFβ-mediated myofibroblast activation, including galectin-3. Inhibitors of galectin promoted fibrosis regression in a rat model [98] and are currently being investigated in a clinical trial (Table 15.3).
Other pro-fibrotic cytokines include PDGF, which drives HSC proliferation and migration, and connective tissue growth factor (CTGF), which stimulates HSC proliferation, migration, adhesion, survival, and extracellular matrix production [92].
Receptor-Mediated Activation
HSCs express a myriad of receptors , several of which can be targeted to reduce HSC activation and hepatic fibrosis. HSCs express integrins that mediate communication between the cytoskeleton and extracellular matrix. Integrins regulate the activation of TGFβ and HSC-specific deletion of integrin αv resulted in reduced fibrosis in a mouse model [99].
HSCs also express G-protein-coupled receptors, including C-C chemokine receptors (CCRs), cannabinoid receptors 1 and 2, and angiotensin II type 1 receptor (AT1R). Chemokine receptors CCR2 and CCR5 were implicated in fibrogenesis through promotion of macrophage recruitment and HSC activation [100,101,102,103]. A dual CCR2-CCR5 antagonist, cenicriviroc, is currently being investigated in clinical trials for patients with NASH (Table 15.3). Cannabinoid receptor 1 promotes fibrosis, whereas cannabinoid receptor 2 has anti-fibrogenic effects [104, 105]. AT1R and its ligand, angiotensin II, promote HSC activation and fibrosis through phosphorylation of Janus kinase 2 [106].
Toll-like receptors (TLRs) are a class of proteins that play an important role in the innate immune system. TLRs recognize structurally conserved damage-associated molecular patterns (DAMPs) released following hepatocyte injury. Activated HSCs express TLR2, TLR3, TLR4, TLR7, and TLR9. TLR4 activation induced chemotaxis of Kupffer cells and promotes TGFβ-induced HSC activation [107]. TLR2 promoted the activation of the inflammasome, resulting in NASH progression in a mouse model [108].
Finally, HSCs express nuclear receptors such as the farnesoid X receptor (FXR), liver X receptor (LXR), peroxisome proliferator-activated receptor gamma (PPARγ), vitamin D receptor (VDR), and nuclear receptor subfamily 4 group A member 1 (NR4A1). FXR inhibits HSC activation [109], and FXR agonists, such as obeticholic acid, improve NAS score and fibrosis stage in patients with NASH [110]. Activation of PPARγ induces HSC inactivation [111], and a dual PPARα-PPARδ agonist improved NASH in a large clinical trial [112]. VDR ligands reduce HSC activation mediated by TGFβ and reduce hepatic fibrosis [113].
Additional Pathways
Several signaling pathways regulate HSC activation, including the Hedgehog pathway and the Hippo pathway. Inhibition of the Hedgehog pathway leads to decreased HSC activation and reduced hepatic fibrosis [114]. The Hippo pathway is a kinase cascade that results in phosphorylation and inactivation of the transcriptional coactivator yes-associated protein (YAP). YAP inhibition inactivates HSCs and reduces fibrosis [115,116,117,118]. The mechanism by which YAP regulates HSC activity is not completely understood.
Regulation of HSC activation may be mediated by microRNAs, including miR-21 [119] and miR-221 [120], as well as histone modifications regulated by myocardin-related transcription factor A (MRTF-A) [121]. In addition, methyl-CpG-binding protein 2 (MECP2) regulates epigenetic signaling by suppressing PPARγ transcription, resulting in increased HSC activation and fibrosis [122].
HSC Clearance Following Injury
In light of clinical data highlighting the regression of fibrosis in patients with liver disease, recent studies elucidated the fate of HSCs following cessation of injury. HSC clearance occurs through apoptosis, senescence, and reversion (Fig. 15.4). Apoptosis, a form of programmed cell death, occurs during resolution of liver injury and results in reduced numbers of activated HSCs. This process is mediated by death receptors expressed by activated HSCs, including first apoptosis signal (FAS) receptor, tumor necrosis factor receptor 1 (TNFR1), neurotrophin receptor p75 (p75NTR), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors [123]. HSCs undergo senescence, which refers to cell-cycle arrest after reaching a finite proliferative limit. HSC senescence occurs in a p53-dependent manner and results in decreased numbers of activated HSCs and reduced expression of extracellular matrix proteins [124]. Fate-tracing studies in mice demonstrated that HSCs can also undergo reversion to an inactivated phenotype [125, 126]. Approximately 50% of activated HSCs undergo reversion after cessation of liver injury. Interestingly, these HSCs do not return to the quiescent state but exist as inactivated HSCs that are primed for reactivation in response to another injury [125, 126].
Liver Fibrosis and Cancer
Fibroblasts, depending on their activation state, are involved in wound healing, mediating pathological tissue fibrosis, and inducing a desmoplastic reaction in the tumor microenvironment (Fig. 15.5) [127]. HSCs, being the fibroblasts of the liver, are involved in all of these processes within the liver. Indeed, stellate cells are critical in regulating liver regeneration after injury [88, 128], and they are chief producers of fibrotic extracellular matrix during liver fibrogenesis [90, 91]. HSCs also play an important role in modulating the tumor microenvironment in the liver during cancer development and progression [129, 130]. The liver is an interesting model organ to investigate the role of the tumor microenvironment because fibrosis-related effects likely contribute to the development of both primary liver cancers and metastatic liver lesions from other primary sites.
Functional and phenotypic differences between normal activated fibroblasts (NAFs) , fibrosis-associated fibroblasts (FAFs) , and cancer-associated fibroblasts (CAFs) . (Reprinted with permission from [127]) CCL5, C-C motif chemokine ligand 5 (also known as RANTES); CTGF, connective tissue growth factor; CXCL, C-X-C motif chemokine ligand; EDA-FN, extradomain A-fibronectin; EGF, epidermal growth factor; FAP, fibroblast activation protein; FGF, fibroblast growth factor; GFs, growth factors; HGF, hepatocyte growth factor; ICAM1, intercellular adhesion molecule 1; IFNγ, interferon-γ; IL, interleukin; LOX, lysyl oxidase; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; ROS, reactive oxygen species; SDF1, stromal cell-derived factor 1; TGFβ, transforming growth factor-β; TIMPs, tissue inhibitors of metalloproteinases; TNF, tumor necrosis factor; VEGFA, vascular endothelial growth factor A; VCAM1, vascular adhesion molecule 1
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is an aggressive primary liver tumor with a poor prognosis. Worldwide, HCC is the fifth most common cancer and the third most common cause of cancer deaths [131]. HCC is one of the few cancers for which incidence is increasing [132]. HCC most commonly occurs in the setting of chronic liver disease, in which cycles of necrosis, inflammation, and tissue repair lead to progression of nodular lesions. Importantly, HCC is strongly associated with liver fibrosis, and 90% of HCCs arise within cirrhotic livers [133]. The oncogenic process begins with a benign liver regenerative nodule, progressing to a low-grade dysplastic nodule, then to a high-grade dysplastic nodule, and ultimately HCC [134]. The earliest mutations in this progression are telomerase promoter mutations that induce telomerase reactivation [135]. Subsequent oncogenic mutations that promote HCC progression are numerous, and the molecular signatures of the tumors fall into two broad subtypes. The aggressive subtype is characterized by activation of cell proliferation signals, including the p53, Ras/ERK, and Akt/mTOR pathways. The less aggressive subtype is characterized by the activation of Wnt/β-catenin signaling [136]. HCC is distinguished from high-grade dysplastic nodules by the invasion of neoplastic cells into the stroma of portal tracts [134]. Early HCC may be treated by surgical resection or by liver transplantation. Current chemotherapeutic and targeted molecular therapeutic options are limited and ineffective. Most HCC patients (85%) present with advanced disease and have median survival times of less than 1 year [137].
Although cirrhosis is one of the most important risk factors for developing HCC [138], the nature of the association between liver fibrosis and HCC is not established. Whether fibrosis actively promotes the development of HCC or is simply a by-product of persistent inflammation related to ongoing hepatocellular injury is controversial. Increased tissue stiffness in fibrotic livers may create an environment permissive for HCC development through several mechanisms. Greater matrix stiffness in fibrotic liver can activate HSCs and portal fibroblasts to produce and deposit fibrillar collagens that promote fibrosis progression [139, 140]. Stiffened extracellular matrix stimulates epithelial cell integrin signaling and cytoskeletal contractility, leading to enhanced proliferation and invasiveness of premalignant and malignant cells [141]. In HCC, increased integrin signaling has been shown to promote tumorigenesis by stimulating cell motility [142] and inhibiting apoptotic pathways [143]. Increased matrix stiffness stimulates the proliferation and chemotherapeutic resistance of HCC cell lines [144]. Focal adhesion kinase (FAK) signaling is required for the progression of a c-Met/β-catenin-driven in vivo mouse model of HCC [145], suggesting that the stiffened matrix of a fibrotic liver may activate FAK signaling to promote HCC progression.
Importantly, increased matrix rigidity has a direct inhibitory effect on expression of hepatocyte nuclear factor 4 alpha (HNF4α) [60], a master transcriptional regulator of hepatic function and a tumor suppressor. Inhibition of HNF4α stimulates hepatocyte proliferation [146] and induces expression of mesenchymal genes [147]. HNF4α expression is inhibited in fibrotic livers, and forced re-expression slows the progression of fibrosis [148, 149]. Similarly, HNF4α expression is reduced in HCC, and forced re-expression inhibits tumor progression [150,151,152,153,154]. These findings suggest that increased matrix rigidity in fibrotic livers may create an environment permissive for HCC development by decreasing HNF4α expression in hepatocytes. Moreover, HNF4α expression in primary hepatocytes cultured on stiff matrix is increased by inhibition of Rho/Rho-associated protein kinase signaling, indicating a critical role of the mechano-signal transduction networks in modulating HNF4α activity in response to tissue stiffness [60].
Liver Metastases
The liver is the most common distant organ site for tumor metastases . While any primary tumor may metastasize to the liver, primary cancers that most frequently present with liver metastases are gastrointestinal, breast, lung, neuroendocrine, and melanoma [155]. Two theories have been proposed to explain why certain tumors have propensity to metastasize to specific organs. The “seed and soil” hypothesis proposed by Paget in 1889 postulates that certain tumor cells (the “seeds”) have affinity for the microenvironment of certain organs (the “soil”) and that metastases occur when seed and soil are compatible. Alternatively, Ewing proposed in 1929 that metastatic spread was determined by mechanical factors related to the vascular system [156]. The two theories are not mutually exclusive, and both mechanisms are likely important in promoting the establishment of metastatic lesions within the liver. The sinusoidal capillary system of the liver can act as a great sieve to entrap circulating tumor cells and facilitate cancer cell infiltration into the liver parenchyma. In addition, stromal cells such as HSCs may provide a prometastatic microenvironment by promoting tumor cell proliferation, recruiting neovascularization, and suppressing antitumor immunity [129].
Cancer-associated fibroblasts (CAFs) are myofibroblasts that associate with tumors and play a role in remodeling the tumor stroma. CAFs appear to have a distinct phenotype from resting and normal activated fibroblasts involved in wound healing [127] (Fig. 15.5). CAFs may enhance tumorigenesis by inducing cancer cell invasion and angiogenesis. YAP, part of the Hippo pathway and a mechano-signal responsive transcriptional coactivator, is activated in CAFs and promotes cancer cell invasion, extracellular matrix stiffening, and angiogenesis [157]. These findings give functional significance to the desmoplastic reaction surrounding many tumor types and suggest that the stroma may be a key regulator of tumor progression. In addition, the desmoplastic tumor stroma may act as a physical barrier to the efficient delivery of cytotoxic chemotherapeutic agents in the treatment of cancers [158]. While there is evidence that the stiffened tumor microenvironment may be pro-tumorigenic, there is also evidence that the role of the stiffened tumor stroma may be more complex. Ablating the peri-tumoral stroma by targeting Hedgehog signaling enhanced cancer cell killing by chemotherapy in preclinical studies [159]. However, targeting CAFs in human pancreatic cancer led to accelerated disease progression and halted clinical trials [160]. Additional studies showed that depletion of CAFs and inhibition of Sonic Hedgehog led to accelerated pancreatic cancer progression and more aggressive disease [161, 162]. These studies suggest that the tumor stroma may have both pro- and antitumor properties that are context dependent. Therefore, rather than ablating the tumor stroma in devising novel treatments for cancer, perhaps it is more important to “reeducate” the stroma to be more anti-tumorigenic [163]. The role of CAFs in the regulation of cancer progression remains an important ongoing area of laboratory investigation.
It is not yet established whether pre-existing liver fibrosis promotes or inhibits the development of liver metastases from other primary sites. There is evidence that activated HSCs become CAFs and promote tumorigenesis by secreting growth factors, remodeling the stroma, promoting angiogenesis, and suppressing the antitumor response [129]. It is possible that activated HSC and cancer cell cross talk results in a feed-forward loop that enhances metastatic tumor growth. On the other hand, there are decades of clinical observational studies suggesting that fibrotic livers are less prone to developing metastases and that the fibrotic liver microenvironment may be poor “soil” for the metastatic implants [164]. An alternative explanation as to why metastases in fibrotic livers are less observed may be that cancer patients with liver fibrosis have shorter life-spans and die before the development of clinical metastases [165]. In addition, it is also possible that because the fibrotic liver is such good “soil” for primary liver cancer development, the chances of finding primary liver tumors in a fibrotic liver are much greater than finding metastatic cancer, thereby giving the impression that fibrotic liver is less prone to metastases. Because the liver is such an important organ for the development of primary cancers as well as secondary spread of metastases, improved understanding of the role of the liver microenvironment in modulating tumor progression is critical for advancing cancer treatment research.
Conclusion
Liver fibrosis is a significant source of human disease, morbidity, and mortality. Shifting patterns in etiological factors indicate that NAFLD/NASH will become a dominant cause of liver fibrosis and cirrhosis worldwide. Advances in understanding the role of extracellular matrix stiffening and the molecular basis of HSC activation in liver fibrosis have led to novel diagnostic modalities and development of targeted anti-fibrotic therapies. Diagnostic tools and treatment options for liver fibrosis are continuing to evolve and are being informed by basic discoveries from the laboratory and outcomes of clinical trials. Determining how the fibrotic liver microenvironment may regulate hepatocyte function and tumorigenesis remains important areas of investigation.
References
Mortality GBD, Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. https://doi.org/10.1016/S0140-6736(14)61682-2.
Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49(8):690–6. https://doi.org/10.1097/MCG.0000000000000208.
Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61(6):1–51.
Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375–82 e1-2. https://doi.org/10.1053/j.gastro.2013.04.005.
El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C-associated diseases in the United States. J Viral Hepat. 2012;19(3):153–60. https://doi.org/10.1111/j.1365-2893.2011.01563.x.
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–31. https://doi.org/10.1016/j.jhep.2005.10.013.
Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int: Off J Int Assoc Stud Liver. 2012;32(1):79–84. https://doi.org/10.1111/j.1478-3231.2011.02517.x.
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. https://doi.org/10.1053/j.gastro.2014.11.039.
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. https://doi.org/10.1002/hep.29466.
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. https://doi.org/10.1038/nrgastro.2013.171.
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–44. https://doi.org/10.1038/nrgastro.2013.41.
Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256(4):624–33. https://doi.org/10.1097/SLA.0b013e31826b4b7e.
Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–53. https://doi.org/10.1053/j.gastro.2011.06.061.
Charlton MR, Kondo M, Roberts SK, Steers JL, Krom RA, Wiesner RH. Liver transplantation for cryptogenic cirrhosis. Liver Transpl Surg. 1997;3(4):359–64.
Gougelet A, Colnot S. MicroRNAs linking cancer and inflammation: focus on liver cancer. In: Babashah S, editor. MicroRNAs: key regulators of oncogenesis. Cham: Springer International Publishing; 2014. p. 183–208.
Baloch Z, Klapper J, Buchanan L, Schwartz M, Amenta PS. Ontogenesis of the murine hepatic extracellular matrix: an immunohistochemical study. Differentiation. 1992;51:209–18. https://doi.org/10.1111/j.1432-0436.1992.tb00698.x.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. Am Soc Biochem Mol Biol. 2000;275:2247–50.
Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9(14):1401–10.
Griffiths MR, Keir S, Burt AD. Basement membrane proteins in the space of Disse: a reappraisal. J Clin Pathol. 1991;44(8):646–8. https://doi.org/10.1136/jcp.44.8.646.
Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21(1):63–71. https://doi.org/10.1136/gut.21.1.63.
Bianchi FB, Biagini G, Ballardini G, Cenacchi G, Faccani A, Pisi E, et al. Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 1984;4(6):1167–72.
Matsumoto S, Yamamoto K, Nagano T, Okamoto R, Ibuki N, Tagashira M, et al. Immunohistochemical study on phenotypical changes of hepatocytes in liver disease with reference to extracellular matrix composition. Liver. 1999;19(1):32–8. https://doi.org/10.1111/j.1478-3231.1999.tb00006.x.
Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44(3):239–42. https://doi.org/10.1001/jama.1963.03700140159106.
Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77(2):314–46.
Muragaki Y, Timmons S, Griffith CM, Oh SP, Fadel B, Quertermous T, et al. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc Natl Acad Sci U S A. 1995;92(19):8763–7. https://doi.org/10.1073/pnas.92.19.8763.
Musso O, Rehn M, Saarela J, Théret N, Liétard J, Hintikka E, et al. Collagen XVIII is localized in sinusoids and basement membrane zones and expressed by hepatocytes and activated stellate cells in fibrotic human liver. Hepatology. 1998;28(1):98–107. https://doi.org/10.1002/hep.510280115.
Rehn M, Pihlajaniemi T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci U S A. 1994;91(10):4234–8.
Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta Mol basis Dis. 2013;1832(7):876–83. https://doi.org/10.1016/j.bbadis.2012.11.002.
Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JMB, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015 (287); https://doi.org/10.1152/ajpgi.00447.2014.
Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21(3):351–72. https://doi.org/10.1055/s-2001-17556.
Friedman SL. The cellular basis of hepatic strategies. N Engl J Med. 1993;328(25):1828–35.
Gallai M, Kovalszky I, Neubauer K, Armbrust T. Expression of extracellular matrix proteoglycans perlecan and decorin. Am J Physiol Gastrointest Liver Physiol. 2007;148(5):1–9.
Roskams T, Rosenbaum J, De Vos R, David G, Desmet V. Heparan sulfate proteoglycan expression in chronic cholestatic human liver diseases. Hepatology. 1996;24(3):524–32. https://doi.org/10.1053/jhep.1996.v24.pm0008781318.
Tátrai P, Dudás J, Batmunkh E, Máthé M, Zalatnai A, Schaff Z, et al. Agrin, a novel basement membrane component in human and rat liver, accumulates in cirrhosis and hepatocellular carcinoma. Lab Inves: J Tech Methods Pathol. 2006;86(11):1149–60. https://doi.org/10.1038/labinvest.3700475.
McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15(6):989–97. https://doi.org/10.1002/hep.1840150603.
Qureshi M, Forouhar F. Cirrhosis: gastrointestinal features. In: Wu GY, editor. Atlas of dermatological manifestations of gastrointestinal disease. New York: Springer Science and Business Media; 2013. p. 177–8.
Clément B, Rescan PY, Baffet G, Loréal O, Lehry D, Campion JP, et al. Hepatocytes may produce laminin in fibrotic liver and in primary culture. Hepatology (Baltimore). 1988;8(4):794–803.
Maher JJ, Friedman SL, Roll FJ, Bissell DM. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988;94(4):1053–62.
Martinez-Hernandez A, Martinez J. The role of capillarization in hepatic failure: studies in carbon tetrachloride-induced cirrhosis. Hepatology. 1991;14:864–74.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. https://doi.org/10.1002/hep.20701.
Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50(1):1–3. https://doi.org/10.1016/j.jhep.2008.10.014.
Mendes LC, Stucchi RS, Vigani AG. Diagnosis and staging of fibrosis in patients with chronic hepatitis C: comparison and critical overview of current strategies. Hepatic Med: Evid Res. 2018;10:13–22. https://doi.org/10.2147/HMER.S125234.
Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307(8):832–42. https://doi.org/10.1001/jama.2012.186.
Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, et al. Biomarkers of liver fibrosis. Adv Clin Chem. 2008;46:131–60.
Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18(1):23–31. https://doi.org/10.1111/j.1365-2893.2009.01263.x.
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–36. https://doi.org/10.1002/hep.24105.
Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46(5):775–82. https://doi.org/10.1016/j.jhep.2006.12.013.
Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102(11):2589–600. https://doi.org/10.1111/j.1572-0241.2007.01466.x.
Simonovsky V. The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. Br J Radiol. 1999;72(853):29–34. https://doi.org/10.1259/bjr.72.853.10341686.
Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18(3):337–57, ix. https://doi.org/10.1016/j.mric.2010.08.013.
Ernst O, Sergent G, Bonvarlet P, Canva-Delcambre V, Paris JC, L’Hermine C. Hepatic iron overload: diagnosis and quantification with MR imaging. AJR Am J Roentgenol. 1997;168(5):1205–8. https://doi.org/10.2214/ajr.168.5.9129412.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–35. https://doi.org/10.1002/hep.28906.
Augustin S, Millan L, Gonzalez A, Martell M, Gelabert A, Segarra A, et al. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60(3):561–9. https://doi.org/10.1016/j.jhep.2013.10.027.
Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607 e2. https://doi.org/10.1053/j.gastro.2016.10.026.
Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56(6):2125–33. https://doi.org/10.1002/hep.25936.
Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22(3):433–46. https://doi.org/10.1016/j.mric.2014.05.001.
Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207–13 e2. https://doi.org/10.1016/j.cgh.2007.06.012.
Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–54. https://doi.org/10.1152/ajpgi.00032.2007.
Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, et al. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28(4):467–74.
Desai SS, Tung JC, Zhou VX, Grenert JP, Malato Y, Rezvani M, et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64(1):261–75. https://doi.org/10.1002/hep.28450.
Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018; https://doi.org/10.1038/nrgastro.2018.10.
Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46(5):927–34. https://doi.org/10.1016/j.jhep.2007.02.006.
Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32(5):930–4. https://doi.org/10.1053/jhep.2000.19322.
Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65(1):82–8. https://doi.org/10.1016/j.gie.2006.08.023.
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–61. https://doi.org/10.1056/NEJMoa044456.
Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38(3):266–72.
Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107(12):1872–8. https://doi.org/10.1038/ajg.2012.313.
Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, et al. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36(2):240–5. https://doi.org/10.1111/liv.12916.
Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;8:CD004544. https://doi.org/10.1002/14651858.CD004544.pub2.
Li L, Yu C, Li Y. Endoscopic band ligation versus pharmacological therapy for variceal bleeding in cirrhosis: a meta-analysis. Can J Gastroenterol. 2011;25(3):147–55.
Garcia-Pagan JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–9. https://doi.org/10.1056/NEJMoa0910102.
Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29(6):1655–61. https://doi.org/10.1002/hep.510290608.
Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. https://doi.org/10.1056/NEJMoa1211801.
Puente A, Hernandez-Gea V, Graupera I, Roque M, Colomo A, Poca M, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int: Off J Int Assoc Stud Liver. 2014;34(6):823–33. https://doi.org/10.1111/liv.12452.
Singh V, Dhungana SP, Singh B, Vijayverghia R, Nain CK, Sharma N, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012;56(2):348–54. https://doi.org/10.1016/j.jhep.2011.04.027.
Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403–9. https://doi.org/10.1056/NEJM199908053410603.
Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51(5):1675–82. https://doi.org/10.1002/hep.23500.
Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–57. https://doi.org/10.1002/hep.26314.
Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–91, 91 e1. https://doi.org/10.1053/j.gastro.2009.05.056.
Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–81. https://doi.org/10.1056/NEJMoa0907893.
Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8(4):283–91.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–75. https://doi.org/10.1016/S0140-6736(12)61425-1.
Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–93. https://doi.org/10.1002/hep.23785.
D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56(2):532–43. https://doi.org/10.1002/hep.25606.
Mazzaferro V, Regalia E, Montalto F, Pulvirenti A, Brunetto MR, Bonino F, et al. Risk of HBV reinfection after liver transplantation in HBsAg-positive cirrhosis. Primary hepatocellular carcinoma is not a predictor for HBV recurrence. The European Cooperative Study Group on Liver Cancer and Transplantation. Liver. 1996;16(2):117–22.
Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–403. https://doi.org/10.1053/jhep.2001.24563.
OPTN. OPTN/SRTR annual data report 2015. Am J Transplant. 2017;17(S1):1–564.
Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72. https://doi.org/10.1152/physrev.00013.2007.
Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161(1):207–18.
Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. https://doi.org/10.1038/ncomms3823.
Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832(7):948–54. https://doi.org/10.1016/j.bbadis.2013.02.019.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411. https://doi.org/10.1038/nrgastro.2017.38.
Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123(4):1323–30.
Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis. 2010;30(3):226–31. https://doi.org/10.1055/s-0030-1255352.
Greenhalgh SN, Conroy KP, Henderson NC. Cre-ativity in the liver: transgenic approaches to targeting hepatic nonparenchymal cells. Hepatology. 2015;61(6):2091–9. https://doi.org/10.1002/hep.27606.
Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274(38):27161–7.
Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274(52):37413–20.
Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8(10):e75361. https://doi.org/10.1371/journal.pone.0075361.
Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–24. https://doi.org/10.1038/nm.3282.
Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–70.
Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50(1):185–97. https://doi.org/10.1002/hep.22952.
Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120(11):4129–40. https://doi.org/10.1172/JCI41732.
Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174(5):1766–75. https://doi.org/10.2353/ajpath.2009.080632.
Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12(6):671–6. https://doi.org/10.1038/nm1421.
Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128(3):742–55.
Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60(1):334–48. https://doi.org/10.1002/hep.27117.
Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–32. https://doi.org/10.1038/nm1663.
Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57(2):577–89. https://doi.org/10.1002/hep.26081.
Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328(1):116–22. https://doi.org/10.1124/jpet.108.144600.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. https://doi.org/10.1016/S0140-6736(14)61933-4.
Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279(12):11392–401. https://doi.org/10.1074/jbc.M310284200.
Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147–59 e5. https://doi.org/10.1053/j.gastro.2016.01.038.
Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–13. https://doi.org/10.1016/j.cell.2013.03.028.
Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123(6):2380–94. https://doi.org/10.1172/JCI66904.
Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7:12502. https://doi.org/10.1038/ncomms12502.
Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63(3):679–88. https://doi.org/10.1016/j.jhep.2015.04.011.
Zhang K, Chang Y, Shi Z, Han X, Han Y, Yao Q, et al. Omega-3 PUFAs ameliorate liver fibrosis and inhibit hepatic stellate cells proliferation and activation by promoting YAP/TAZ degradation. Sci Rep. 2016;6:30029. https://doi.org/10.1038/srep30029.
Swiderska-Syn M, Xie G, Michelotti GA, Jewell ML, Premont RT, Syn WK, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232–44. https://doi.org/10.1002/hep.28542.
Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, et al. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288(52):37082–93. https://doi.org/10.1074/jbc.M113.517953.
Ogawa T, Enomoto M, Fujii H, Sekiya Y, Yoshizato K, Ikeda K, et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut. 2012;61(11):1600–9. https://doi.org/10.1136/gutjnl-2011-300717.
Tian W, Fan Z, Li J, Hao C, Li M, Xu H, et al. Myocardin-related transcription factor A (MRTF-A) plays an essential role in hepatic stellate cell activation by epigenetically modulating TGF-beta signaling. Int J Biochem Cell Biol. 2016;71:35–43. https://doi.org/10.1016/j.biocel.2015.12.005.
Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138(2):705–14, 14 e1–4. https://doi.org/10.1053/j.gastro.2009.10.002.
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–94. https://doi.org/10.1038/nri3623.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67. https://doi.org/10.1016/j.cell.2008.06.049.
Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(24):9448–53. https://doi.org/10.1073/pnas.1201840109.
Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143(4):1073–83 e22. https://doi.org/10.1053/j.gastro.2012.06.036.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98. https://doi.org/10.1038/nrc.2016.73.
Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. https://doi.org/10.1002/jcp.21172.
Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases? Hepatology. 2011;54(2):707–13. https://doi.org/10.1002/hep.24384.
Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56(2):769–75. https://doi.org/10.1002/hep.25670.
Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3(4):353–67. https://doi.org/10.1586/egh.09.35.
Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016; https://doi.org/10.1200/jco.2015.64.7412.
Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387(4):349–60. https://doi.org/10.1515/BC.2006.047.
Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 2010;30:17–25.
Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60(6):1983–92. https://doi.org/10.1002/hep.27372.
Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–39. https://doi.org/10.1053/j.gastro.2015.05.061.
Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol. 2014;10(3):153–61.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50.
Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–56. https://doi.org/10.1002/hep.21792.
Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G110–8. https://doi.org/10.1152/ajpgi.00412.2010.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. https://doi.org/10.1016/j.ccr.2005.08.010.
Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49(3):839–50. https://doi.org/10.1002/hep.22731.
Zhang H, Ozaki I, Mizuta T, Matsuhashi S, Yoshimura T, Hisatomi A, et al. Beta 1-integrin protects hepatoma cells from chemotherapy induced apoptosis via a mitogen-activated protein kinase dependent pathway. Cancer. 2002;95(4):896–906. https://doi.org/10.1002/cncr.10751.
Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192–205. https://doi.org/10.1002/hep.24108.
Shang N, Arteaga M, Zaidi A, Stauffer J, Cotler SJ, Zeleznik-Le NJ, et al. FAK is required for c-Met/β-catenin-driven hepatocarcinogenesis. Hepatology. 2015;61(1):214–26. https://doi.org/10.1002/hep.27402.
Bonzo Ja FCH, Matsubara T, Kim J-H, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4α in adult mice. J Biol Chem. 2012;287(10):7345–56. https://doi.org/10.1074/jbc.M111.334599.
Santangelo L, Marchetti A, Cicchini C, Conigliaro A, Conti B, Mancone C, et al. The stable repression of mesenchymal program is required for hepatocyte identity: a novel role for hepatocyte nuclear factor 4alpha. Hepatology. 2011;53(6):2063–74. https://doi.org/10.1002/hep.24280.
Nishikawa T, Bell A, Brooks JM, Setoyama K, Melis M, Han B, et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015;125(4):1533–44. https://doi.org/10.1172/JCI73137.
Yue HY, Yin C, Hou JL, Zeng X, Chen YX, Zhong W, et al. Hepatocyte nuclear factor 4alpha attenuates hepatic fibrosis in rats. Gut. 2010;59(2):236–46. https://doi.org/10.1136/gut.2008.174904.
Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, et al. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology. 2004;39(4):1038–47. https://doi.org/10.1002/hep.20155.
Lazarevich NL, Shavochkina DA, Fleishman DI, Kustova IF, Morozova OV, Chuchuev ES, et al. Deregulation of hepatocyte nuclear factor 4 (HNF4)as a marker of epithelial tumors progression. Exp Oncol. 2010;32(3):167–71.
Ning BF, Ding J, Yin C, Zhong W, Wu K, Zeng X, et al. Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res. 2010;70(19):7640–51. https://doi.org/10.1158/0008-5472.CAN-10-0824.
Späth GF, Weiss MC. Hepatocyte nuclear factor 4 provokes expression of epithelial marker genes, acting as a morphogen in dedifferentiated hepatoma cells. J Cell Biol. 1998;140(4):935–46. https://doi.org/10.1083/jcb.140.4.935.
Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, et al. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48(5):1528–39. https://doi.org/10.1002/hep.22510.
Vidal-Vanaclocha F. The prometastatic microenvironment of the liver. Cancer Microenviron. 2008;1(1):113–29. https://doi.org/10.1007/s12307-008-0011-6.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8. https://doi.org/10.1038/nrc1098.
Calvo F, Ege N, Grande-garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–46. https://doi.org/10.1038/ncb2756.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. https://doi.org/10.1016/j.ccr.2012.01.007.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. https://doi.org/10.1126/science.1171362.
Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19(11):1410–22. https://doi.org/10.1038/nm.3389.
Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–34. https://doi.org/10.1016/j.ccr.2014.04.005.
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–47. https://doi.org/10.1016/j.ccr.2014.04.021.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. https://doi.org/10.1038/nm.3394.
Augustin G, Bruketa T, Korolija D, Milosevic M. Lower incidence of hepatic metastases of colorectal cancer in patients with chronic liver diseases: meta-analysis. Hepato-Gastroenterology. 2013;60(125):1164–8. https://doi.org/10.5754/hge11561.
Fisher ER, Hellstrom HR, Fisher B. Rarity of hepatic metastases in cirrhosis – a misconception. JAMA. 1960;174:366–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, J.Y., Thakar, D., Chang, T.T. (2019). Liver Fibrosis: Current Approaches and Future Directions for Diagnosis and Treatment. In: Willis, M., Yates, C., Schisler, J. (eds) Fibrosis in Disease . Molecular and Translational Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-98143-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-98143-7_15
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-98142-0
Online ISBN: 978-3-319-98143-7
eBook Packages: MedicineMedicine (R0)