Abstract
Wildrice (Zizania spp.) is an annual aquatic grain, occurring naturally in shallow waters of lakes and streams. Zizania palustris is found mainly in the Great Lakes region of the USA and Canada. This species of wildrice has been harvested from natural stands for many centuries (and still is) by certain groups of Native Americans who consider it sacred. It has also been cultivated in paddies since 1950 and is still undergoing domestication as a crop. Two other species are present in North America: Z. aquatica and Z. texana. The former occurs throughout the Great Lakes, St. Lawrence Seaway, Atlantic Coast, and Gulf Coast regions. The latter is endangered, being present only in a small stretch of the San Marcos River in Texas, as well as in several refugia populations. Genetic studies suggest Z. palustris has a strong syntenic relationship to Oryza sativa. Genetic diversity varies widely among and within stands but is generally high, although inbreeding is higher than expected in certain populations. A recently identified potential threat is the toxic effects of sulfide in sediments under certain conditions. Major preservation concerns include declining or disappearing stands due to hydrology issues and shoreland development, difficulty storing seeds either short term or long term, and narrow stratification and seed moisture requirements to break dormancy. There are no accessions currently being conserved in the US National Plant Germplasm System. Development of ex situ storage protocols should continue while pursuing strategic preservation and restoration of natural stands, guided by knowledge of their population genetics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Early voyageurs and settlers who encountered this plant called it “wildrice” because it grew in water, similar to Oryza sativa. This is how it is commonly identified in the marketplace, although it may not be wild and is not strictly rice (Oelke and Porter 2016). In some scientific literature, a single word is used (e.g., Hayes and Stucker 1989) to avoid confusion with wild species of Oryza, a convention that we will use here. Wildrice is an annual aquatic grain, occurring naturally in shallow waters of lakes and streams, primarily of the Great Lakes region of the USA and Canada, but also extending along the St. Lawrence Seaway and along the Eastern Seaboard and Gulf Coast of the USA (Aiken et al. 1988; Terrell et al. 1997). Wildrice tolerates a wide range of water depths (0.05–2.50 m), sediments (clay to peat), and latitudes (30° to 56°N) (Aiken et al. 1988).
Taxonomically, four species are recognized in the genus Zizania. Three are native to North America—Z. aquatica L. (Fig. 3.1), Z. palustris L. (Fig. 3.2), and Z. texana Hitch. (Fig. 3.3); the fourth, Z. latifolia (Griseb.) Turcz. ex Stapf, is native to eastern Asia. Zizania palustris, an annual plant, has larger grains than the other three and is the species that has been harvested in the wild and domesticated as a crop. Two botanical varieties of Z. palustris occur naturally in North American waters: var. palustris and var. interior (Fig. 3.4). Zizania aquatica is also annual, but the grains are of smaller size and are not harvested for food. The two varieties of Z. aquatica are var. aquatica and var. brevis (Fig. 3.5). Zizania texana and Z. latifolia are also small-seeded, but unlike Z. aquatica, they are perennial (Terrell et al. 1997). Cultivated or harvested wildrice that is now known as Z. palustris was often called Z. aquatica in older scientific literature; Fassett (1924) recognized one species, Z. aquatica, with four varieties, and Gleason and Cronquist (1963) continued that convention. Aiken et al. (1988) and Terrell et al. (1997) described the four distinct species that are currently widely accepted. Zizania palustris and Z. aquatica in particular have been distinguished as separate species on the basis of spikelet anatomy (Duvall and Biesboer 1988a), allozymes (Warwick and Aiken 1986), crossability (Duvall and Biesboer 1988b), and plastid DNA restriction sites (Duvall et al. 1993).
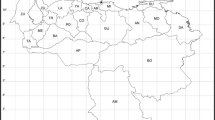
Modeled potential distribution of (a) Zizania palustris L. var. palustris and (b) Z. palustris L. var. interior (Fassett) Dore and Z. texana Hitchc., based on climatic and edaphic similarities with herbarium reference localities. Full methods for generation of maps and data providers are given in Appendix 1
1.1 Recent Cultivation, Domestication, and Breeding
Cultivation in paddies began in northern Minnesota in the early 1950s, with selection for domestication traits following in the late 1960s. The cultivated crop subsequently spread to California, mainly in the Sacramento Valley and Fall River Valley, and on a limited basis in the Willamette Valley of Oregon and along the St. Joe and St. Maries Rivers near Coeur d’Alene, Idaho (Oelke 2007).
In parts of Canada, lakes without wildrice have been seeded with wild-type (shattering) seeds collected from other lakes, and the resulting stands are managed for wildrice production. Canadian lake populations are typically harvested by airboat (Aiken et al. 1988). Wildrice has also been established and grown on a limited basis in New South Wales, Australia, and in eastern Hungary (Oelke 2007).
General reviews of wildrice include Aiken et al. (1988), Oelke (2007), and Oelke and Porter (2016). Domestication of wildrice has been reviewed by de Wet and Oelke (1978) and by Hayes and Stucker (1989). Breeding has been reviewed by Grombacher et al. (1997). Domestication appears to have been initiated by the discovery of qualitative “nonshattering” in a paddy-grown wild population around 1969, permitting the grain to remain on the plant long enough to be harvested in one pass by a combine (Fig. 3.6). Elliott and Perlinger (1977) concluded that the nonshattering phenotype appears to be controlled by two complementary genes. There is quantitative variability for shattering resistance beyond these two or three genes (Everett and Stucker 1983).
Comparative genetic studies have indicated a strong syntenic relationship with Oryza sativa; all ten rice linkage groups are represented in wildrice, with three being duplicated in wildrice (Kennard et al. 2000; Hass et al. 2003). Kennard et al. (2002) found that three QTLs had major effects for shattering, possibly orthologous to the shattering loci in Oryza. Inbred lines have been developed and crossed to produce hybrid varieties, facilitated by a cms-restorer system (Foster 1998; Foster and Zhu 1999). Some hybrid varieties have been grown for commercial production in California. Crosses between specific breeding populations in Minnesota have shown evidence of hybrid vigor for grain yield, indicating possible heterotic groupings (Porter and Kahler 2010).
1.2 Seed Storage Issues
Lack of reliable seed storage has hampered breeding progress, as well as both short- and long-term seed preservation. Wildrice seeds don’t appear to tolerate drying well, although factors such as heterogeneous seed maturity or development of dormancy during desiccation may affect their response to drying. But even if dormancy is broken to remove it as a germination-inhibiting factor (Probert and Longley 1989) and freshly harvested seeds are tested at different developmental stages (Probert and Brierley 1989), seeds still do not tolerate drying.
Although seeds can retain viability for up to 6 months when stored at temperatures as high as 30 °C and seed moistures down to 30% (fwb), stratification is still necessary to break dormancy. Conversely, hydrated seeds can be frozen to −10 °C without damage but still require stratification at temperatures between 0 °C and 10 °C to break dormancy (Kovach and Bradford 1992a). Kovach and Bradford (1992b) found that the reported desiccation intolerance of wildrice can be mitigated by proper control of temperature of dehydration (>25 °C) and temperature and rate of rehydration (10–25 °C over at least 3 weeks). They were able to maintain viability by dehydrating seeds in this way to a seed and embryonic axis moisture content as low as 6–8% (fwb). They conclude the classification of wildrice as recalcitrant is unwarranted. Vertucci et al. (1995) flash dried excised embryos at 35 °C or room temperature to different moisture contents. More mature embryos survived to lower temperatures (−50 °C) than the least mature embryos (−18 °C). They concluded that long-term preservation of wildrice seeds is possible at −20 °C, depending on the maturity of the embryo.
2 Wild Relatives of the Crop
Genetic diversity within and among wild populations of Z. palustris, the source from which the crop was selected, has been of particular interest for research aimed at conservation efforts. Using 13 isozyme markers, Lu et al. (2005) found the overall genetic diversity of 17 Wisconsin populations to be moderate (0.15) compared with other wind-pollinated species but low compared to the mean of Poaceae. Population size and degree of isolation were major factors contributing to genetic variability; gene diversity in turn showed significant positive correlations with several fitness traits that were measured. Gene flow between populations was low. Inbreeding within populations (f) was also low, averaged among the populations studied, but varied greatly, with a high of 0.52, suggesting differences in outcrossing rates, disturbance, and human influence. Kern and Kahler (2011) found higher-than-expected levels of inbreeding in two large wildlife refuge populations in Minnesota, especially compared with other natural populations. They also found greater genetic diversity and less inbreeding in river populations within the refuge than in their respective lake populations. When Kern and Kahler (2014) studied genetic diversity of six separated bays within the St. Louis River estuary, they found two of the sites were genetically differentiated from each other and from the other four, possibly a result of historical reseeding efforts using seed from elsewhere, and different sedimentation and water chemistries.
Biesboer et al. (2014) sought to document genetic diversity using SSR markers in a large study of 70 wild Z. palustris populations across Minnesota. They found a high degree of heterozygosity within wildrice populations, averaging 0.54 with a range of 0.37–0.73. Based on allele frequencies, the populations were grouped into four major clades and ten sub-clades. Genetic distance coefficients (Nei83) ranged from 0.22 to 0.83, indicating a wide amount of genetic variability among populations. Using Wright’s Fixation Index (FST) to compare heterozygosity of each population to the expected total heterozygosity across all populations, they identified six clades.
Counts and Lee (1987, 1988a, b, and 1990) grew wildrice populations from various lakes in Ontario, Canada, together in a common greenhouse or lake environment, to study the responses of a number of morphological and phenological traits to various environmental and cultivation factors. Their results suggested that phenotypic plasticity in wildrice buffers the populations from directional selection pressures. Counts (1993) followed with a study of genetic variability (using isozymes) and phenotypic plasticity among two Z. palustris and four Z. aquatica populations collected along the Atlantic seaboard and grown together in varying greenhouse conditions. She observed no relationship between heterozygosity and degree of phenotypic plasticity of stem size, flowering, and reproductive traits, but Z. palustris populations responded to temperature differences with greater plasticity than Z. aquatica populations.
Because of its endangered status, Z. texana has received research attention aimed at its preservation. Richards et al. (2007) assessed its genetic diversity using microsatellite markers. The larger, demographically stable stands along its 4-km range of the San Marcos River in Texas contained the greatest genetic diversity. Stratified sampling of such stands captured all the microsatellite alleles in fewer individuals, where random sampling did not. The population had a high degree of heterozygosity overall.
2.1 Use of Wild Relatives for Crop Improvement
Anecdotally, wildrice cultivars may trace their origins from few or a single lake population. Wildrice breeders have collected accessions from many natural stands, primarily from Minnesota lakes, to form gene pools as a source of breeding materials (Elliott 1980; Porter et al. 2001), but these have not been a major source of new traits or varieties. Varietal development efforts have relied heavily on recurrent phenotypic selection within already adapted open-pollinated populations, in order to maintain genetic diversity within populations and because of the limited ability to reliably store seeds for several generations. Kahler et al. (2014) used highly polymorphic SSR markers derived from Z. texana (Richards et al. 2004; Kern et al. 2011) to confirm these relationships among advanced breeding populations by constructing a phylogenetic tree based on Nei’s genetic distances; one breeding population appearing to have a closer genetic distance to several natural populations than to the other breeding populations.
Nonshattering phenotypes are occasionally found in Z. palustris stands. Seed size is generally greater in lake populations than in river populations (Eule-Nashoba et al. 2012). Some lake populations are known anecdotally by ricers (hand harvesters) for their greater size. Wild populations vary in many morphological traits but have not been explicitly sought for introgression of traits, because considerable genetic diversity still exists within breeding populations (R. Porter, personal observation).
Other species may have traits of interest, but they have not been extensively utilized. Grombacher et al. (1997) described previously unpublished work in which accessions of Z. aquatica from Florida were crossed successfully with several Z. palustris lines, using Z. aquatica as the female parent (per Duvall and Biesboer 1988b). Reduced dormancy was introgressed into several breeding populations by backcrossing; nondormancy appeared to be dominant and simply inherited (Porter 1998). Grombacher et al. (1997) also suggest that Z. aquatica var. brevis could be a source of short awns, short seeds, short height, and salinity tolerance due to its adaptation to tidal habitats. Z. texana and Z. palustris were crossed successfully by Duvall and Biesboer (1988a), for phylogenetic studies, but not for utilization. In the future, Z. texana could be a source of perenniality, if this were to become a breeding objective.
3 Wild Utilized Species
For wildrice, the wild relatives have a longer history of use than cultivated wildrice. Native Americans continue to harvest the grain from natural stands (Fig. 3.2); their treaty-recognized right to do so both on-reservation and in ceded territories has been upheld by the US Supreme Court (Minnesota v. Mille Lacs 1999). Others can obtain state permits in Minnesota or Wisconsin to harvest the crop from public waters. The Minnesota Department of Natural Resources (Minnesota DNR 2008) estimated that 4,000 to 5,000 individuals participate in wildrice harvesting annually, 3,000 of whom are tribal members. Individual tribal departments of natural resources and inter-band agencies such as the Great Lakes Indian Fish and Wildlife Commission (GLIFWC) study and manage the health of natural stands of wildrice, mostly in Minnesota and Wisconsin. Reservation wildrice committees, as well as the departments of natural resources of key states like Minnesota and Wisconsin, regulate wildrice harvesting and educate the public on the allowed method and harvest season. The Minnesota DNR has frequently assessed and published the stand densities of a number of key lakes. Recent assessments found over 64,000 acres of wildrice stands in Minnesota on 1,200 lakes and rivers (Minnesota DNR 2008).
Some Native American groups have expressed concern that cultivated wildrice pollen flow to natural stands could occur and affect their genetic integrity or even cause a genetic collapse. A comprehensive study to identify threats to natural wildrice concluded that conventional breeding does not pose such a threat, since no novel genes or alleles have been brought into cultivars from outside the natural Zizania gene pool (Minnesota DNR 2008). Also, limited pollen travel studies suggest there is a significant decrease in the amount of gene flow at distances of up to 2 miles from wildrice paddies (Cregan 2004). Therefore, it seems unlikely that any genetic migration from paddies would change the genetic structure of natural stands.
3.1 Archaeological Record of Utilization
McAndrews (1969) estimated wildrice pollen in a Minnesota lake beginning about 1935 years ago. Huber (2000) summarized a number of studies of pollen in Minnesota lake sediments and concluded that wildrice was present in those lakes in the last 10,000 years “in quantities large enough to provide a considerable subsistence component” to the Paleoindian cultures present during that time. Mather and Thompson (2000) reviewed archaeological evidence for the use of wildrice as a food and cited evidence (in the form of wildrice phytoliths) of periods of “intensified use” of wildrice approximately 2000 years before European contact at Mille Lacs Lake in Minnesota. Valppu (2000) also cited evidence of the beginnings of wildrice processing on Big Rice Lake, St. Louis Co., Minnesota, about 2000 years ago.
3.2 Cultural Significance for Native Americans
Wildrice has been harvested by Native Americans from natural stands for centuries, having been recognized as a valuable source of nutrition. It is called manoomin by the Ojibwe (Anishinaabe); considered a sacred grain, it is a very important part of their cultural activities (Vennum 1988). It is still harvested the traditional way: while one person poles a canoe through a stand, another dislodges the ripe grains from the plants by tapping the stems with ricing sticks, allowing the grains to fall into the canoe. The grains are then parched to gelatinize the starch, allowing for long-term storage. It is boiled like rice to be consumed as a whole grain in various ways (Oelke and Porter 2016).
4 Conservation Status of CWR and WUS
Decline and disappearance of historic wildrice stands have been a concern for some time, although natural stands do fluctuate from year to year. One case documented a return of wildrice after at least a 5-year absence, following a major flooding event that resulted in significant sediment disturbance (Dukerschein 2000).
Threats to wildrice were identified and reported under mandate of the Minnesota State Legislature (Minnesota DNR 2008). The primary threats include “changes in local hydrology due to dams and channelization, water-based recreation and shoreland development, and mining and other industrial activities,” but hydrology issues and shoreland development were identified as especially important at the local level. The study also identified the statewide and regional threats of most importance as loss of genetic integrity, invasive species, and climate change.
More recently, the Minnesota Pollution Control Agency was authorized to more closely study the possible impact on wildrice of sulfate-containing effluents from mining or municipal sources (MPCA 2014). Research was funded to investigate the issue through extensive field surveys of wildrice stands and sediments, laboratory hydroponics studies, outdoor container experiments simulating natural conditions, analysis of sediments from the rooting zones of wildrice lakes, and laboratory sediment incubation experiments to observe sulfate movement and conversion to sulfide. Sulfate per se was determined to have minimal effect on wildrice growth but could prove toxic under conditions where it is converted to sulfide. Results of these studies are being used to refine rulemaking about allowable sulfide levels in specific sediment conditions. Data from this comprehensive study (particularly the field survey) should prove useful as a baseline for understanding other factors affecting natural wildrice stands, aiding in conservation efforts.
In the study of threats to wildrice, possible effects of climate change were discussed (Minnesota DNR 2008). Seed set could be reduced if hot, dry conditions coincided with pollination. Carp and invasive plant species could spread into wildrice habitats with warming waters. Warm, humid weather favors certain plant diseases such as Bipolaris spp. that occur naturally in wild populations. Severe weather could damage stands during the more vulnerable floating leaf and seed production stages. The southern edge of the species’ natural range may already be shifting northward.
Zizania texana is listed as endangered (USFWS 1978, 2013). Its range is limited to the upper 2 miles of the San Marcos River in central Texas (Terrell et al. 1978, 1997; Figs. 3.3 and 3.4). Conservation efforts include both in situ preservation and maintaining ex situ refugia populations collected from—and adequately representing the genetic diversity of—the extant San Marcos River population (Wilson et al. 2017). Pollen longevity is short (10–60 min) and is released between 0200 and 0400, limiting sexual reproduction of this perennial species (Power and Oxley 2004); by comparison, pollen longevity in cultivated Z. palustris has been estimated to be less than 2 h after another extrusion (Page and Stucker 1990).
Currently there are no Zizania accessions in the US National Plant Germplasm System. More work is needed to develop reliable protocols for long-term storage of whole seeds (Christina Walters, personal communication). Accessions have been collected directly from public waters at various times by the Minnesota wildrice breeding program. Since short- to medium-term seed storage has been unreliable for plant breeders and other researchers, individual accessions have had to be maintained by being grown out. Those that were not grown out eventually lost seed viability in storage. As another approach to utilization, many were allowed to inter-mate in research paddies each year in a “common garden” approach to maintaining a dynamic germplasm pool.
Conservation efforts should focus on improving ex situ preservation methodology—both short-term and long-term—but also on maintaining or improving in situ population health. Regarding fitness-related traits in natural stands, “Higher levels of genetic variability may translate into improved population persistence for wildrice in natural environments” (Lu et al.). In situ preservation of populations, particularly those that are recognized as declining, should recognize the dynamic nature of this outcrossing species. Seeding new lakes or reseeding declining or disappeared populations has been done by agencies such as GLIFWC, as well as tribal and state DNRs. Restoration efforts may need to take into consideration the need for an adequate population size and the addition of new alleles from other populations in order to reverse inbreeding of isolated stands in particular.
Biesboer et al. (2014) gave recommendations to guide wildrice preservation and restoration. For preservation, priority should be placed on populations that have a high degree of genetic variability as a potential source of seed for restoration of other stands. For restoration, they identified two distinct issues. First, genetic accuracy is the goal, but where populations have disappeared, judgment is needed to determine what might be the closest match. Second, the aim of restoration should be a functional population, perhaps employing a range of genotypes to maximize the likelihood of success. They cite Falk et al. (2001) as providing good principles to guide restoration efforts. Finally, they point out that restoration of the population must be preceded by understanding and correcting the reasons for the decline.
For this iconic North American grain, preservation as both a CWR and WUS is affected by its unique features: its aquatic habitat, its seed storage difficulties, its recent history of domestication, and its cultural importance to Native Americans. All these make its conservation challenging but not impossible.
References
Aiken S, Lee P, Punter D, Steward J (1988) Wild rice in Canada. NC Press, Toronto
Biesboer D, Kahler A, Kern A (2014) Understanding threats, genetic diversity, and conservation options for wild rice. Report to Minnesota Legislature, Environmental and Natural Resources Trust Fund. M.L. 2011, First Special Session, Chp. 2, Art.3, Sec. 2, Subd. 040
Counts R (1993) Phenotypic plasticity and genetic variability in annual Zizania spp. along a latitudinal gradient. Can J Bot 71(1):145–154
Counts R, Lee P (1987) Patterns of variation in Ontario wild rice (Zizania aquatica L.). 1. The influence of some climatic factors on the differentiation of populations. Aquat Bot 28(3):373–392
Counts R, Lee P (1988a) Patterns of variation in Ontario wild rice (Zizania aquatica L.). 2. Differential responses of populations to varying cultivation conditions. Aquat Bot 31(1):23–36
Counts R, Lee P (1988b) Patterns of variation in Ontario wild rice (Zizania aquatica L.). 3. Similarity between source and introduced populations pairs under experimental and field conditions. Aquat Bot 31(1):37–55
Counts R, Lee P (1990) Patterns of variation in Ontario wild rice (Zizania aquatica L.) 4. Influence of regional and local environmental factors on variation within and among field populations. Aquat Bot 36(2):193–205
Cregan J (2004) Aspects of seed storage, pollen travel and population dynamics of wildrice (Zizania palustris). MS Thesis, University of Minnesota
de Wet M, Oelke E (1978) Domestication of American wild rice (Zizania aquatica L, Gramineae). J Agric Tradit Bot Appl 25:67–84
Dukerschein T (2000) Flooding prior to the growing season: a potentially important factor for the growth of wild rice in navigation pool 8, Upper Mississippi River. In: Proceedings of the wild rice research and management conference. Great Lakes Indian Fish and Wildlife Commission, pp 92–106
Duvall M, Biesboer D (1988a) Anatomical distinctions between the pistillate spikelets of the species of wild-rice (Zizania, Poaceae). Am J Bot 75(1):157–159
Duvall M, Biesboer D (1988b) Nonreciprocal hybridization failure in crosses between annual wild rice species (Zizania palustris X Z. aquatica: Poaceae). Syst Bot 13(2):229–234
Duvall M, Peterson P, Terrell E, Christensen A (1993) Phylogeny of North American oryzoid grasses as construed from maps of plastid DNA restriction sites. Am J Bot 80(1):83–88
Elliott W (1980) Wild rice. In: Fehr W, Hadley H (eds) Hybridization of crop plants. American Society of Agronomy, Madison, pp 721–731
Elliott W, Perlinger G (1977) Inheritance of shattering in wild rice. Crop Sci 17:851–853
Eule-Nashoba A, Biesboer D, Newman R (2012) Seed size in lacustrine and riverine populations of wild rice in northern Minnesota and Wisconsin. Botany 90(1):27–33
Everett L, Stucker R (1983) A comparison of the selection methods for reduced shattering in wild rice. Crop Sci 23:956–960
Falk D, Knapp E, Guerrant E. 2001. An introduction to restoration genetics: why is genetic diversity important? Society for Ecological Restoration, U.S. National Park Service Report, pp 17–18
Fassett N (1924) A study of the genus Zizania. Rhodora 26:153–160
Foster K (1998) Hybrid wild rice production utilizing cytoplasmic-genetic male sterility system. U.S. Patent 5,773,680 filed April 28, 1995 and issued June 30, 1998
Foster K, Zhu Z (1999) Hybrid wild rice production utilizing cytoplasmic-genetic male sterility system. U.S. Patent 5,955,648 filed February 12, 1997 and issued September 21, 1999
Gleason H, Cronquist A (1963) Manual of the vascular plants of the northeastern United States and adjacent Canada. Van Nostrand, Princeton
Grombacher A, Porter R, Everett L (1997) Breeding wild rice. Janick J (ed) Plant Breed Rev 14:237–265
Hass B, Pires J, Porter R, Phillips R, Jackson S (2003) Comparative genetics at the gene and chromosome levels between rice (Oryza sativa) and wildrice (Zizania palustris). Theor Appl Genet 107:773–782
Hayes P, Stucker R (1989) The domestication of American wildrice (Zizania palustris, Poaceae). Econ Bot 43(2):203–214
Huber J (2000) Archaeological implications of pollen evidence for wild rice (Zizania aquatica) during the Paleoindian, Archaic, and Woodland periods in Northeast Minnesota. In: Williamson L, Dlutkowski L, McCammon Soltis A (eds) Proceedings of the wild rice research and management conference. Great Lakes Indian Fish and Wildlife Commission, Odanah, pp 40–53
Kahler A, Kern A, Porter R, Phillips R (2014) Maintaining food value of wild rice (Zizania palustris L.) using comparative genomics. In: Tuberosa R, Graner A, Frison E (eds) Genomics of plant genetic resources, vol 2: crop productivity, Food security and nutritional quality. Springer, Dordrecht, pp 233–248
Kennard W, Phillips R, Porter R, Grombacher A (2000) A comparative map of wild rice (Zizania palustris L. 2n=2x=30). Theor Appl Genet 101:677–684
Kennard W, Phillips R, Porter R (2002) Genetic dissection of seed shattering, agronomic, and color traits in American wildrice (Zizania palustris var. interior L.) with a comparative map. Theor Appl Genet 105:1075–1086
Kern A, Kahler A (2011) Wild rice (Zizania palustris L.) genetic diversity at Rice Lake National Wildlife Refuge and Tamarac National Wildlife Refuge. Final technical report for U.S. Fish and Wildlife Service challenge cost share program MOA # 2008CCS-01KF and MOA #2008CCS-04DS
Kern A, Kahler A (2014) Genetic structure and diversity of wild rice in the St. Louis River estuary. Technical report to the Minnesota Department of Natural Resources and Cardno JFNew
Kern A, Kahler A, Phillips R (2011) Using microsatellite markers to characterize the genetic diversity of wild rice in Great Lakes coastal habitats: implications for restoration. Proc Am Water Reso Assoc (Wisconsin Section) 35:62
Kovach D, Bradford K (1992a) Temperature dependence of viability and dormancy of Zizania palustris var. interior seeds stored at high moisture contents. Ann Bot 66:297–301
Kovach D, Bradford K (1992b) Imbibitional damage and desiccation intolerance of wild rice Zizania palustris seeds. J Exp Bot 43(251):747–757
Lu Y, Waller D, David P (2005) Genetic variability is correlated with population size and reproduction in American wild-rice (Zizania palustris var. palustris, Poaceae) populations. Am J Bot 92(6):990–997
Mather D, Thompson R (2000) Archaeological perspectives on wild rice. In: Williamson L, Dlutkowski L, McCammon Soltis A (eds) Proceedings of the wild rice research and management conference. Great Lakes Indian Fish and Wildlife Commission, Odanah, pp 15–26
McAndrews J (1969) Paleobotany of a wild rice lake in Minnesota. Can J Bot 47(11):1671–1679
Minnesota DNR (2008) Natural wild rice in Minnesota: a wild rice study document submitted to the Minnesota Legislature by the Minnesota Department of Natural Resources February 15, 2008
Minnesota v. Mille Lacs Band of Chippewa Indians (1999) 526 U.S. 172
MPCA (2014) Wild rice sulfate study. Minnesota Pollution Control Agency, Saint Paul
Oelke E (2007) Saga of the grain: a tribute to Minnesota cultivated wild rice growers. Hobar, Lakeville
Oelke E, Porter R (2016) Wildrice, Zizania. In: Wrigley C, Corke H, Seetharaman K, Fabioun J (eds) Encyclopedia of food grains. Academic, Oxford, pp 130–139
Page N, Stucker R (1990) An evaluation of factors affecting success of controlled crosses of wild rice (Zizania palustris L.) in the greenhouse. Can J Plant Sci 70:677–681
Porter R, Kahler A (2010) Can population crosses be a means to exploit heterosis in American wildrice? Abstract 184–7. In: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America 2010 International Annual Meetings
Porter R, Kennard W, Phillips R, Oelke E (1998) Seed nondormancy and the domestication of American wildrice. Agron Abstr. p 83
Porter R, Boedigheimer D, Schumer H (2001) Wildrice breeding and germplasm improvement (2000). In: Minnesota wild rice research 1999/2000. University of Minnesota-NCROC, Grand Rapids MN, pp 43–53 Grand Rapids, MN
Power P, Oxley F (2004) Assessment of factors influencing Texas wild-rice (Zizania texana) sexual and asexual reproduction. Final Report prepared for Edwards Aquifer Authority, San Antonio
Probert R, Brierley E (1989) Desiccation intolerance in seeds of Zizania palustris is not related to developmental age or the duration of post-harvest storage. Ann Bot 64:669–674
Probert R, Longley P (1989) Recalcitrant seed storage physiology in three aquatic grasses (Zizania palustris, Spartina anglica and Porteresia coarctata). Ann Bot 63:53–63
Richards C, Reilley A, Touchell D, Antolin M, Walters C (2004) Microsatellite primers for Texas wild rice (Zizania texana), and a prelimary test of the impact of cryogenic storage on the allele frequency at these loci. Conserv Genet 5:853–859
Richards C, Antolin M, Reilley A, Poole J, Walters C (2007) Capturing genetic diversity of wild populations for ex situ conservation: Texas wild rice (Zizania texana) as a model. Genet Resour Crop Evol 54(4):837–848
Terrell E, Emery W, Beaty H (1978) Observations on Zizania texana (Texas wildrice), an endangered species. Bull Torrey Bot Club 105:50–57
Terrell E, Peterson P, Reveal J, Duvall M (1997) Taxonomy of North American species of Zizania (Poaceae). SIDA 17(3):533–549
U.S. Fish and Wildlife Service (USFWS) (1978) Determination that 11 plant taxa are endangered species and 2 plant taxa are threatened species. Fed Regist 43(81):17910–17916
U.S. Fish and Wildlife Service (USFWS), Department of the Interior (2013) Final environmental impact statement and record of decision on the Edwards Aquifer recovery implementation program habitat conservation plan for incidental take of 11 species (8 federally listed) in 8 Texas counties. Fed Regist 78(32):11218
Valppu S (2000) Paleoethnobotanical investigations at the Big Rice site: Laurel culture use of wild rice and associated radiocarbon dates. In: Williamson L, Dlutkowski L, McCammon Soltis A (eds) Proceedings of the wild rice research and management conference. Great Lakes Indian Fish and Wildlife Commission, Odanah, pp 27–39
Vennum T (1988) Wild rice and the Ojibway people. Minnesota Historical Society Press, St. Paul
Vertucci C, Crane J, Porter R, Oelke E (1995) Survival of Zizania embryos in relation to water content, temperature and maturity status. Seed Sci Res 5(01):31–40
Warwick S, Aiken S (1986) Electrophoretic evidence for the recognition of two species in annual wild rice (Zizania, Poaceae). Syst Bot 11(3):464–473
Wilson W, Hutchinson J, Ostrand K (2017) Genetic diversity assessment of in situ and ex situ Texas wild rice (Zizania texana) populations, an endangered plant. Aquat Bot 136:212–219
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Porter, R. (2019). Wildrice (Zizania L.) in North America: Genetic Resources, Conservation, and Use. In: Greene, S., Williams, K., Khoury, C., Kantar, M., Marek, L. (eds) North American Crop Wild Relatives, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-319-97121-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-97121-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97120-9
Online ISBN: 978-3-319-97121-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)