Abstract
Eosinophilic esophagitis (EoE) is an increasingly common esophageal disorder that presents with symptoms similar to those seen in patients with GERD, including dysphagia, heartburn, and atypical chest pain. The diagnosis is confirmed by EGD and esophageal mucosal biopsies demonstrating an elevated number of intraepithelial eosinophils. Treatment strategies include diet modification, oral topical steroid therapy, and proton pump inhibitors. Endoscopic balloon dilation is used to treat esophageal remodeling and structuring that can result from long-standing EoE. There is no role for surgical intervention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Eosinophilic esophagitis (EoE) is a chronic immune/antigen-mediated esophageal disease that presents as esophageal dysfunction in the setting of localized eosinophilic predominant inflammation. The most recent practice guideline from the American College of Gastroenterology defines EoE by the following criteria: symptoms related to esophageal dysfunction and the presence of eosinophil-predominant inflammation isolated to the esophagus that persists after a trial of proton pump inhibitors (PPI) in the absence of a secondary cause of esophageal eosinophilia [1]. Its prevalence has been increasing, due in part to increased awareness of the condition and more frequent diagnosis, but also as a result of a true increase in the incidence of disease. The estimated prevalence of EoE in the United States between 2010 and 2015 was 30.0/100,000 for adults age 18–65 years and 12.8/100,000 for adults over the age of 65 [2].
Diagnosis
History and Physical Exam
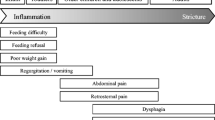
The typical EoE patient is a young or middle-aged male with a history of atopy. Men outnumber women (3:1). The most common presenting symptom of EoE is dysphagia due to esophageal dysfunction or food impaction. These symptoms are more often experienced with solid foods than with liquids, and patients may have a history of avoiding high-consistency foods. Dysphagia in patients with EoE is a result of chronic inflammation, dysmotility, and fibrostenotic remodeling. Additional behavior modifications related to dysphagia are commonly reported, such as eating slowly or needing to swallow multiple times to fully clear the food bolus from the esophagus. Patients may also report heartburn or atypical chest pain.
EoE is increasingly recognized as a manifestation of a food sensitization or allergy. A history of atopy should alert the treating physician to the possibility of EoE. As many as 70% of children and adults with EoE have a history of asthma, allergic rhinitis, and/or atopic dermatitis [3].
The Eosinophilic Esophagitis Activity Index (EEsAI) is a validated, patient-reported survey instrument that characterizes symptom severity and may be used for ongoing evaluation during and after therapy [4]. This survey is based on seven items assessing the frequency and duration of dysphagia, the severity of dysphagia when eating foods of eight different consistencies, and behavioral changes (avoidance, modification, and slow eating of certain foods) as a result of their dysphagia. The EEsAI evaluates these items over the past 24 h, 7 days, and 30 days. The Dysphagia Symptom Questionnaire (DSQ) and the Mayo Dysphagia Questionnaire (MDQ) are alternative survey measures that are used to assess EoE severity, but they are less specific in regard to food consistency and behavioral modifications [5, 6]. While the EEsAI, DSQ, and MDQ are useful in following symptom evolution over time, they are not sufficient to indicate endoscopic or histologic severity or remission, as symptoms and objective findings are inconsistently correlated [7].
Physical examination is generally unremarkable in patients with EoE, though 10% of patients with EoE also have eczema [8]. Despite its relationship with other allergic conditions, allergy testing either by skin prick or serum tests for IgE is not useful and is generally not recommended [9].
Differential Diagnosis
Patients presenting with dysphagia or food impaction should be evaluated for mechanical causes of obstruction such as a neoplasm, esophageal stricture, or epiphrenic diverticula, as well as primary esophageal motility disorders such as achalasia or systemic sclerosis. A complete history and physical can help narrow the differential diagnosis, but additional diagnostic measures are often required to reach a final diagnosis, including upper endoscopy, esophageal biopsies, esophageal manometry, and barium esophagram. Elevated levels of eosinophils found in the esophageal epithelium, the hallmark of eosinophilic esophagitis, may be seen in a variety of other conditions. These include inflammatory bowel diseases, IgE-mediated food allergies, celiac disease, hypereosinophilic syndrome, GERD, infectious diseases, and toxic injury.
Upper Endoscopy
Endoscopy is used to diagnose EoE, monitor disease progression and remission, and guide therapy. EoE manifests a variety of endoscopic findings, including mucosal edema, esophageal rings (also known as trachealization or corrugation), furrows (also known as felinization), exudates or plaques, luminal narrowing, and mucosal fragility during endoscopic evaluation (Fig. 8.1) [1]. Due to the wide range of endoscopic features and the variability in endoscopists’ evaluation and descriptive terminology, a classification and scoring system for EoE was developed and validated in 2013 [10, 11]. This score, known as the EoE Endoscopic Reference Score (EREFS) system, encompasses the five primary endoscopic findings of EoE: edema, rings, exudate, furrows, and strictures [12]. Each of these parameters is given a grade and used to generate a score (Table 8.1). A score of 2.0 or greater has an 88% sensitivity and 92% specificity for diagnosing EoE [13].
(a) Longitudinal furrows (arrow) and exudates (dashed arrow) are visible throughout the esophagus; (b) fixed concentric rings (arrows) are seen down the length of the esophagus. (Images courtesy of Dr. Anurag Soni, Department of Medicine, Division of Gastroenterology and Hepatology, University of Wisconsin)
A history suggestive of EoE and supporting endoscopic findings must be confirmed by mucosal biopsies demonstrating eosinophil-predominant inflammation. Biopsies should be taken at the time of endoscopy from at least two different locations in the esophagus, usually in the proximal and distal halves of the esophagus. This point is especially important because while patients with GERD may have esophageal eosinophilia located in the distal esophagus, patients with EoE will have diffuse eosinophilia throughout the esophagus. It is recommended to obtain multiple biopsies due to the patchy and heterogeneous nature of EoE. Though the number of biopsies needed is debatable, diagnostic sensitivity can approach 100% with 6–9 biopsies [14, 15].
Other Imaging and Diagnostic Tools
Barium esophagram may be useful to detect esophageal narrowing not appreciated on endoscopy. Compared to barium esophagram, endoscopy has a sensitivity of only 14.7% and a specificity of 79.2% for detecting esophageal narrowing [16]. Its use may be reserved for patients presenting with persistent dysphagia and normal endoscopic findings. Endoscopic and endoluminal ultrasonography may detect esophageal mural thickening, though this has been used primarily in studies examining steroid effectiveness [17, 18].
Patients with EoE may have decreased esophageal distensibility and abnormal motility, which can be detected with esophageal manometry or by using a functional luminal imaging probe (FLIP). Documented derangements of esophageal motility include hypertensive or weak peristaltic function and poor esophageal shortening upon swallowing [18]. Distensibility measured by FLIP is significantly decreased throughout the length of the esophagus and at the gastroesophageal junction in patients with EoE [19, 20].
Histology
The primary histologic finding of EoE on histology is an elevated number of intraepithelial eosinophils (Fig. 8.2). Most diagnostic criteria use a threshold of at least 15 eosinophils per high-powered field [1, 21]. Eosinophils may be clustered in microabscesses (aggregates of four or more eosinophils) and are often located at or near the epithelial surface, a phenomenon called “surface layering.” The basal layer of the epithelium may be thickened to a significant degree, comprising nearly the entire epithelium. Intracellular edema can be significant, making intercellular bridges that are normally invisible to light microscopy readily apparent. The normally thin and loose connective tissue of the lamina propria can become thick and dense with collagen fibers. Eosinophils and other inflammatory cells may also be seen in the lamina propria, though these do not contribute to the intraepithelial eosinophil count used to make the diagnosis of EoE [21].
(a) Esophageal biopsy showing basal cell hyperplasia (solid arrow), intercellular edema or spongiosis (arrowhead), and marked increase in intraepithelial eosinophils with eosinophilic microabscesses (dashed arrow); (b) increased intraepithelial eosinophils (solid arrow) and eosinophilic microabscesses (dashed arrow) at greater magnification. (Images courtesy of Dr. Rao Watson, Department of Pathology and Laboratory Medicine, University of Wisconsin)
Distinction from GERD
The presentation of EoE can be very similar to that of gastroesophageal reflux disease (GERD). EoE was initially believed to be a marker for GERD [22, 23]. Both can be characterized by heartburn, chest pain, or dysphagia. The most commonly reported symptom in EoE is dysphagia with food impaction, whereas patients with GERD more frequently complain of heartburn and regurgitation. It is important to differentiate EoE patients from patients with GERD because their treatments differ substantially.
Attwood et al. first described EoE as being separate from GERD in 1993 in a case series of patients who presented with dysphagia but whose endoscopic images and pH monitoring demonstrated no evidence of acid reflux. Esophageal biopsies of these patients showed elevated eosinophils in the esophageal epithelium compared to a control cohort of patients known to have GERD (56 eosinophils/HPF in patients with EoE vs. 3.3 eosinophils/HPF in patients with GERD) [24]. The following year, Straumann et al. termed this “idiopathic eosinophilic esophagitis” [25]. The endoscopic findings of EoE are typically evenly distributed across the esophagus and are characteristically different than those of GERD, which are localized to the distal esophagus.
Treatment
Treatment of EoE is directed at symptom reduction, remission of endoscopic and histologic disease manifestations, and prevention of long-term sequelae (e.g., strictures, luminal narrowing). Several therapeutic options are available, which may be used alone or in combination.
Diet Modification
Diet modification may be attempted to alleviate symptoms and reverse esophageal fibrostenosis. Accepted regimens include elemental diets, allergy testing-directed elimination diets, and empiric food elimination. Because elemental diets are poorly tolerated, and the predictive value of allergy testing is limited, empiric food elimination has become the preferred method. The most popular regimen is the six-food elimination diet, removing the six most common food allergens (milk protein, wheat, eggs, soy, peanuts/tree nuts, and seafood) for 6 weeks (the induction phase). Following this period, foods are sequentially reintroduced with repeated endoscopies to monitor for disease recurrence (the reintroduction phase). Once the food trigger is identified, patients are counseled to continue avoiding it in their diets (the maintenance phase).
Prospective randomized clinical trials have demonstrated that the six-food elimination diet decreases the level of eosinophilia in 65–75% of patients and decreases symptom scores by up to 94% [26, 27]. Milk protein and wheat are the most frequently identified food triggers. This approach is especially useful for patients seeking non-pharmacologic treatments, though the need for frequent endoscopies and their associated cost are notable drawbacks. Alternatives to the six-food elimination diet are the four-food elimination diet (eliminating milk protein, wheat, eggs, and soy) and empiric elimination of cow’s milk alone. These have the advantage of being less restrictive, and they can identify the food trigger faster. Most patients who fail can be rescued with the full six-food elimination diet [28].
Medications
Swallowed topical steroids are used as first-line therapy. Budesonide and fluticasone are most commonly prescribed. In a prospective randomized controlled trial of 36 patients, treatment with a 15-day course of budesonide decreased eosinophilia (47.8–17.7 eosinophils/HPF), induced full histologic remission in 72%, and significantly decreased reported symptoms [29]. These results were durable out to 50 weeks, and long-term therapy showed a trend toward normalization of any evidence of esophageal remodeling prior to initiation [17]. Fluticasone has been shown to induce complete histologic remission in 65–68% of participants, but does not cause a significant reduction in reported symptoms [30, 31]. Cessation of either therapy results in relapse in nearly all patients, and thus patients should continue topical steroids as maintenance therapy. Esophageal candidiasis is a potential adverse outcome of topical steroids (found in up to 30% of patients), though this is often asymptomatic and detected on endoscopy alone. Because EoE is localized to the esophagus, systemic steroids are reserved for patients with severe symptoms and in need of rapid therapy [9].
Proton pump inhibitors were historically the first-line therapy for EoE. Initially, it was believed that patients who had symptom relief with PPI therapy had GERD and those who saw no benefit had true EoE. However, there is a growing awareness of a subset of patients with esophageal eosinophilia whose symptoms respond to PPI, but have no evidence of GERD. This condition has been termed PPI-responsive esophageal eosinophilia (PPI-REE) [32]. Three mechanisms of action for this effect have been proposed. The first is that PPIs themselves have anti-inflammatory properties and can reduce eosinophil migration into the esophageal epithelium [33]. The second is that patients with PPI-REE have improved epithelial barrier function after receiving PPI therapy, preventing potential food allergens from crossing the mucosal layer [34]. A final proposed mechanism is that some patients with EoE may also have a component of acid reflux that responds to PPI therapy. Regardless of mechanism, it is reasonable to initiate PPI therapy for patients with EoE as a first step, reserving topical steroid therapy for those who do not respond.
Endoscopic Therapy
In addition to its role in diagnosis and disease surveillance, endoscopy has important therapeutic uses. More than 70% of patients with EoE have evidence of decreased esophageal distensibility. Long-standing EoE can result in esophageal remodeling leading to strictures, [35] which are identified in 30–80% of adults with EoE. The risk of each of these changes increases with disease duration [18]. Endoscopic balloon dilation is an effective treatment of these complications and the resultant dysphagia. In a retrospective study of 10 patients with steroid-refractory EoE, all patients improved their dysphagia scores after 1–5 dilation sessions [36]. Another study of 207 patients found that esophageal dilation increased esophageal diameter between 5 and 7 mm. This correlated with a significant improvement in dysphagia symptoms in 93% of patients, with a median follow-up of 17 months [37]. More than half of patients require more than one dilation session to achieve success [38]. The best predictor of success is the esophageal caliber achieved at the end of dilation therapy. Despite initial safety concerns raised due to the mucosal fragility seen in EoE, complication rates are similar to those undergoing esophageal dilation for other causes [39].
A subset of patients with EoE have a diffusely stenotic, extremely narrow-caliber esophagus. These patients are typically older and have had a longer symptom duration. They are often resistant to steroid therapy and require multiple dilations to achieve symptom relief [40].
Conclusion
EoE is an increasingly common disease whose hallmark symptoms overlap with GERD. Surgeons who perform endoscopy may be involved in its diagnosis and endoscopic treatment of complications resulting from long-standing EoE. There is no role for surgical intervention in the management of EoE.
References
Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92; quiz 693
Mansoor E, Cooper GS. The 2010-2015 prevalence of eosinophilic esophagitis in the USA: a population-based study. Dig Dis Sci. 2016;61:2928–34.
Hirano I. 2015 David Y. Graham lecture: the first two decades of eosinophilic esophagitis-from acid reflux to food allergy. Am J Gastroenterol. 2016;111:770–6.
Schoepfer AM, Straumann A, Panczak R, Coslovsky M, Kuehni CE, Maurer E, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–66. e21
Dellon ES, Irani A-M, Hill MR, Hirano I. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634–42.
Grudell ABM, Alexander JA, Enders FB, Pacifico R, Fredericksen M, Wise JL, et al. Validation of the Mayo dysphagia questionnaire. Dis Esophagus. 2007;20:202–5.
Safroneeva E, Straumann A, Coslovsky M, Zwahlen M, Kuehni CE, Panczak R, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology. 2016;150:581–90. e4
Miehlke S. Clinical features of eosinophilic esophagitis. Dig Dis. 2014;32:61–7.
Richter JE. Current Management of Eosinophilic Esophagitis 2015. J Clin Gastroenterol. 2016;50:99–110.
Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–96. e5
Peery AF, Cao H, Dominik R, Shaheen NJ, Dellon ES. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:475–80.
Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95.
Dellon ES, Cotton CC, Gebhart JH, Higgins LL, Beitia R, Woosley JT, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol. 2016;14:31–9.
Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130:798–800.
Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–9.
Gentile N, Katzka D, Ravi K, Trenkner S, Enders F, Killian J, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40:1333–40.
Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9. e1
Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin N Am. 2014;43:297–316.
Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90.
Nicodème F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–7. e1
Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. viii – ix
Winter HS, Madara JL, Stafford RJ, Grand RJ, Quinlan JE, Goldman H. Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology. 1982;83:818–23.
Brown LF, Goldman H, Antonioli DA. Intraepithelial eosinophils in endoscopic biopsies of adults with reflux esophagitis. Am J Surg Pathol. 1984;8:899–905.
Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16.
Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124:1419–29.
Gonsalves N, Yang G-Y, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–9.e1; quiz e14–5
Lucendo AJ, Arias Á, González-Cervera J, Yagüe-Compadre JL, Guagnozzi D, Angueira T, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804.
Molina-Infante J, Arias A, Barrio J, Rodríguez-Sánchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol. 2014;134:1093–9. e1
Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37, 1537.e1.
Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147:324–33. e5
Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:742–9. e1
Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–7.
Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32.
Van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RMJGJ, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1815–23.e2.
Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85.e4.
Schoepfer AM, Gschossmann J, Scheurer U, Seibold F, Straumann A. Esophageal strictures in adult eosinophilic esophagitis: dilation is an effective and safe alternative after failure of topical corticosteroids. Endoscopy. 2008;40:161–4.
Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon H-U, Straumann A, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70.
Runge TM, Eluri S, Cotton CC, Burk CM, Woosley JT, Shaheen NJ, et al. Outcomes of esophageal dilation in eosinophilic esophagitis: safety, efficacy, and persistence of the Fibrostenotic phenotype. Am J Gastroenterol. 2016;111:206–13.
Jacobs JW, Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci. 2010;55:1512–5.
Eluri S, Runge TM, Cotton CC, Burk CM, Wolf WA, Woosley JT, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc. 2016;83:1142–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 SAGES
About this chapter
Cite this chapter
Gunter, R.L., Funk, L.M. (2019). Identifying Patients with Eosinophilic Esophagitis. In: Grams, J., Perry, K., Tavakkoli, A. (eds) The SAGES Manual of Foregut Surgery . Springer, Cham. https://doi.org/10.1007/978-3-319-96122-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-96122-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96121-7
Online ISBN: 978-3-319-96122-4
eBook Packages: MedicineMedicine (R0)