Abstract
The history of surgery for epilepsy is dominated by the treatment of temporal lobe epilepsy (TLE). The studies of Penfield in 1936 [1] on cortical excisions and Penfield and Jaspers in 1954 [2] on the functional anatomy of the temporal lobe are historical landmarks in this field, followed by the development of the temporal lobectomy by Falconer in 1953 [3, 4], the amygdalo-hippocampectomy by Niemeyer in 1958 [5] and, more recently, the microsurgical selective amygdalo-hippocampectomy by Yasargil in 1982 [6–8].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Temporal lobectomy
- Amygdalo-Hippocampectomy
- Amygdalo-Hippocampotomy
- Temporal lobe epilepsy
- Epilepsy surgery
The history of surgery for epilepsy is dominated by the treatment of temporal lobe epilepsy (TLE) . The studies of Penfield in 1936 [1] on cortical excisions and Penfield and Jaspers in 1954 [2] on the functional anatomy of the temporal lobe are historical landmarks in this field, followed by the development of the temporal lobectomy by Falconer in 1953 [3, 4], the amygdalo-hippocampectomy by Niemeyer in 1958 [5] and, more recently, the microsurgical selective amygdalo-hippocampectomy by Yasargil in 1982 [6,7,8].
It is widely accepted that 5%–6% of all the pharmacoresistent epilepsies are susceptible to surgical treatment and 70%–75% out of these have limbic mesial or medial TLE frequently with mesial temporal lobe sclerosis; this means that all over the world 20–25 new cases per million habitants per year have TLE with surgical indications [9,10,11]. That is why the mesial temporal lobe (MTL) is by far the main and most frequent target for the surgical treatment of epilepsy. It is also the best target because its surgical removal or inactivation is the procedure with the highest chance of controlling the refractory mesial TLE. After the MTL resection, epileptogenic conditions like mesial temporal lobe sclerosis (MTLS) , hippocampal ganglioglioma , or dysembryoplasic neuroectodermic tumors (DNETs) have long-term epilepsy control rates higher than 80% in most centers (Table 14.1) [12].
To achieve such therapeutic success, the surgical procedure must be safe, complete, and adequate, which means it must be uneventful, comprehend the main MTL structures, and follow the most appropriate approach route. It is worth noting that the hippocampus excision is often restricted to its anterior part on the dominant hemisphere in order to avoid resulting serious verbal memory deficits.
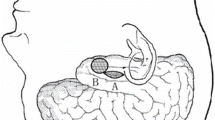
The MTL structures involved in epilepsy [13,14,15,16,17] are the hippocampus proper or Cornu Ammonis with the dentate gyrus and fimbria, the parahippocampal gyrus with the entorhinal cortex, and the amygdala or amygdaloid nucleus within the uncus (Figs. 14.1 and 14.2). As the amygdala continues medially and upward to the pallidum with no clear-cut separation, its removal is usually confined to the ventrolateral part (generally lateral to a straight line drawn between the inferior choroidal point and the M2 segment of the middle cerebral artery). This usually includes the dissection of the uncus, which sometimes falls behind the tentorial notch.
There are several surgical strategies to treat temporal lobe epilepsy. We must distinguish the selective MTL approaches from the enlarged ones; the latter usually involve a temporal lobectomy or at least the removal of the anterior part of the temporal lobe, including the temporal pole or temporal polectomy. In the selective approaches the extent of the hippocampus resection may vary [18, 19], but it usually includes all the MTL structures; the selective cortico-amygdalectomy has been shown to result in less seizure control [20, 21].
The temporal lobectom y is mainly indicated for the neocortical temporal epilepsy and for the mixed origin epilepsy, although in the majority of cases of purely temporal medial epilepsy its practice depends essentially on the operative tradition of each center or even on the preference of the neurosurgeon. For some surgeons it is still regularly performed, whereas for others it is only done when it is suspected that the epileptic zone extends to the temporal neocortex and both the temporal pole and the lateral or the inferior temporal lobe cortex. Therefore when an extensive temporal neocortical epileptic focus or lesion is verified, there is a formal indication to perform a temporal lobectomy. We generally use the operative technique of Falconer [3, 4] with good results (Fig. 14.3).
The extension of the temporal lobectomy differs according to the cerebral hemisphere involved: if it is the language-dominant hemisphere, it should be more restricted posteriorly to avoid the Wernicke area that comprehends the posterior part of the superior temporal gyrus (up to 4.5 cm from the temporal pole instead of up to 5.5 cm on the nondominant side). In case of doubt, the functional MRI helps to localize the Wernicke area. In addition, the sodium amytal carotid Wada test may be useful to verify which cortical zone is transitorily inactivated; if necessary, the language may be tested by intraoperative electric stimulation with the patient awake.
The removal or sectioning of the MTL structures is represented in the Fig. 14.4. There are different selective approach routes to the MTL [22,23,24]. The following should be considered (Fig. 14.5): the superior approach, through the sylvian fissure or along its margins; the anterior one, by the rostral part of the sylvian fissure and the limen insulae; the lateral, through the temporal lobe convexity; and the inferior, underneath the temporal lobe. The posterior interhemispheric supratentorial or transtentorial routes provide a more difficult access to the anterior TML structures, which mainly concern the most caudal hippocampal formation [25, 26].
Several anatomic studies made in vitro at the Anatomy Laboratory of the Faculty of Medicine of the University of Lisbon (FMUL) with microdissection of a large number of human brains [22] provided data concerning distances and dimensions of the MTL structures that are useful to consider for the different surgical procedures (Table 14.2). The mean length of the normal human hippocampus is 4 cm, and its maximum width is 1.5 cm at its head and 1 cm in its body; the distance from the temporal horn of the ventricle to the temporal pole is around 3 cm; to the rhinal sulcus, including the entorhinal cortex, the distance is 1.5 cm (see Fig. 14.2). The photograph registration of the main operative steps illustrates the most significant features of each approach route, as can be seen in the figures. Furthermore, in vivo observations obtained during surgery of many epileptic patients has led to some relevant conclusions concerning the comparative evaluation of advantages and disadvantages among such approach routes.
The superior approach (access from above) to the MTL includes the subpial anterior trans-T1 (superior temporal gyrus) route of Olivier and the transylvian route of Yasargil [6,7,8, 21, 27]. Both use a frontotemporal curved (or straight vertical temporal) skin incision and a pterional bone flap (or a well-centered keyhole craniotomy), with the head turned 45 to 60 degrees to the other side. The trans-T1 Olivier route [21, 27] goes through peeling or removing (in variable amounts) the anterior part of T1 along the inferior edge of the sylvian fissure; it provides an almost direct access to the amygdala and allows a longitudinal exposure of the hippocampus from ahead (it is worth noting that Olivier changed from his initial entry route to a trans-sulcal T1–2 and more recently to a small anterior trans-T2 one [21]). The Yasargil route [6,7,8] goes through the opening of the middle part of the sylvian fissure and the microdissection between the temporal branches of the middle cerebral artery; it requires the smallest brain tissue (temporal stem) transection to reach the hippocampus (Table 14.2) that is approached more perpendicularly to its main axis. It provides a limited manoeuvering space for the surgical instruments between the sylvian vessels and within the ventricle, which may be difficult for the less experienced neurosurgeon (it may not be easy to move a standard ultrasonic aspirator handpiece through this route). The anterior route used by Schramm [18, 19] is a rostral variant of the previous two, as it goes through the anterior curved part of the sylvian fissure that must be opened and the limen insulae that is sectioned laterally. This approach avoids cutting the temporal stem to a large extent but provides a limited angle view to the hippocampal tail.
The lateral approaches comprehend the trans-sulcal T1–2 (or T2–3) routes (probably the most commonly used) and the trans-T2 through the middle temporal gyrus, the widest gyrus of this lobe. This trans-T2 route [5, 21] is the oldest one (Niemeyer) used to reach the hippocampus, still based on an older principle that it was safer to cross the gyrus than to penetrate the “forest” of the sulci vessels; conversely, with the development of microsurgery and other technical refinements, the cerebral sulci became like “highways” that drive the dissection deeper in the attempt to avoid cutting so much brain tissue. These lateral approach routes use a typical curved (or straight vertical) temporal skin incision and a temporal bone flap (or a keyhole flap), with the head in a pure lateral horizontal position, sometimes with a slight posterior tilt. The trans-sulcal approach (Rougier), when performed through the anterior part of the T1–2 sulcus [28] where there is often more space free of important veins, is actually among the shortest and easiest ways to obtain wide access to the lateral ventricle (see distances in Table 14.2).
The inferior, subtemporal approach routes may be performed through the fusiform gyrus [29] or through the collateral sulcus and the parahippocampal gyrus [30]. These subtemporal routes use a temporal curved (or square) skin incision and a low temporal craniotomy, with the head turned laterally and tilted posteriorly toward the surgeon. Even with this head inclination, some degree of temporal lobe retraction from the middle cranial fossa floor is required; this may be hazardous to some major bridging veins draining to the lateral sinus like the Labbé vein.
All these approach routes are much easier to perform with the help of a neuronavigation system (Fig. 14.6). This allows centering of the skin incision and the craniotomy, pointing accurately at the target, and most important a choice of the best route to go through. This can be verified during the operation, provided no excessive brain shift occurs.
The imaging results of the amygdalo-hippocampectomy are well seen on postoperative MRI (Fig. 14.7): in this example, obtained in a left MTL of a right-handed patient, we can see the void resulting from the removal of the anterior half of the hippocampus and the ventral-lateral amygdala.
When evaluating the results and the morbidity of these operations and their mechanisms [12, 31,32,33,34,35,36], both common and specific features must be looked at. Whichever approach route is used, the MTL resection may result in some common deficits and disorders, which are essentially dependent on the removal of the hippocampus and the amygdala; these mainly concern memory deficits and depression. The memory deficits [21, 34, 35, 37] are dependent on the side of the hippocampus operated on. The verbal memory is mostly affected when the dominant hemisphere for language is involved, usually the left one. That is why by precaution the hippocampal resection on the dominant hemisphere is often restricted to its anterior half to two thirds; this is also why in some cases a selective (cortico)amygdalectomy is performed without a hippocampectomy to avoid additional memory deficits [20, 21]. Indeed, the Wada test for memory with perfusion of the posterior cerebral artery is seldom done to check the effect of the selective inactivation of the hippocampus, and there is not yet an adequate paradigm to selectively label the hippocampus in functional MRI [38]. The depression after the amygdalo-hippocampectomy is a distinct problem because it is mainly a late consequence, and it is not clear whether the left cerebral hemisphere plays a major decisive role in this occurrence [39, 40].
A specific morbidity related to the variable approach routes to the MTL is the optic field defect caused by the sectioning of the temporal stem that includes a segment of the Meyer loop of the optic radiations [41,42,43,44]. This is most likely to occur in the superior trans-sylvian and lateral routes because their approach angles are more transverse and require a wider opening of the lateral ventricle; the more anterior the approach to the ventricle, the less the optic radiation is severed. Therefore the rostral and the inferior trans-parahippocampal routes tend to spare these radiations the most. Nevertheless, the resultant optic field defect is often not clinically significant, even after a wide ventricle opening if it is very anterior.
Whatever approach route is chosen, another most sensible part of these operations is related to the subarachnoidal dissection to complete the MTL removal on the medial side. Such dissection is performed through the choroidal fissure between the plexus and the hippocampus to avoid damaging the brain tissue above. This subarachnoidal space contains many important structures (Fig. 14.8) [16, 21, 45,46,47,48,49]: the basal vein of Rosenthal, the posterior cerebral and the anterior choroidal arteries, more rostrally the third cranial nerve, superiorly the optic tract, and deeper the midbrain. All these structures must be absolutely spared!
In an attempt to avoid damaging such intra-arachnoidal structures in those cases where there is no expansive lesion, we have lately developed an alternative technique to the amygdalo-hippocampectomy , the amygdalo-hippocampotomy (Figs. 14.8 and 14.9). With this technique, instead of removing the hippocampus it is disconnected while still removing the lateral amygdala. The principle is the same as that applied to treat other types of epilepsy by disconnecting the epileptogenic brain with the same clinical results as resecting it; well-known examples are the hemispherotomies of Delalande [50], Villemure [51], or Schramm [52], performed instead of the classic hemispherectomy; the temporal lobotomy of Benabid et al. [53] instead of the temporal lobectomy; and the focal disconnections of Ng [54] and Mohamed [55]. The amygdalo-hippocampotomy is a safer operation because the choroidal fissure and the structures inside it are not dissected; the hippocampus is completely separated (from inside the ventricle until the pia mater) around its body and head, and its tail is cut as far as desirable. This way the surgery becomes both easier and somewhat shorter in time. The good results obtained after the first 20 patients operated on with more than 2 years of follow-up are clinically equivalent to the last 100 cases previously operated on with complete MTL removal [56].
A final note to be kept in mind by all neurosurgeons involved in this type of surgery: The key to the success of the surgical treatment of the TLE is to tailor the best operative strategy to each patient; to achieve this, one must be familiar with a variety of surgical approach routes and their relative advantages. It is crucial that every surgeon become acquainted and skilled with one technique that is efficient, secure, and reliable.
(a–d), Left medial temporal and neighboring structures: medial view in different angles, from rostral to caudal. CF – Choroidal fissure; CS – Collateral sulcus; DG – Dentate gyrus; Ent – Entorhinal cortex; F – Fimbria; Fus – Fusiform gyrus; LG – Lateral geniculated body; Mid – Midbrain; MD – Mamillary body; OC – Optic chiasm; OT – Optic tract; PG – Para-hippocampal gyrus; Pulv – Pulvinar; Rh S – Rhinal sulcus; TP – Temporal pole; Un – Uncus
Selective surgery of the hippocampus : in dotted lines with arrows, the peri-hippocampal dissection; in oval, shaded, pink area with dotted limits, the projection of the amygdala. 1 – Head of the hippocampus; 2 – Body of the hippocampus; 3 – Tail of the hippocampus; 4 – Fimbria; 5 – Para-hippocampus; 6 – Posterior cerebral artery; 7 – Basal vein (Rosenthal)
Different approach routes to the medial temporal lobe (for the names of the authors, see text and references). F 3 – Inferior frontal gyrus; T1, 2, 3 – Superior, middle, inferior temporal gyri; Chor Fis – Choroidal fissure; Col Sulc – Collateral sulcus; DG – Dentate gyrus; F – Fimbria; Fus – Fusiform gyrus; HIP – Hippocampus; Hip sulc – Hippocampal sulcus; MCA – Middle cerebral artery; Parahip – Para-hippocampal gyrus; SV – Sylvian vein
Transventricular features of the mesial temporal region microdissection. Left: with the hippocampus in situ; Right: after the hippocampus removal. 1 – Fimbria; 2 – Hippocampus head; 3 – Hippocampus body; 4 – Choroidal plexus; 5 – Choroidal fissure; 6 – Optic tract; 7 – Basal vein; 8 – Posterior cerebral artery; 9 – Basilar artery; 10 –Oculomotor nerve
References
Penfield W. Epilepsy and surgical therapy. Arch Neurol Psychiatr. 1936;36:449–84.
Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. London: J & A Churchill Ltd; 1954.
Falconer MA, Pond DA, Meyer A, Woolf AL. Temporal lobe epilepsy with personality and behaviour disorders caused by an unusual calcifying lesion. J Neurol Neurosurg Psychiatry. 1953;16:234–44.
Falconer MA, Hill D, Meyer A, Mitchell W, Pond DA. Treatment of temporal lobe epilepsy by temporal lobectomy: survey of findings and results. Lancet. 1955;1:827–35.
Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M, Bailey P, editors. Temporal lobe epilepsy. Springfield, IL: Charles C Thomas; 1958. p. 461–82.
Wieser HG, Yasargil MG. Selective amygdalo-hippocampectomy as a surgical treatment of medio-basal limbic epilepsy. Surg Neurol. 1982;17:445–7.
Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy: operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93–123.
Yasargil MG, Wieser HG, Valavanis A, von Amon K, Roth P. Surgery and results of selective amygdalo-hippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin North Am. 1993;4:243–61.
Luders HO. Mesial temporal sclerosis. In: Luders HO, editor. Epilepsy surgery. London: Informa UK Ld; 2008. p. 249–51.
Wiebe S, Blume W, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8.
Wieser HG. ILAE commission report: mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714.
Jehi L. Mesial temporal lobectomy: post-surgical seizure frequency. In: Luders HO, editor. Epilepsy surgery. London: Informa UK Ld; 2008. p. 1223–35.
Duvernoy HM. The human hippocampus. 3rd ed. Berlin: Springer; 2005.
Lopes da Silva F, Witter MP, Boeijinga PH, AHM L. Anatomic organization and physiology of the limbic cortex. Physiol Rev. 1990;70:453–511.
Sindou M, Guenot M. Surgical anatomy of the temporal lobe for epilepsy surgery. Adv Tech Stand Neurosurg. 2003;28:315–43.
Ulm AJ III, Tanriover N, Rothon AL, Roper S. In: Starr PA, Barbaro NM, Larson PS, editors. Surgical anatomy of the temporal lobe. New York: Thieme; 2009. p. 16–28.
Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–9.
Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296–307.
Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509–16.
Kim HI, Olivier A, Jones-Gotman M, Primrose D, Andemann F. Corticoamygdalectomy in memory-impaired patients. Stereotact Funct Neurosurg. 1992;58:162–7.
Olivier A. Surgery of temporal lobe epilepsy: cortico-amygdalohippocampectomy, transcortical selective amygdalohippocampectomy. In: Olivier A, Bolling WW, Tanriverdi T, editors. Techniques in epilepsy surgery: the MNI approach. New York: Cambridge University Press; 2012. p. 97–131. (Ch. 9–10).
Gonçalves-Ferreira A, Miguéns J, Farias JP, Levy-Melancia J, Andrade M. Selective amygdalo-hippocampectomy: which route is the best? Stereotact Funct Neurosurg. 1994;63:182–91.
Campero A, Tróccoli G, Martins C, Fernandez-Miranda JC, Yasuda A, Rhoton AL Jr. Microsurgical approaches to the medial temporal region: an anatomical study. Neurosurgery. 2006;59:279–308.
Ojeman JG, Silbergeld DL. Approaches to epilepsy surgery. Neurosurg Clin North Am. 1993;4:183–91.
Oliveira JG, Parragua RG, Chaddad-Neto F, Ribas GC, Oliveira GPL. Supracerebellar transtentorial approach: resection of the tentorium instead of an opening – to provide broad exposure of the mediobasal temporal lobe: anatomical aspects and surgical applications. J Neurosurg. 2012;116:764–72.
Ture U, Harput MV, Kaya AH, Baimedi P, Firat Z, Ture H, et al. The paramedian supracerebellar-transtentorial approach to the entire length of the mediobasal temporal region: an anatomical and clinical study. J Neurosurg. 2012;20:1–19.
Olivier A. Commentary on cortical resections. In: Engel Jr J, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1987. p. 405–16.
Rougier A, Saint-Hilaire J, Loiseau P, Bouvier G, Baulac M, Bouthillier A, et al. Evaluation and surgical treatment of the epilepsies. Neurochirurgie. 1992;38(suppl.1):3–112.
Hori T, Tabuchi S, Kurisaki M, Kondo S, Takenobu A, Watanabe T. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33:50–6.
Park TS, Bourgeois BF, Silbergeld DL, Dodson WE. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. J Neurosurg. 1996;85:1172–6.
Behrens E, Schramm J, Zentner J, Konig R. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1–10.
Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal sclerosis. J Neurosurg. 2006;104:513–24.
Nayel MH, Awad IA, Luders HL. Extent of mesiobasal resection determines output after temporal lobectomy for intractable partial seizures. Neurosurgery. 1991;29:55–61.
Pimentel J, Bentes C, Campos A, Gonçalves-Ferreira A. Long-term and late seizure outcome after surgery for temporal lobe epilepsy. Epileptic Disord. 2010;12:54–8.
Rydenhag B, Silander HC. Complications of epileptic surgery after 654 procedures in Sweden, September 1990-1995: a multicentric study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49:51–6.
Sasaki-Adams D, Hadar EJ. Temporal lobe epilepsy surgery: surgical complications. In: Luders HO, editor. Epilepsy surgery. London: Informa UK Ld; 2008. p. 1288–99.
Hermann BP, Seidenberg M, Dohan FC, Wyler AR, Haltiner A, Bobholz J, et al. Reports by patients and their families of memory change after left anterior temporal lobectomy: relationship to degree of hippocampal sclerosis. Neurosurgery. 1995;36:39–45.
Ojeman JG. Will fMRI replace the Wada test? In: Miller JW, Silbergeld DL, editors. Epilepsy surgery: principles and controversies. New York: Taylor & Francis; 2006. p. 336–41.
Kanner AM, Balabanov AJ. Psychiatric outcome of epilepsy surgery. In: Luders HO, editor. Epilepsy surgery. London: Informa UK Ld; 2008. p. 1254–62.
Naylor AS, Rogvi-Hansen B, Kessing L, Krause-Larsen C. Psychiatric morbidity after surgery for epilepsy: short term follow-up of patients undergoing amygdalohippocampectomy. J Neurol Neurosurg Psychiatry. 1994;57:1375–81.
Choi C, Rubino PA, Fernandez-Miranda JC, Abe H, Rhoton AL Jr. Meyer’s loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery. 2006;59:228–36.
Peuskens D, Van Loon J, Van Calenbergh F, Van den Bergh R, Goffin J, Plets C. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55:1174–84.
Rubino PA, Rhoton AL Jr, Tong X, Oliveira E. Three-dimensional relationships of the optic radiations. Neurosurgery. 2005;57(suppl 4):219–27.
Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches of the temporal horn. J Neurosurg. 2004;101:739–46.
Bolling WW. Selective amygdalohippocampectomy. In: Baltuch GH, Villemure J-G, editors. Operative techniques in epilepsy surgery. New York: Thieme Medical Publishers Inc.; 2009. p. 41–50.
Erdem A, Yasargil MG, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993;79:256–65.
Renella RR. Microsurgery of the temporo-medial region. Vienna: Springer; 1998.
Roper SN, Rhoton AL Jr. Surgical anatomy of the temporal lobe. Neurosurg Clin North Am. 1993;4:223–31.
Wen HT, Rhoton AL Jr, Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe, part I: mesial temporal lobe and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45:549–92.
Delalande O, Pinard JM, Basdevent C, Gauthe M, Plouin P, Dulac O. Hemispherotomy Epilepsy. 1992;33(Suppl 3):99–100.
Villemure JG, Mascott CR. Peri-insular hemispherotomy. Neurosurgery. 1995;37:975–81.
Schramm J, Lehmann TN, Zentner J, Mueller CA, Scorzin J, Fimmers R, et al. Randomized controlled trial of 2.5 cm versus 3.5 cm mesial temporal resection in temporal lobe epilepsy – part 1: intent-to-treat analysis. Acta Neurochir. 2011;153:209–19.
Chabardès S, Minotti L, Hamelin S, Hoffman D, Seigneuret E, Carron R, et al. Temporal disconnection as an alternative treatment for intractable temporal lobe epilepsy. Neurochirurgie. 2008;54:297–302.
Ng WH, Valiante T. Lateral temporal lobectomy with hippocampal disconnection as an alternative surgical technique for temporal lobe epilepsy. J Clin Neurosci. 2010;17:634–5.
Mohamed AR, Freeman JL, Maixner W, Bailey CA, Wrennal JA, Harvey AS. Temporoparietooccipital disconnection in children with intractable epilepsy. J Neurosurg Pediatr. 2011;7:660–70.
Gonçalves-Ferreira A, Rainha Campos A, Herculano-Carvalho M, Pimentel J, Bentes C, Peralta AR, et al. The epilepsy surgery group. Amygdalohippocampotomy, surgical technique and clinical results. J Neurosurg. 2013;118:1107–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Gonçalves-Ferreira, A. (2019). Anterior Temporal Lobectomy and Amygdalo-Hippocampectomy. In: Fountas, K., Kapsalaki, E. (eds) Epilepsy Surgery and Intrinsic Brain Tumor Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-95918-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-95918-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95917-7
Online ISBN: 978-3-319-95918-4
eBook Packages: MedicineMedicine (R0)