Abstract
Background
Hippocampal sclerosis is the most common cause of drug-resistant epilepsy amenable for surgical treatment and seizure control. The rationale of the selective amygdalohippocampectomy is to spare cerebral tissue not included in the seizure generator.
Method
Describe the selective amygdalohippocampectomy through the trans-superior temporal gyrus keyhole approach.
Conclusion
Selective amygdalohippocampectomy for temporal lobe epilepsy is performed when the data (semiology, neuroimaging, electroencephalography) point to the mesial temporal structures. The trans-superior temporal gyrus keyhole approach is a minimally invasive and safe technique that allows disconnection of the temporal stem and resection of temporomesial structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Relevant surgical anatomy
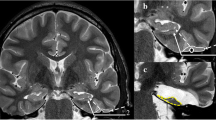
The neural structures constituting the mesial temporal lobe are the parahippocampal gyrus (PHG), uncus, hippocampus, fimbria, dentate gyrus and amygdala [6, 10] (Fig. 1). With the temporal horn of the lateral ventricle opened, the hippocampus appears clearly visible. The hippocampus lies just lateral to the cerebral peduncle. Its head occupies the rostral extent of the temporal horn and curves mesially to form the uncus. Its body extends posteriorly into the tail that curves backward and upward to the trigone of the ventricle [2]. Inside the temporal horn, the choroid plexus that partially covers the hippocampus and the choroid fissure are important surgical landmarks. The inferior choroidal point marks the beginning of the choroid fissure that lies between the fimbria and the thalamus. The hippocampal cisternal aspect faces the brainstem from which it is limited by the transverse fissure [2]. The crural cistern contains the P2 segment of the posterior cerebral artery and the oculomotor nerve. The hippocampal sulcus, containing the hippocampal arteries and veins, is perpendicular to the ambient cistern and separates the dentate gyrus superiorly from the parahippocampal gyrus inferiorly [8].
Morphological and surgical anatomy of mesial temporal lobe. a Axial slice of a right temporal lobe. 1 Temporal pole, 2 hippocampal uncus, 3 amygdala, 4 hippocampus body (cornu ammonis), 5 parahippocampal gyrus (T5), 6 lateral temporal neocortex. b Frontal slice of a left mesial temporal lobe. 1 Hippocampus body (cornu ammonis), 2 parahippocampal gyrus (T5), 3 temporal horn of the lateral ventricle, 4 collateral sulcus. c Anatomical pathway of a right trans-STG selective amygdalohippocampectomy. The amygdalohippocampal formation appears in yellow. The yellow bars delimit the trans-STG corridor
Description of the technique
Standard endotracheal general anaesthesia is used. The patient is positioned supine, with the shoulder elevated and the head turned. The patient’s head is immobilised either in a Mayfield pin head holder or with adhesive tape. The head is turned about 75° contralaterally while being extended to make the zygomatic arch the highest point of the operative field.
Scalp incision is a small curvilinear shape that starts at the superior border of the zygomatic arch just in front of the tragus, extends to the superior temporal line and anteriorly to the hairline (Fig. 2a). The temporalis muscle is incised via a curvilinear incision and retracted anteriorly.
Two burr holes are placed on each side of the sphenoid wing. A small temporal craniotomy is fashioned to expose the superior temporal gyrus (STG), the superior temporal sulcus and the Sylvian fissure. The sphenoid wing is flattened with a rongeur to allow adequate exposure to the anterior part of the STG (Fig. 2b). Haemostasis of the middle meningeal artery with cautery and sphenoid wing with bone wax is achieved.
The dura is opened in a semicircular fashion and reflected over the temporalis muscle (Fig. 3a). A corticectomy measuring about 2 cm is made, as anterior as possible, along the upper border of the STG and just below the Sylvian fissure (Fig. 3b). Using an ultrasonic aspirator, a corridor is made along and directed by the arachnoid of the Sylvian fissure that points to the temporal horn (Fig. 3c). The ventricle is opened and retractors are positioned into the temporal horn to better visualise the choroid plexus and choroidal fissure marking the medial boundary of the hippocampal dissection (Fig. 3d). The hippocampus becomes visible.
Intraoperative view of left selective amygdalohippocampectomy via trans-STG keyhole approach (first part). a The craniotomy is centred on the STG. The dura is opened in semicircular fashion and reflected over the temporalis muscle. b A corticectomy is made along the upper border of the STG and just below the Sylvian fissure. c A trans-STG corridor is made along and directed by the arachnoid of the Sylvian fissure that points to the temporal horn. d The retractors are positioned into the temporal horn of the ventricle. The choroidal fissure marks the medial boundary of the hippocampal dissection. e A disconnection is made lateral to the hippocampus along the ventricular border lateral to the collateral eminence. f The hippocampus is retracted laterally to facilitate dissection of the fimbria attaching to the choroid fissure and disconnection mesially
The resection of the mesial structures is subpial using the ultrasonic dissector. The resection is started by entering the lateral ventricular sulcus at the junction of the hippocampus with the collateral eminence. A disconnection is made lateral to the hippocampus along the ventricular border lateral to the collateral eminence, in the anteroposterior axis, aiming to the level of the collateral sulcus (Fig. 3e). This allows the hippocampus to be retracted laterally to facilitate dissection of the fimbria attaching to the choroid fissure and disconnection mesially (Fig. 3f). The medial part of the parahippocampal gyrus (PHG) is also separated from the arachnoid of the hippocampal sulcus. The combined hippocampus/PHG is separated from the hippocampal tail posteriorly by mediolateral transverse section. The specimen is freed from its arachnoidal cover by rolling it from mesial to lateral and separated from its vascular pedicle with a dissector (Fig. 4a and b). The hippocampal formation should be removed en bloc to allow the finest histological examination [1] (Fig. 4c and f). The typical posterior limit of the resection corresponds to the midbrain tectum (Fig. 4d). The anterior limit is the level of the rhinal sulcus that includes the uncus. The uncus and the amygdala are removed subpial. The mesial boundary of the amygdala resection is not precise. Additional pieces of the hippocampal formation can be removed piecemeal, taking care to keep the pial surface intact.
Intraoperative view of left selective amygdalohippocampectomy via trans-STG keyhole approach (last part). a and b The hippocampus is freed from its arachnoidal cover by rolling it from mesial to lateral and separated from its vascular pedicle with a dissector. c The hippocampus is removed en bloc. d The posterior limit of the resection corresponds to the midbrain tectum. e Final view of the temporal neocortex after trans-STG selective amygdalohippocampectomy. f Coronal section of the hippocampus allowing histological examination
Haemostasis is accomplished with Surgicel®, cottonoid packing and irrigation. The resection cavity is backfilled with irrigation (Fig. 4e). Dura is closed with running sutures in watertight fashion. Fibrin glue can be added on the dural closure to enhance watertightness. If epidural haemostasis needs to be achieved, dural tack-up sutures are placed along craniotomy edges. The bone flap is reaffixed with four non-absorbable sutures. The bone defect from removal of sphenoid wing can be eliminated with the bone powder. The wound is closed in standard fashion.
Indications
Selective amygdalohippocampectomy is indicated for patients presenting medically drug-resistant epilepsy localised to the temporomesial structures associated with hippocampal sclerosis.
Transcortical STG keyhole approach is a minimally invasive technique that allows disconnection of the temporal stem (unlike an MTG approach) and resection of temporomesial structures (Fig. 5).
Limitations
Patients without clear evidence of mesial temporal lobe seizure foci or involvement of lateral temporal neocortex and patients with cryptogenic temporal epilepsy are not ideal candidates for selective amygdalohippocampectomy. In such cases, anterolateral temporal lobectomy or tailored temporal cortectomy should be chosen as the appropriate technique.
The procedure we have described is strictly guided by anatomical landmarks, but image-guidance may help to localise these landmarks. This approach requires training, habit and a perfect knowledge of the temporal lobe anatomy. However, we recommend to use an image-guidance system for the first cases in order to better locate the landmarks of the corticectomy.
How to avoid complications
-
Do not try to do the amygdalohippocampectomy without appropriate transventricular exposure.
-
Subpial dissection technique is a key to temporomesial resection. Take care to use the ultrasonic aspirator on low settings of suction and vibration to preserve the arachnoid protecting the critical underlying structures (cranial nerve III, posterior cerebral artery, anterior choroidal artery, midbrain peduncle).
-
Take care not to extent the amygdala resection too superiorly into the globus pallidus.
-
Be careful not to resect any tissue posterosuperior to the temporal horn to avoid damaging the fibres of Meyer’s loop. Moreover, excessive posterolateral opening of the ventricle wall can result in damage to the optic radiations and leads to visual field defect.
-
The hippocampal vessels typically can be separated from the hippocampus without coagulation. However, if coagulation and section of the hippocampal vessels in the hippocampal sulcus is necessary, take care to preserve the anterior choroidal artery and the posterior cerebral artery (P2 segment).
-
Haemostasis of the resection cavity is accomplished with Surgicel® and cottonoid packing. Use of bipolar cautery in the arachnoid resection can be dangerous.
Specific perioperative considerations
Presurgical non-invasive evaluation comprised: complete neurological, neuropsychological and psychiatric examination, scalp video-electroencephalogram (EEG) recording of habitual seizures, brain 3-T MRI, and sometimes functional MRI, subtraction ictal single photon emission computed tomography (SPECT) co-registered to MRI and interictal positron emission tomography (PET) [5].
In the postoperative period, the patient is monitored at the intensive care unit and extubated when fully awake. Anti-epileptic drugs must be resumed at full dose. A postoperative imaging (e.g. MRI) is performed to eliminate the occurrence of intracranial complication and evaluate the extent of temporomesial resection. The patient is carefully monitored for seizures.
Specific information to give to the patient about surgery and potential risks
Patients should be informed that the seizure-free rate after surgical resection for hippocampal sclerosis is about 70 % [3, 4]. Mortality is very rare (<1 %) [9] and the rate of complication is low: definitive hemiparesis or third cranial nerve paralysis occurs in 1 % of the cases [7]. Visual field defects occur frequently after surgery for hippocampal sclerosis and are usually contralateral superior quadrantanopia due to direct trauma to the optic radiations while accessing the mesial temporal structures.
References
Blumcke I, Cross JH, Spreafico R (2013) The international consensus classification for Hippocampal sclerosis: an important step towards accurate prognosis. Lancet Neurol 12:844–846
Destrieux C, Bourry D, Velut S (2013) Surgical anatomy of the hippocampus. Neurochirurgie 59:149–158
Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M (2006) Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia 47:2115–2124
Mathon B, Bedos Ulvin L, Adam C, Baulac M, Dupont S, Navarro V, Cornu P, Clemenceau S (2015) Surgical treatment for mesial temporal lobe epilepsy associated with Hippocampal sclerosis. Rev Neurol (Paris) 171:315–325
Mathon B, Bedos-Ulvin L, Baulac M, Dupont S, Navarro V, Carpentier A, Cornu P, Clemenceau S (2015) Evolution of ideas and techniques, and future prospects in epilepsy surgery. Rev Neurol (Paris) 171:141–156
Sajko T, Skoro I, Rotim K (2013) How I do it - selective amygdalohippocampectomy via subtemporal approach. Acta Neurochir (Wien) 155:2381–2387
Tanriverdi T, Ajlan A, Poulin N, Olivier A (2009) Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg 110:1111–1123
Wen HT, Rhoton AL Jr, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, Marino R Jr (1999) Microsurgical anatomy of the temporal lobe: part 1: mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery 45:549–591, discussion 591–542
Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study G (2001) A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318
Wieser HG, Yasargil MG (1982) Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol 17:445–457
Acknowledgments
The authors thank Dr. Dominique Hasboun and Dr. Anne Bertrand (Department of Neuroradiology, La Pitié-Salpêtrière Hospital, Paris, France) for anatomical views and imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Key points
- Knowledge of mesial temporal lobe anatomy is fundamental.
- Place the retractor to secure an unobstructed view of the intraventricular mesial temporal lobe structures.
- Visualise the choroid plexus and the choroid fissure that are most important surgical landmarks.
- Subpial dissection technique is a key to all aspects of temporomesial epilepsy surgery.
- Be careful not to resect any tissue posterosuperior to the temporal horn to avoid damaging the fibres of Meyer’s loop.
- Take care not to extend the amygdala resection too superiorly into the globus pallidus.
- Perform the posterior disconnection at the level of the midbrain tectum.
- The hippocampal vessels typically can be separated from the hippocampus without coagulation.
- Haemostasis of the resection cavity is accomplished with Surgicel® and cottonoid packing.
- In postoperative period, antiepileptic drugs must be resumed at full dose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The video shows the major steps of a left selective amygdalohippocampectomy via trans-superior temporal gyrus keyhole approach. In our institution, the actual length of the whole procedure is 75 min on average (MP4 50.7 mb)
Rights and permissions
About this article
Cite this article
Mathon, B., Clemenceau, S. Selective amygdalohippocampectomy via trans-superior temporal gyrus keyhole approach. Acta Neurochir 158, 785–789 (2016). https://doi.org/10.1007/s00701-016-2717-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2717-4