Abstract
Asphalt binders remain exposed to aging from mixing and placement operations throughout their service life. The aging process is a chemical event, often known as oxidization, which hardens asphalt binders by causing changes to their chemical compositions. Stiffening also occurs when they are modified chemically to make them fit in certain environmental and loading conditions. Aging can accelerate stiffening of the modified binders because of the simultaneous presence of the modifier and oxidizing agents. This study evaluates the changes in the chemical fingerprints of asphalt binders in terms of their Saturates, Aromatics, Resins, and Asphaltenes (SARA) fractions and Fourier Transformation Infrared Spectroscopy (FTIR)—based functional groups. Two selected performance grade (PG) binders (PG 64-22) modified with Polyphosphoric Acid (PPA) and Styrene Butadiene Styrene (SBS) have been subjected to Rotational Thin Film Oven (RTFO) and Pressure Aging Vessel (PAV) aging. Asphalt binders from two different crude sources modified with different percentages of PPA, SBS and a combination of PPA and SBS were considered in this study. Due to the aging effects, the modified asphalt binders became abundant in solid phase than the unaged binders at a lower modification level, which in turn made the binders stiff. The changes in pattern were different for binders from two different crude origins. The findings from this study can help setting guidelines for level of chemical modification to asphalt binders.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Asphalt binder is complex mixture of numerous hydrocarbons. The chemical complexity of asphalt binder is inherited from the maturation of the ancient living organisms, which is very susceptible to chemical oxidation by reaction with the atmospheric oxygen. The oxidative aging causes a mass embrittlement of asphalt binder, which is not desirable as far as the serviceability is the concern. Oxidation is also influenced a great deal by the mineral aggregates that stay together with the asphalt binder in the mixture and act like a mineral catalyst toward oxidation (Petersen 2009). Although, oxidation is not the sole cause of the mass embrittlement of the asphalt binder, it is still an area to be research on. The aging of asphalt binder happens when heat and air (i.e. oxygen) are present together. The pavement temperature is usually higher than that of the ambient temperature, since it absorbs all the heat around and thus becomes highly susceptible to oxidative aging. The sensitivity of asphalt binder to oxidative hardening is widely variable with the asphalt source and its chemical compositions (Petersen 2000). The Zaca-Wigmore experimental road test is regarded as the classic demonstration of this source dependence (Hveem et al. 1959). This aging, though happens during hot summer days, influences the low temperature susceptibilities of an asphalt binder. A pavement with less elasticity is vulnerable to traffic loadings. Sometimes, loading is not necessarily required for a crack to occur during the winter days.
An asphalt binder is often modified to achieve some rheological improvement both in the cases of low and high temperature seasons. According to the National Center for Asphalt Technology (NCAT), some of the specific reasons that justifies a modification of asphalt binder includes stiffening binders and mixtures to minimize rutting and softening the binders at low temperatures to improve the relaxation properties and strain tolerance (King 1999). With that motive, asphalt binder is often supplied to the highway agencies with chemical modification. Polyphosphoric Acid (PPA) and Styrene Butadiene Styrene (SBS) are some of the modifiers that have long been used in asphalt modification by refineries to achieve those above-mentioned superiorities. However, with all these modification asphalt binder is in fact pushed a little further toward the oxidative aging. The aging that might cause the hardening through molecular association in an unmodified binder can get a head start with the stiffening effect of these modifiers. It is very likely to expect the detrimental effects way before the termination of the life of a pavement. Therefore, the effect of aging on chemically modified asphalt binders are needed to be explored from an engineering point of view.
2 Literature Review
Asphalt binder is analyzed based on the family of like chemical compounds namely SARA fractions due to its inherent chemical complexity from the crude parent. The families are termed as Saturates (S), Aromatics (A), Resins (R), and Asphaltenes (A). All these compounds stay together as a mixture. The molecules in the complex mixture stay as agglomerates and bonded together by polar association forces such as hydrogen bonding and dipole moments (Petersen 2009). As temperature increases, this polar association forces gets weakened and broken and eventually increase the global hardening of the asphalt binder. The saturates fraction is a light straw colored oil, primarily hydrocarbon in nature and having a little aromaticity. The saturates fractions highly resistant to ambient air oxidation (Corbett and Merz 1975). The other three fractions remains at a trend of moving from more non-polar fractions to more polar fractions. The oxygen containing functional groups are formed due to this oxidation reaction. The amount of ketones that form due to oxidation is linearly related to the log viscosity of the asphalt binder (Lee and Huang 1973; Epps et al. 1986). Although the Asphaltenes fraction has been considered to be chemically inert by some researchers (e.g., Rostler and White 1959), the data presented in many literatures indicates that Asphaltenes are inherently extremely reactive with oxygen.
The aging of bitumen can be categorized into two groups. The main aging that happen due to oxidation is a irreversible process and ends up resulting in changes in the rheology of the binder. The other is termed as reversible one, which is the physical hardening (Bahia and Anderson 1993). The physical hardening may be attributed to molecular structuring which implies that the reorganisation of bitumen molecules (or bitumen microstructures) to approach an optimum thermodynamic state under a specific set of conditions (Branthhaver et al. 1993). The bitumen aging is a very complex process, which includes multiple variables as a aging contributor. The bitumen aging happens during the handling and storage operation as well as in field exposure for a longer period. In storage tanks, the asphalt is kept heated all the time so that it remains sufficiently fluid and ready to be used. The other variables that govern the aging are the mix, nature of aggregates, and even the particle size distribution, void content of the mix, production related factors, temperature and time. All these factors operate at the same time making the process of aging very complex (Lu and Isacsson 2002).
PPA and SBS have long been used in asphalt modification by refineries while some state departments of transportation (DOTs) have bans on PPA alleging it to be the cause of some premature pavement distresses such as striping. However, PPA and SBS have some beneficial implications too. PPA is an oligomer of H3PO4, which is obtained through dehydration of H3PO4 at high temperatures or by heating P2O5 dispersed in H3PO4 (Jameson 1959). SBS is an elastomer, which is frequently used in asphalt modification. SBS increases the kinematic and dynamic viscosity values of asphalt binders (Lu and Isacsson 1997). The aging induced the changes in the components of base binder and degradation of the SBS modifiers (Wu et al. 2009).
The aging is an oxidation phenomenon and this oxidation causes the formation of certain functional groups such as carbonyl and sulfoxides in asphalt binder (Lu and Isacsson 2002). The degree of formation of carbonyl and the sulfoxide functional groups can easily be identified using FTIR spectroscopy. Hossain et al. (2012) studied FTIR-based functional groups that developed due to warm-mix additive modification of. The FTIR gives signal with a peak at certain wavenumber values for certain functional groups.
3 Objectives
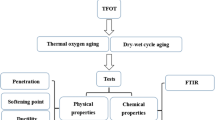
The study focused on finding the effects of chemical modification at macroscale. Asphalt binders from two different crude origins were analyzed for detecting the change in chemical composition at different aging conditions and also the functional groups that might occur upon both modification and aging. The objectives of this study were as follows: (i) perform short-term and long-term aging of the sample binders; (ii) measure the chemical composition of the both unaged and aged binders; (iii) detect any functional groups using IR spectroscopy; and (iv) analyze and interpret the data to observe any changes or pattern due to aging.
4 Materials and Methodology
Asphalt binder samples were collected from two approved suppliers of Arkansas Department of Transportation (ArDOT). They supply binders that fulfill the PG requirements as warranted by ArDOT. The unmodified and modified asphalt binder samples used in this study are listed in Table 1. The PPA used in modifying the asphalt binders in this study was of 105% grade. The SBS was commercially available as Vector Dexco 2411.
4.1 SARA Analysis
The SARA analysis was intended for determining the percentages of certain families of chemical constituents in the tested asphalt binders. Any improvement in rheological properties happens through certain alteration of chemical constituents, which lead to a change in the percentages of chemical constituent fractions. The analysis was performed in accordance with “ASTM D 4124-09: Standard Test Method of Separating Asphalt into Four Fractions” (ASTM D4124-09 2009). The test specimen was put into reflux with n-Heptane for at least 3 h. To start a reflux, an asphalt specimen weighing 2.00 ± 0.30 g was taken in a round bottom flask. For each grams of asphalt specimen, 100 mL of n-Heptane (HPLC grade) was added to it. A stirring magnet was put into the flask. A Liebig condenser was fitted to the opening of that flask. The assembly was fastened with a clamp and set on a heating bath containing silicone oil or sand minus #20 US standard sieve. The heating bath was placed on hot plate and the temperature was set at 200 ± 50 °C and the stirring was set at 300 ± 50 rpm. This reflux operation caused the highly polar or the most solid fraction (i.e. the Asphaltenes) to precipitate. The n-Heptane dissolved the other three fractions except the Asphaltenes. Although the standard recommended to use iso-Octane, it was not capable enough to entirely dissolve the specimen. Therefore, n-Heptane was used to get the entire specimen dissolved. The other three fractions are collectively termed as Maltenes. The Maltenes were loaded onto a chromatographic column containing activated alumina (pH 9–10) of particle size 50–200 μm and allowed to elute under gravity. The Maltenes came out in a sequence as the Saturates, the Aromatics and the Resins. The Saturates fraction came first out of the activated alumina with n-Heptane. The napthene Aromatics fraction was eluted with consecutive application of toluene and toluene:methanol (50:50) solvents. A ultraviolet (UV) light of 366 nm wavelength was shined onto the column to monitor advancement of the Aromatics fraction. A fluorescent band was progressing down. After collecting all the fluorescent bands, the polar Aromatics or the Resins started to elute. It was collected lastly with trichloroethylene. The Saturates was colorless. The Aromatics was yellow or red in color while the polar aromatics or the Resins were black in color. All the eluted fractions were completely dried using a rotary evaporator and were reported as the percent fractions of the original sample. Sometimes a drying with chloroform was required to escape all the solvents out of the eluted fractions.
4.2 Fourier Transform Infrared Spectroscopy
The FTIR test is a spectroscopy technique applied on an asphalt binder to detect the presence or change in quantities of functional groups that might have occurred due to the modification (Yildirim 2007). In this test, a vibrational Infra-Red (IR) light is passed through the tested sample. When the natural vibrational frequency of a specific molecule matches the frequency of the IR radiation, the molecule absorbs the energy and increases the amplitude of the vibrational motion and detected as a peak in the interferogram. In this study, disposable Real Crystal IR cards were used for preparing the samples. The IR cards contained a KBr substrate. FTIR functions as a fingerprinting tool such as the case of humans. In FTIR, the natural vibrations of the covalent bonds among the molecules are exploited. Every type of bond has a certain natural frequency of vibration. Two of the same type of bond in two different compounds can exist in two slightly different molecular environments. Therefore, no two molecules of different structures have the same IR absorption pattern, which serves as the fingerprint for that specific compound (Pavia 2008). A Nicolet 8700 spectrometer was used in this study. The data acquisition and analysis was done using the Omnic 6.2 software.
5 Results and Discussion
The percentages SARA fractions for all the binder samples have been reported in Figs. 1 and 2 as stacked charts. Asphalt binder samples from the Canadian crude source (Source-1) were high in Asphaltenes content than those from the Arabian crude source (Source-2). Even though both binders were marketed as PG 64-22, the neat binder from Source-2 (S2B1) had an Asphaltenes content of 13.2% and the neat binder from Source-1 (S1B1) had an Asphaltenes content of 19.9%. On the other hand, S1B1 binder had a low polar fraction (Resins) compared to S2B1.
The Asphaltenes fractions seemed to serve as a viscosity building component, which resulted in an improved rutting resistance. Asphaltenes and Resins fractions are the solid and nearly solid fractions respectively in an asphalt colloidal structure. The overall strength is typically provided by the overall solid phase (Asphaltenes and Resins combined) of the binder. Therefore, despite having a lower Asphaltenes content, Source-2 binders were not poor in rutting resistance. Thus, despite having different amounts of solid species, both were marketed as PG 64-22 binders with nearly similar rutting resistances. As per Robertson et al. (2001), an increase in polarity results in an increase in association and hence increases the stiffness. Source-2 samples had more polar Aromatics (i.e. Resins), which ensured the polar environment. The amount of PPA required to bump the PG from a neat binder could be observed in the test matrix (Table 1). A little higher amount of PPA was required for Source-2 binder to bump one PG grade than that from Source-1.
Asphalt binders modified with SBS were different in composition from that of PPA modified ones. In the case of Source-1 binders, Asphaltenes content remained the same as that of the neat binder (Fig. 1). There was no noticeable effect on the Resins and Aromatics contents either. Contents remained almost the same although the PG rose to 70-22. The addition of SBS did not take part in the transformation of compounds, rather induced a different modification mechanism. The colloidal structure of asphalt advocates such occurrence. When a polymer is added as a modifier, it is swollen by the light Aromatics components from the parent bitumen (Kraus 1982). Asphaltenes and polymers do not mix, but they create a phase separation, leaving the polymer swollen by the Aromatics on one side and the Asphaltenes on the other side (Lesueur 2009). Consequently, the colloidal matrix becomes depleted in Maltenes and hence enriched relatively in Asphaltenes content. Thus, an Asphaltenes rich phase occurs and the desired hardening is achieved. However, in the case of S2B5, the above-stated mechanism was not noticeable. The constituent fractions were changed although there was no externally added acid. The occurred change was somewhat like an acid modified binder. This could possibly be the effect of the inherent acidic environment of Source-2 asphalt binders, which promoted the association of compounds and made the conversion of Aromatics toward Asphaltenes easier. Although the neat binder from Source-2 contained a lower amount of Asphaltenes, a 2% addition (same as that of Source-1) of SBS resulted in a PG 70-22 binder. This occurrence could be attributed to the continuous formation of Asphaltenes due to the inherently acidic environment, which was detailed by the same authors in another article (Alam et al. 2017).
The combined effect of PPA and SBS (the polymer) is stated as synergetic and contribute more efficiently in asphalt modifications (Lesueur 2009). The Asphaltenes rich phase experiences a two-fold increase due to the combined effect. PPA causes Asphaltenes to rise which is later reinforced by the addition of SBS. SBS causes the “physical distillation” of the phases (Lesueur 2009). As evident in Figs. 1 and 2, both S1B6 and S2B6 had an increased Asphaltenes and Resins contents that resulted in a PG 76-22 binder through the so-called physical distillation process.
The SARA fractions of unaged, RTFO-aged, and PAV-aged binder samples have also been reported in Figs. 1 and 2. The only distinguishable thing was a change in Asphaltenes content. The Asphaltenes content had increased upon the short-term aging in the case of both binder sources. The changes in other fractions did not happen in any consistent pattern and that is expected since the Asphaltenes are the final phase of the constituent fractions. All other constituent fractions were either continuously fed by the previous fractions or continuously contributing to the formation of the subsequent polar fractions. Hence, the change in their constituent fractions was not consistent. However, there was a small but consistent change in the Saturates content. The Saturates content was reduced upon the RTFO-aging process. This phenomenon is analogous to the reduction in the sample mass in the RTFO-aging process. Upon PAV aging Asphaltenes content reached to a considerably higher percentage. The binders S1B6 and S2B6 were modified with the same amount of PPA as that of S1B3 and S2B3. In B6 binders from both sources, there was SBS in combination with PPA. It is claimed that SBS does not contribute to any sort of association process that might cause the formation of more Asphaltenes or polar species.
FTIR tests were conducted on both unaged and aged binder samples from both sources. From the FTIR spectra it was revealed that neither of PPA and SBS modification added any new functionality to the binder samples. The peak at certain wavenumbers displayed a higher signal, which implied that the aging introduced some increment in certain functional groups. Per Lamontagne et al. (2001), the change in the quantities of the functional groups could be obtained using the following equations.
Carbonyl Index (C=O),
Sulfoxide-Index (S=O),
Tables 2 and 3 list the areas under the curves corresponding to the 1700 and 1030 cm−1 peaks and the corresponding indices based on Eqs. 1 and 2. The carbonyl index was the one that appeared in the spectra only after the PAV aging process. The oxygen molecules from the air got attached to the carbons upon aging. In the process of aging, the oxidation, dehydrogenation and crosslinking reactions occur at the same time (Siddiqui and Ali 1999). This occurrence leads to the appearance of more carbonyl (C=O) and sulfoxide (S=O) groups. Both the carbonyl and the sulfoxide indices increased upon the PAV aging. Like the Source-1 binders, both the carbonyl and sulfoxide indices increased upon aging. The carbonyl group was only visible for the PAV aged binders.
6 Conclusion and Recommendation
The Canadian crude binders were high in Asphaltenes fraction, whereas the Arabian Crude binders were much lower in the Asphaltenes content. However, both binders met the required Performance Grade (PG) specifications. The solid fraction alone does not contribute in the physical properties rather a combination of all the components implement changes in the physical properties of an asphalt binder. The required amount of PPA to cause a grade bump for Arabian crude source binders was higher than that of the Canadian crude source binders for similar grade bumps. It implies the influence of crude origin in setting up a binder modification methodology. The FTIR test data revealed that PPA modification did not introduce any new functional groups in the asphalt binder samples. Only the short-term and long-term aging caused the increase in certain functional groups especially the species that respond to oxidative aging. The recommended action for an asphalt binder to have the enhanced capability of sustaining severe aging could be making the binder deficient of polar species. This action could be performed either by taking out the highly polar fractions or adding the some less polar oil fractions like Aromatics from. This could prolong the saturation of the binder’s colloidal structure and thus delay the aging.
References
Alam, S., Hossain, Z.: Changes in fractional compositions of PPA and SBS modified asphalt binders. J. Constr. Build. Mater. 152, 386–393 (2017). http://dx.doi.org/10.1016/j.conbuildmat.2017.07.021
ASTM D4124-09.: Standard Test Method for Separation of Asphalt into Four Fractions. ASTM International, West Conshohocken, PA, USA (2009). https://doi.org/10.1520/d4124-09
Bahia, H.U., Anderson, D.A.: Glass transition behavior and physical hardening of asphalt binders (with discussion). J. Assoc. Asph. Paving Technol. 62 (1993)
Branthhaver, J.F., Petersen, J.C., Robertson, R.E., Duvall, J.J., Kim, S.S., Harnsberger, P.M., Scharbron, J.F.: Binder Characterization and Evaluation. Volume 2: chemistry (1993)
Corbett, L.W., Merz, R.E.: Asphalt binder hardening in the Michigan test road after 18 years of service. Trans. Res. Rec. (1975)
Epps, J., Petersen, J.C., Kennedy, T.W., Anderson, D., Haas, R.: Chemistry, rheology, and engineering properties of manganese-treated asphalts and asphalt mixtures. Trans. Res. Rec. (1986)
Hossain, Z., Lewis, S., Zaman, M., Buddhala, A., O’Rear, E.: Evaluation for warm-mix additive-modified asphalt binders using spectroscopy techniques. J. Mater. Civ. Eng. 25(2), 149–159 (2012)
Hveem, F.N., Zube, E., Skog, J.: Progress report on the Zaca-Wigmore experimental asphalt test project. In: Symposium on Road and Paving Materials, ASTM International (1959)
Jameson, R.F.: The composition of the “strong” phosphoric acids. J. Chem. Soc. (Resumed), 752–759 (1959)
King, G.K.: Additives in asphalt. J. Assoc. Asph. Paving Technol. 68, 32–69 (1999)
Kraus, G.: Modification of asphalts by block plymers of butadiene and styrene. Rubber Chem. Technol. 55(5), 1389–1402 (1982). https://doi.org/10.5254/1.3535936
Lamontagne, J., Dumas, P., Mouillet, V., Kister, J.: Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: application to road bitumens. Fuel 80(4), 483–488 (2001)
Lee, D.Y., Huang, R.J.: Weathering of asphalts as characterized by infrared multiple internal reflection spectra. Appl. Spectrosc. 27(6), 435–440 (1973)
Lesueur, D.: The colloidal structure of bitumen: consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 145(1), 42–82 (2009)
Lu, X., Isacsson, U.: Influence of styrene-butadiene-styrene polymer modification on bitumen viscosity. Fuel 76(14–15), 1353–1359 (1997)
Lu, X., Isacsson, U.: Effect of ageing on bitumen chemistry and rheology. Constr. Build. Mater. 16(1), 15–22 (2002)
Petersen, J.C.: Chemical composition of asphalt as related to asphalt durability. Dev. Pet. Sci. 40, 363–399 (2000)
Petersen, J.C.: A review of the fundamentals of asphalt oxidation: chemical, physicochemical, physical property, and durability relationships. Transportation Research E-Circular, Transportation Research Board (2009)
Rostler, F.S., White, R.M.: Influence of chemical composition of asphalts on performance, particularly durability. In: Symposium on Road and Paving Materials, ASTM International (1959)
Robertson, R.E., Branthhaver, J.F., Harnsberger, P.M., Peterson, J.C., Dorrence, S.M., McKay, J.F., Tauer, J.E.: Fundamental properties of asphalts and modified asphalts. Volume I: Interpretive report (No. FHWA-RD-99-212) (2001)
Siddiqui, M.N., Ali, M.F.: Investigation of chemical transformations by NMR and GPC during the laboratory aging of Arabian asphalt. Fuel 78(12), 1407–1416 (1999)
Wu, S.P., Pang, L., Mo, L.T., Chen, Y.C., Zhu, G.J. Influence of aging on the evolution of structure, morphology and rheology of base and SBS modified bitumen. Constr. Build. Mater. 23(2), 1005–1010 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Alam, S., Hossain, Z. (2019). Changes in Chemical Fingerprints of Asphalt Binders Due to Aging. In: Zhang, K., Xu, R., Chen, SH. (eds) Testing and Characterization of Asphalt Materials and Pavement Structures. GeoChina 2018. Sustainable Civil Infrastructures. Springer, Cham. https://doi.org/10.1007/978-3-319-95789-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-95789-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95788-3
Online ISBN: 978-3-319-95789-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)