Abstract
We studied the bioethanol production in molasses-based medium by yeast Saccharomyces cerevisiae immobilized in calcium alginate magnetite beads (CAMB). The yeast was isolated from soil samples collected near a local sugar mill, and identified as S. cerevisiae. We synthesized magnetite nanoparticles and immobilized yeast in CAMB. The media components and environmental parameters were statistically screened and optimized for better ethanol production, using statistical design methodologies—factorial designs and response surface methodology. The factors of molasses concentration, temperature and incubation time were found to have significant effect on ethanol production. The immobilized cells could be reused for more than 120 days, retaining its original activity. The CAMBs with immobilized yeast cells were analysed by ESEM with EDAX, after 96 h of fermentation to observe the surface structure of the beads. It can be observed that yeast was immobilized in the beads and actively growing. Further ethanol production was carried out in packed-bed column reactor using yeast immobilized in CAMB, under fed-batch mode. The average ethanol produced by fed-batch fermentation was 1.832 g% ± 0.103, and the average ethanol yield was 81.420% ± 4.6. Further studies using yeast immobilized in CAMB are recommended to carry out continuous fermentation, and further scale up bioethanol production in a magnetically stabilized fluidized bed reactor (MSFBR), where the position of the beads in the system can be controlled and maintained by the application of oscillating electric field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioethanol Production
- Bioethanol Yield
- Molasses Concentration
- Ethanol Production
- Magnetite Nanoparticles
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nanotechnology and biofuels are two research fields which are exponentially growing. In past few years, nanoparticles have been found to be useful in various applications like in electronics, as catalysts and as an antimicrobial agent, photocatalytic degradation of organic dyes, in enhancing oil recovery, in health and environmental applications, to list few (Roy et al. 2014; Vanaja et al. 2014; Muthukrishnan et al. 2015). Generally, nanoparticles are chemically synthesized using processes involving reducing agents and capping agents under controlled conditions, or green synthesis using microorganisms or plant-based products (Abdul Rahman et al. 2014; Priyadarshini et al. 2014; Padman et al. 2014; Singh et al. 2015). The world economy has been dominated by technologies that depend solely on fossil energies, such as natural gas, coal or petroleum to produce chemicals, fuels, materials and power. A 50% rise in worldwide marketed energy expenditure has been projected by the US Energy Information Administration between 2005 and 2030. This growth will be predominantly observed in the non-OECD (Organization for Economic Co-operation and Development) or developing world countries where energy consumption is expected to increase by 85%, corresponding to the data collected by EIA in 2008 about 40.1% of world consumption (Mino 2010). Energy security and environmental concerns are largely the reason behind the growth of biofuels around the globe. To facilitate their growth, a wide range of incentives, market mechanisms and subsidies have been put in place. Biofuels provide an alternative to fossil fuel dependency and emit fewer pollutants (De Carvalho et al. 1993). Apart from these considerations, underdeveloped countries also view biofuels as a potential means to create employment opportunities as well as stimulate rural development. For example, India ranks sixth in terms of energy demand, accounting for 3.6% of the total global energy demand. Crude oil has been the major resource to meet the energy demand, and the demand for oil and its products is increasing dramatically every year. In India, biofuels are based mainly on feedstocks which are non-food based, to avoid a possible conflict of fuel versus food security. By year 2025, most of the petroleum in India will be imported. Estimates have indicated that more than 150 million tonnes of crude oil was consumed in 2007–08. The domestic crude oil is only able to meet around 23% of the actual demand, while the rest of the demand is fulfilled by importing crude oil from other countries. In 2008, in an effort to increase its energy security and independence, the National Policy on Biofuels was announced by the Government of India, mandating a phase-wise implementation of the programme of ethanol blended in petrol in various states. The oil marketing companies (OMCs) were to take up the blending of ethanol at 5% with petrol in 20 states and four national territories. However, due to shortage in ethanol, the implementation of this policy has not had much success (Ray et al. 2012). Bioethanol is an eco-friendly alternate biofuel that can be used in unmodified petrol engines with current fuelling infrastructure and it is easily applicable in the present-day combustion engine, as mixing with gasoline (Hansen et al. 2005). Relatively low emission of carbon monoxide, oxides of nitrogen and other volatile organic compounds are the product of ethanol combustion. Emission from ethanol combustion is lower compared to the emission of fossil fuel combustions such as diesel and gasoline, and its toxicity is also low (Wyman and Hinman 1990).
2 Substrates Used for Bioethanol Production
Bioethanol can be produced from sugar, biomass and wastes. However, the nature of the substrate greatly affects the processes of the ethanol fermentation. Therefore, the raw materials selected for ethanol fermentation have great importance in the fermentation process (Baptista et al. 2006). Hydrolysed enzymes ferment the complex sugars to reducing sugars and then to high concentrations of ethanol. It is also being made from a variety of agricultural by-products such as grain, fruit juices, fruit extracts, whey, sulphite waste liquor and molasses (Nigam et al. 1998). Generally, molasses is extracted from different agricultural sources such as sugarcane and sugar beet. It is a sugary–syrupy dark material left after the extraction of sugar from the mother syrup, and it is very rich in nutrients required by most microorganisms. Molasses are generally found to contain 45–60% total sugars, 20–25% reducing sugars, 25–35% sucrose, 10–16% ash, 0.4–0.8% calcium, 0.1–0.4% sodium, 1.5–5% potassium and pH 5–5.5 (Chen and Chou 1993). Molasses has no furfural, which is toxic to most of fermenting microorganisms (El-Gendy et al. 2013). Generally, cane molasses is reported to contain less sucrose and more invert sugar, and lower nitrogen and raffinose, dark colour and extra buffer capacity (Wang et al. 1984; Borzani et al. 1993, Borzani 2001). Although Brazil produces the most sugarcane, India is the world’s largest producer of sugar. The majority of the sugarcane grown in India is used by sugar mills to produce sugar and its main by-products: molasses and bagasse. Currently, 70% of the harvested sugarcane is utilized by regulated mills to produce sugar. The other 20-30% is used for the production of alternate sweeteners: gur and khandsari (Jaggery) and for seeds (Raju et al. 2009).
3 Saccharomyces Cerevisiae for Bioethanol Production
Worldwide demand of ethanol is generally satisfied by biotechnological fermentation process but various processes have been developed for ethanol production. Screening of a number of organisms for ethanol production has been performed, which include fungi, yeast and bacteria. These organisms have been studied extensively to determine their ethanol fermentation capabilities, especially yeast cells (Bajaj et al. 2001). S. cerevisiae is one such highly studied and utilized eukaryotic microorganism—yeast is a unicellular microorganism. S. cerevisiae cells measure 5–10 microns wide and 5–12 µm long. S. cerevisiae was originally believed to have been isolated from the skin of grapes (Pretorius 2000). It has an optimum temperature growth range at 30 °C, and it is tolerant of a wide pH range (2.4–8.2), being the optimum pH for growth between values of 3.5 and 3.8 (Gray 1941, 1948). With respect to the nutritional requirements, all strains can grow aerobically on glucose, fructose, sucrose and maltose and fail to grow on lactose and cellobiose. Also, all strains of S. cerevisiae can use ammonia and urea as the sole nitrogen source but cannot use nitrate since they lack the ability to reduce them to ammonium ions. They can also use most amino acids, small peptides and nitrogen bases as a nitrogen source (Bisson 1999). Ethanol is produced by fermentation when certain species of yeast (notably S. cerevisiae) metabolize sugar in the absence of oxygen, producing ethanol and carbon dioxide. Ethanol is well known as an inhibitor of growth of microorganisms. It has been reported to damage mitochondrial DNA in yeast cells (Ibeas and Jimenez 1997) and to cause inactivation of some enzymes. Nevertheless, some strains of the yeast S. cerevisiae show tolerance and can adapt to high concentrations of ethanol (Ghareib et al. 1988; Alexandre et al. 1994). Many studies have documented the alteration of cellular lipid composition in response to ethanol exposure (Mishra and Prasad 1989; Ingram 1976). It has been found that S. cerevisiae cells grown in the presence of ethanol appear to increase the amount of monounsaturated fatty acids in cellular lipids (Beaven et al. 1982).
S. cerevisiae can be used as either free cells or as immobilized to different matrices for ethanol production. Immobilization is a general term describing a wide variety of the cell or particle attachment or entrapment (López et al. 1997). It can be applied to basically all types of biocatalysts including enzymes, cellular organelles, animal cells and plant cells. The major advantage of immobilized cells, in contrast to free-living cells and immobilized enzymes, is reduction of the cost of bioprocessing as there is no involvement of pure enzymes, which are very costly even when procured in small quantities, and no requirement for additional steps of cell separation. The biocatalyst can be used repeatedly and continuously, and high cell density is maintained. In addition, immobilization can provide resistance to shear for shear-sensitive cells such as those from plants and animals. Different immobilization types have been defined: covalent coupling/cross-linking, capture behind semipermeable membrane or encapsulation, entrapment and adsorption (Mallick 2002). The types of immobilization can be grouped as ‘passive’ (using the natural tendency of microorganisms to attach to surfaces—natural or synthetic, and grow on them) and ‘active’ (flocculent agents, chemical attachment and gel encapsulation) (Cassidy et al. 1996; Cohen 2001; Moreno-Garrido 2008). The use of calcium alginate for immobilization of yeast cells has been around since 1980s. Calcium alginate is preferred because beads made of alginate can be stable for a period of more than 90 days (Nagashima et al. 1983). Cells immobilized on a variety immobilization matrix show comparatively higher yield when utilized for ethanol production (Black et al. 1984; McGhee et al. 1984) as compared to free-living cells. A variety of supports for the immobilization of S. cerevisiae were studied, such as spheres of stainless steel (Black et al. 1984), cellulose (Okita et al. 1985), calcium alginate (McGhee et al. 1984), synthetic commercial sponge (Del Borghi et al. 1985) cotton cloth (Joshi and Yamazaki 1984), immobilized cell reactor (Najafpour et al. 2004), yeast anchored on calcium alginate and clay support (Osawemwenze and Adogbo 2013). S. cerevisiae cells were entrapped in a matrix of alginate and magnetic nanoparticles (CAMB) and covalently immobilized on magnetite-containing chitosan (CHMM) and cellulose-coated magnetic nanoparticles (CCMN) (Ivanova et al. 2011).
4 Magnetite Nanoparticles
Magnetite (Fe3O4) is a biocompatible material, with low toxicity and strong magnetic properties, which responds to an external magnetic field, but not interacting in the absence of magnetic field. It has (Huang et al. 2003). It has been widely used for in vivo examination including magnetic resonance imaging, contrast enhancement, tissue-specific release of therapeutic agents, gene therapy (Berry and Curtis 2003), hyperthermia (Tartaj et al. 2006) and magnetic field assisted radionuclide therapy (Pankhurst et al. 2003), as well as in vitro binding of proteins and enzymes.
It also has biological and medical applications which include tissue repair, immunoassay, detoxification of biological fluids and cell separation (Gupta and Gupta 2005). Due to ferromagnetic properties of magnetite and diamagnetic properties of accompanying molecules and particulate matter, loaded magnetic adsorbents and carriers can be separated from suspensions with the use of magnetic fields (Šafarík and Šafaríková 1999, 2001, 2002). There are a variety of methods for the synthesis of magnetite nanoparticles in various irregular shapes, such as co-precipitation, ultrasound irradiation, hydrothermal and electrochemical synthesis, and pyrolysis, which produce nanoparticles with sizes ranging from ≈5 to 100 nm (Nyirő-Kósa et al. 2012; El Ghandoor et al. 2012).
5 Case Study
We studied immobilization of locally isolated S. cerevisiae yeast strain in calcium alginate magnetite beads (CAMB), to produce ethanol. The media components and environmental parameters were statistically screened and optimized for better ethanol production, using statistical design methodologies. Further, ethanol production was carried out in packed-bed column reactor using yeast immobilized in CAMB, under fed-batch mode.
5.1 Materials and Chemicals
All the chemicals were of analytical grade, purchased from Loba Chemie, HiMedia, Baroda Chemical Industries Ltd., SuLab, and Fisher Scientific, India Ltd. Rose bengal chloramphenicol (RBC) HiVeg agar was purchased from HiMedia, India. Sugarcane molasses and sugarcane bagasse were procured from local farmers and market. When not in use, the molasses was stored at 4 °C.
5.2 Isolation and Maintenance of Yeast
The yeast S. cerevisiae was isolated using RBC HiVeg agar medium from soil samples collected near a local sugar mill. The culture was maintained on RBC HiVeg agar medium by sub-culturing every 15 days and incubating at 30 °C for 24 h. The yeast culture was also preserved in 25% glycerol solutions for long-term preservation at 5 °C.
5.3 Synthesis of Magnetite (Fe3O4) Nanoparticles
Ultra-fine particles of magnetite nanoparticles (Fe3O4) were prepared by co-precipitating aqueous solutions of (NH4)2Fe(SO4)2 and FeCl3 mixtures, respectively, in alkaline medium. (NH4)2Fe(SO4)2 and FeCl3 solutions were mixed in their respective stoichiometry (i.e. ratio Fe+2: Fe+3 = 1:2). The mixture was kept at 80 °C. This mixture was added to the boiling solution of NaOH (0.5 mol. was dissolved in 600 mL of distilled water) within 10 s under constant stirring. Magnetite was formed by conversion of metal salts into hydroxides, which took place immediately, and transformation of hydroxides into ferrites. The solution was maintained at 100 °C for 1.5 h. The Fe3O4 particles were washed several times by distilled water (El Ghandoor et al. 2012) and dried in an oven.
5.4 Immobilization of S. Cerevisiae
Active cultures of S. cerevisiae for fermentation were prepared in Wickerham WH media: 2 g/L, KH2PO4); 10 g/L, (NH4)2SO4; 1 g/L, MgSO4·7H2O; 2 g/L, Yeast extract; and 10 g/L, Glucose (Haynes et al. 1955) for 48 h at 30 °C. Cells were harvested by centrifugation and were washed twice with sterile saline (0.85 g NaCl in 100 mL distilled water) and then suspended in sterile saline to be used later for inoculation. The S. cerevisiae cells were harvested at exponential phase to be utilized for immobilization. Sodium alginate (2%) was completely dissolved over a period of 4 h by continuous stirring on magnetic stirrer and then autoclaved at 121 °C for 15 min. The cells were mixed with the sodium alginate slurry. The final inoculum size was 10 mg of cell dry weight/mL of gel, and the final content of nanoparticles was 2.5% (w/v). The contents were thoroughly mixed for even dispersal of cells as well as magnetite nanoparticles. To prepare the beads, the slurry, containing yeast cells and magnetite nanoparticles, was dispersed dropwise using a sterile syringe and plunger into chilled 2% CaCl2 solution which was previously sterilized. As soon as the drops of sodium alginate came in contact with the chilled calcium chloride solution, spherical beads were formed as the sodium ion was replaced by calcium ions. The calcium alginate magnetite beads (CAMB) containing the cells were incubated at 4 °C for proper chelation and then thoroughly washed with distilled water repeatedly. The beads were then placed in fresh sterile CaCl2 solution and refrigerated to be used for further studies.
5.5 Analytical Methods
Reducing sugars were analysed by dinitrosalicylic acid (DNS) method (Miller 1959). The reducing sugar concentration in the sample was calculated using the standard curve of D-glucose. Total soluble carbohydrate in molasses was determined by the phenol–sulfuric acid method (Dubois et al. 1956; Joshi et al. 2008; Al-Bahry et al. 2013), and concentration of carbohydrates was estimated by comparing it with standard sucrose and glucose solutions. The ethanol concentration was determined by dichromate oxidation and thiosulphate titration (Marcelle et al. 2007; Ingale et al. 2014).
5.6 Experimental Designs
5.6.1 Screening Design
Screening was carried out by Plackett–Burman design of the most important components affecting bioethanol production by S. cerevisiae using sugarcane molasses. For the designing of experiments, Design Expert software 9.0.4.1 (Stat-Ease, Inc., Minneapolis, MN, USA) was utilized. A total of 11 components were evaluated, with each being represented at two levels, High (H) and Low (L). In the design, it is assumed that the main factors have individual effects but no interactions, and a first-order polynomial equation is appropriate (Eq. 1):
where Y represents the response, β0 is the model coefficient, βi is the linear coefficient, xi is the variables and n is the number of parameters (variables). The effect of each variable was determined by Eq. 2:
where E(xi) is the response value effect of the tested variable, ΣMi+ is the summation of the response value at high level, ΣMi− is the summation of the response value at low level and N is the number of experiments. Table 1 represents the factors to be evaluated. Table 2 shows the design matrix built by statistical software Design Expert software 9.0.4.1 (Stat-Ease, Inc., Minneapolis, MN, USA) for the evaluation of 11 variables in 20 experimental runs. Variables A through L represent the 11 medium components (actual variables) and D1 through D8 represent dummy variables (used to reduce error in data). Data were analysed through analysis of variance (ANOVA).
5.6.2 Optimization Design
After selecting the most important variables which influenced the bioethanol production by S. cerevisiae, response surface methodology (RSM) was used for optimization of the process. The central composite design (CCD) was applied to study the different process variables. The behaviour of the system was demonstrated by the following quadratic equation (Eq. 3):
where Y is the predicted response, β0 is a constant, βi is the linear coefficient, βii is the squared coefficient, βij is the cross-product coefficient and xi is the dimensionless coded value of (Xi). The above equation was solved by using the statistical software Design Expert software 9.0.4.1 (Stat-Ease, Inc., Minneapolis, MN, USA). A 25 factorial design with five replicates at the centre point with a total number of 20 trials were employed.
5.7 Packed-Bed Fermentation Under Fed-Batch Mode
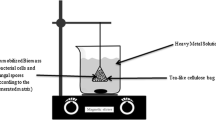
After optimization of bioethanol production by batch fermentation, fed-batch bioethanol fermentations were also carried out. The diameter of the column was 3 cm, and the length of the column was 45 cm. The volume of the column without beads was 230 mL. When the column was packed with CAMB, the void volume of the column was 100 mL. The column was packed to 70% of the column volume. The reactor was set up using standard IV (Intravenous) infusion set to control the feed rate (Fig. 1). The fed-batch fermentation was carried out at molasses concentration of 20 g% (w/v), temperature of 28 °C and incubation time of 72 h. The feeding rate was 0.06 g mL−1 h−1.
5.8 Scanning Electron Microscopy of CAMB
For electron microscope scanning, samples of calcium alginate magnetite beads (CAMB) immobilized with S. cerevisiae were taken after 96 h of ethanol fermentation. The samples were examined under a scanning electron microscope model-XL 30 ESEM with EDAX (Philips, Netherlands). The resolution of the instrument was up to 2 Å, acceleration voltage of 0.2–30 kV and up to 2,50,000× magnification. The analysis was performed at Charutar Vidya Mandal’s SICART (Sophisticated Instrumentation Centre for Applied Research and Testing) facility, Gujarat, India.
6 Results and Discussions
6.1 Isolation of Yeast and Immobilization in Calcium Alginate Magnetite Nanoparticles
The yeast was locally isolated and identified as S. cerevisiae (Fig. 2). The synthesized and dried magnetite nanoparticles used to prepare calcium alginate beads are shown in Fig. 3. S. cerevisiae was immobilized in calcium alginate magnetite beads as described and was stored in fridge prior to the experiment (Fig. 4). The concentrations of total sugars as well as reducing sugar present in molasses were analysed by different methods. The concentration of total sugar present in cane molasses analysed in the present study was 29.42%, which was more or less similar to that reported by others (Nofemele et al. 2012; Bajaj et al. 2003). Sugarcane bagasse was pretreated with 5% H2SO4 for 2 h, was neutralized with NaOH and was used as a pretreated hydrolysate in the optimization experiments to see its effect on ethanol production. There was no requisite for pretreatment of the molasses as it does not contain complex compounds such as cellulose and lignin.
6.2 Screening of Variables by Plackett–Burman Design
The statistical design used for the optimization of ethanol production was an 11-factor system with eight dummy variables, with the factors being molasses, potassium di-hydrogen phosphate, ammonium sulphate, magnesium sulphate, yeast extract, pH, temperature, incubation time, agitation and pretreated sugarcane bagasse hydrolysate. The responses of the system were the ethanol production and ethanol yield. The design summary is shown in Table 3.
The design was used to identify the most important factors early in the experimentation phase in order to screen out the factors which have significant impact on bioethanol production as compared to other less significant factors. It was observed that runs 6, 8, 10, 17 and 20 had maximum ethanol production and maximum ethanol yield.
The adequacy of the factorial model for the experimental responses (ethanol production R1 and yield R2) was checked using the analysis of variance (ANOVA), which was verified using the Fisher’s statistical model (F-value). Table 4 shows the ANOVA for R1 response. ANOVA of the factorial model for ethanol production had the ‘Model F-value’ of 3.24, which implied the model was significant. There was only a 4.98% chance that a ‘Model F-value’ this large could occur due to noise.
Since ‘p-value’ of the model was less than 0.0500, it indicated that the model was significant. The ‘p-value’ of molasses (A) was 0.004, which is less than 0.0500, which indicated that the factor had a significant effect on the production of ethanol.
A normal probability of the standardized residuals for ethanol production is shown in Fig. 5. A normal probability plot indicates that if the residuals follow a normal distribution, in which case, the points will follow a straight line. Since some scattering is expected even with the normal data, it can be assumed that the data is normally distributed. Thus, it indicates a good validity for the approximation of factorial model.
Table 5 shows the ANOVA for R1 response. ANOVA of the factorial model for bioethanol yield had the ‘Model F-value’ of 7.64, which implied the model was significant. There was only a 0.22% chance that a ‘Model F-value’ this large could occur due to noise. Since ‘p-value’ of the model was less than 0.0500, it indicated that the model was significant. The ‘p-value’ of molasses (A) was 0.0022, which is less than 0.0500, which indicated that the factor had a significant effect on the yield of ethanol.
A normal probability of the standardized residuals for ethanol production is shown in Fig. 6. A normal probability plot indicates that if the residuals follow a normal distribution, in which case, the points will follow a straight line. Since some scattering is expected even with the normal data, as shown in Fig. 6, it can be assumed that the data is normally distributed. Thus, the obtained probability plot indicates a good validity for the approximation of the factorial model. Based on the results obtained from the Plackett–Burman design, we selected three variables, namely, molasses concentration, temperature and the incubation time. Molasses concentration, temperature and the incubation time have positive influence on bioethanol production and yield hence higher levels of all the three variables resulted in higher bioethanol production. The other components of the production medium, such as KH2PO4, (NH4)2SO4, MgSO4.7H2O, yeast extract, pH, pretreated hydrolysate, immobilized yeast and agitation, were found to be insignificant, so their concentrations were set at their middle level in central composite design.
6.3 Optimization of Bioethanol Production and Yield by Response Surface Methodology (RSM)
6.3.1 Statistical Analysis and Validation of Model
The statistical design used for the bioethanol production is a three-factor (Molasses concentration, temperature and the incubation time) system. A total of 20 experiments with three variables and five coded levels (five different concentrations) were performed. Table 6 shows the coded and actual values of the variables. The response of the production was based on the ethanol and yield. The design summary is shown in Table 7.
The design was a set of 20 runs, combinations of three-factor experimental design, based on the RSM and CCD as shown in Table 8. The RSM is a mathematical-based system utilized to study the interactions between the factors, while the CCD enables the deduction of optimal condition for bioethanol production. CCD contains an embedded factorial or fractural factorial design with centre points that is augmented with a group of ‘star points’ that allow estimation of curvature. As shown in Table 8, runs 12, 13, 17 and 20 had maximum bioethanol production and maximum yield. The quadratic polynomial equations describe the correlation between the significant coefficients, i.e. p-value (Prob > F) less than 0.05, and are used to obtain the regression values of coefficients where only significant coefficients are considered. Since this model supports hierarchy, the insignificant coefficients were not omitted. This equation was used to derive the predicted responses for ethanol (Eq. 4) and yield (Eq. 5).
The adequacy of the quadratic model for the experimental responses (Ethanol R1 and Yield R2) was checked using the analysis of variance (ANOVA), which was verified using the Fisher’s statistical model (F-value). Table 9 shows the ANOVA for R1 (Ethanol) response. ANOVA of the response surface quadratic model for response R1 had an ‘F-value’ of 8.80, which implied that the model was significant. There was only a 0.11% chance that an F-value this large could occur due to noise. The ‘p-value’ of the model was 0.00107, which is less than 0.05; this indicates that the model terms are significant and imply that the bioethanol production is sensitive to the factors/coefficients in the model. The factors which have the most significant influence on bioethanol production are molasses (A) and incubation time (C). The ‘Lack of Fit F-value’ of 0.40 implies that the lack of fit is not significant relative to the pure error. There is an 83.37% chance that a ‘Lack of Fit F-value’ this large could occur due to noise. Non-significant lack of fit is good as we want the model to fit. Signal-to-noise ratio can be measured by another statistical measurement which is known as the ‘Adequate precision’. A ratio greater than 4 is desirable. The ratio of our model is 10.804, which indicates an adequate signal. This model can be therefore used to navigate the design space as well as for further optimization.
‘Coefficient of determination or R2’ value gives information about the goodness of fit of a model. It indicates the correlation between experimental and predicted values. If the value of R2 is closer to 1, it indicates that the filled model explains most of the variability, while a value closer to 0 indicates that there is no linear relationship between values. In the current study, the R2 value is 0.89, which indicates that the experimental and predicted values are in reasonable agreement. The ‘coefficient of variation (CV)’ is the standardized measure of dispersion; it indicates the degree of precision to which the experiments are compared. The higher reliability of the experiment is usually signified by a high value of CV. In the present study, the CV% value is low (16.67), which implies a good reliability and precision of the experiment.
The ‘Predicted coefficient of determination (Pred R2)’ of 0.6398 is in reasonable agreement with the ‘Adjusted coefficient of determination (AdjR2)’ of 0.7870, i.e. the difference is less than 0.2. This suggests that the data fits well with the model and gives a decisively good estimate of response for the system. The contour plots below show the interactive effect of incubation time and molasses concentration on bioethanol production when the temperature is 32.5 °C (Fig. 7), and the interactive effect of temperature and molasses concentration on bioethanol production when the incubation time is 72 h (Fig. 8).
From the figures, it can be observed that significantly higher production of bioethanol was obtained with proportional increase in molasses concentration and increase in incubation time (Fig. 7). Substantial production of bioethanol was obtained with increase in molasses concentration and no corresponding increase in temperature (Fig. 8). The perturbation plot (Fig. 9) shows the comparative effects of the three variables on bioethanol production. The sharp curvature of two factors—Molasses (A) and incubation time (C)—shows that the bioethanol yield was sensitive to these variables. The comparatively almost flat curve for temperature (B) shows less sensitivity of the response towards this factor. Thus, the temperature of fermentation is not a major variable when immobilized cells are applied for bioethanol production.
The three-dimensional diagram (Fig. 10) displays the interactive effects of molasses concentration and incubation time on bioethanol production at a constant temperature of 32.5 °C. It can be observed from the graph that as the molasses concentration increases, the bioethanol production also increases. The bioethanol concentration also increases when the incubation time is elongated. The simultaneous increase in both the molasses concentration and incubation time shows their cooperative effect on bioethanol production as it increases proportionally to the increase in these two variables. The maximum ethanol production was observed when the molasses concentration was 20 g% (w/v) and the incubation time was 96 h. On the other hand, temperature played no significant role in bioethanol production by immobilized cells of S. cerevisiae.
The second response considered is the bioethanol yield (R2). ANOVA of the response surface quadratic model for bioethanol yield is shown in Table 10. The model is a significant model with Fisher F-test value of 3.55, with only a 3.07% chance that an F-value this large could occur due to noise. Values of ‘Prob > F’ less than 0.0500 indicate that model terms are significant. In this case, C is a significant model term with a ‘p-value’ of 0.0005. The goodness of fit for the model was analysed by the value of the coefficient of determination (R2). In this response, the value of R2 is 0.76, which indicates that the experimental and predicted values are in reasonable agreement. The ‘Lack of Fit F-value’ of 0.32 implies the lack of fit is not significant relative to the pure error. There is an 88.15% chance that a ‘Lack of Fit F-value’ this large could occur due to noise. Non-significant lack of fit is good as we want the model to fit. The CV% value for the model is low at 16.16, which implies a good reliability and precision of the experiment.
The ‘Predicted coefficient of determination (Pred R2)’ of 0.6398 is in reasonable agreement with the ‘Adjusted coefficient of determination (Adj R2)’ of 0.5466, i.e. the difference is less than 0.2. This suggests that the data fits well with the model and gives a decisively good estimate of response for the system. ‘Adequate precision’ measures the signal-to-noise ratio. A ratio greater than 4 is desirable. The ratio of our model is 6.485, which indicates an adequate signal. This model can be therefore be used to navigate the design space as well as for further optimization.
Figure 11 shows a contour plot which shows the effect of incubation time and molasses concentration on bioethanol yield. It can be observed that incubation time is the major variable that affects the yield, while the effect of molasses concentration on the yield is not as significant. From the colour of the graph, it can be deduced that the incubation time between 80 and 96 h is adequate to increase the ethanol concentration to 75% or above. The perturbation plot (Fig. 12) shows the comparative effects of the three variables on bioethanol production. The sharp curvature of one factor, incubation time (C), shows that the bioethanol yield was sensitive to this variable. The comparatively almost flat curve for molasses (A) and temperature (B) showed less sensitivity of the response (i.e. yield) towards those factors. Thus, the molasses concentration and temperature of fermentation are not major variables when bioethanol yield is concerned. The 3D plot (Fig. 13) shows the interactive effects of molasses concentration and incubation time on bioethanol production at a constant temperature of 32.5 °C. It can be observed that as the incubation time increases, the bioethanol yield also increases.
6.3.2 Optimization of Fermentation Process and Model Verification
Statistical methods such as factorial designs and response surface methodologies are widely used for the improvement of several bioproducts, including bioethanol (Joshi et al. 2007; Kshirsagar et al. 2015; Raheem et al. 2015; Turhan et al. 2015). The process of optimization was carried out to determine the optimum value of bioethanol production, using the Design Expert software 9.0.4.1, Stat-Ease, Inc. According to the built-in optimization step, the desired goal for each operational condition, i.e. Molasses (A), temperature (B) and incubation time (C), was chosen within the studied range. The response (bioethanol production) was defined as ‘maximum’ to achieve the highest performance. The programme combines the individual desirability into a single number and then searches to optimize this function based on the response goal. Accordingly, the optimum working conditions and respective bioethanol production were established, and the results are presented in Table 11. The average bioethanol production after optimization was 2.3 g% ± 0.14. Similarly, for the optimization of bioethanol yield, the response was defined as ‘maximum’ to achieve the highest performance. The optimum working conditions and respective bioethanol yield are presented in Table 12.
The maximum bioethanol yield observed in the study after optimization, which is 91.0245% ± 0.51, was comparable to the theoretical yield in the work done by Göksungur and Zorlu (2001) in Turkey using Ca-alginate immobilized S. cerevisiae with beet molasses serving as the substrate. It was also similar to the yield obtained by Ivanova et al. (2011), who obtained an average of 90% of the theoretical yield using CAMB for simultaneous ethanol fermentation and starch saccharification. It was greater than the theoretical yield observed by Limtong et al. (2007), who had utilized Kluyveromyces marxianus as the fermentation organism and sugarcane juice as the substrate. The validation of the RSM was carried out to confirm the results of ethanol production and ethanol yield. The maximum ethanol production and yield obtained were 2.75 g% and 91.85%, respectively, at the molasses concentration of 20 g% (w/v), temperature 28 °C and incubation time of 96 h. Due to the incorporation of magnetite in immobilized beads of S. cerevisiae, it was observed that when the immobilized beads were added into the production media, the beads settled at the bottom of the conical flask, while the immobilized beads lacking magnetite did not settle to the bottom of the flask and were instead observed to be floating on the surface of the media. Alcohol production is an anaerobic process, so when the beads settle at the bottom of the flask, where there is less oxygen available, they are able to produce alcohol more efficiently.
6.4 Fed-Batch Packed-Bed Fermentation
The ethanol produced and the yield were almost constant in every batch. The average ethanol produced by fed-batch fermentation was 1.832 g% ± 0.103. The average ethanol yield was 81.420% ± 4.6. Prakasham et al. (1999) investigated the catalytic role of various inert solid supports on the acceleration of alcoholic fermentation by S. cerevisiae. The tested supports were de-lignified sawdust, de-lignified wheat bran, river sand, chitin, chitosan and titanium oxide. The results of the alcoholic fermentation showed that all carriers stimulated ethanol production, which was attributed to the attachment of the cells to these materials. Bekers et al. (1999) used porous spheres of stainless steel treated by oxidation with TiCl4 or aminopropyltrietoxilase, as carriers for yeast cells. The assays of batch fermentation using an inoculum of immobilized cells showed an increase of yeast cell stability and ethanol production. The authors suggested that the increase of ethanol synthesis by cell immobilization in porous treated stainless steel could be the result of catalytic action of some carrier surface element on metabolism. Nigam et al. (1998) carried out alcoholic fermentation using agar-immobilized yeast cells. A packed-bed reactor was employed, and cane molasses was utilized. They obtained a maximum productivity of 79.5 g ethanol/L h with 195 g/L reducing sugar as feed. Low dilution rates are allowed for proper utilization of sugar, which in turn affected the ethanol concentration and volumetric ethanol productivity. The process was continued for 100 days, and the beads remained stable over the course of the fermentation. In the study conducted by Göksungur and Zorlu (2001), it was found that on employing continuous immobilized packed-bed reactor for ethanol production, ethanol concentration of 4.43% and a theoretical yield of 79.5% were observed at the end of 25 days. The 2% calcium alginate beads also retained their structure over the course of fermentation. Osawemwenze and Adogbo (2013) studied the ethanol synthesis using yeast anchored on calcium alginate and clay support. They observed that immobilized yeast cells using clay support gave higher ethanol product yield in both batch and fed-batch processes as compared to calcium alginate support Ivanova et al., (2011). S. cerevisiae cells were entrapped in a matrix of alginate and magnetic nanoparticles (CAMB) and covalently immobilized on magnetite-containing chitosan (CHMM) and cellulose-coated magnetic nanoparticles (CCMN). These immobilized cells were applied in column reactors for ethanol fermentation. The type of immobilization affected the ethanol fermentation along with other factors such as feed sugar concentration, initial particle loading and the dilution rate. The overall ethanol yield of 88.8% was obtained using CAMB for ethanol fermentation from starch hydrolysates. Table 13 shows the ethanol production by different microorganisms immobilized on different substrates.
6.5 Evaluation of Calcium Alginate Magnetite Beads (CAMB)
The hardness and rigidity of the CAMB were tested manually by the application of pressure. There was sufficient substrate penetration into the beads due to better porosity, and the beads were strong enough to hold the weight of packing in the column. They were also stable and active for a long time period. The beads could be stored by refrigeration for more than 100 days. After the CAMB were used for ethanol fermentation, some swelling in the size of the beads was observed. An increase in the size of the beads by almost 10% was observed after repeated ethanol fermentations. The CAMBs with immobilized yeast cells were analysed by ESEM with EDAX to observe the surface structure of the beads. It can be observed that yeast is immobilized in the beads and is actively growing (Fig. 14).
7 Conclusion and Future Outlook
Biofuels are derived from renewable biomass resources, so they are a definite strategic advantage for the promotion of sustainable development of renewable energy resources. They can supplement conventional energy sources in meeting the rapidly increasing requirements for transportation fuels, which can be associated with high economic growth, as well as in meeting the energy needs of any countries vast agrarian, suburban and metropolitan population. To a greater extent, biofuels can satisfy these energy needs in an environmentally benign and cost-effective manner while reducing dependence on import of fossil fuels and thereby providing a higher degree of National Energy Security.
In this study, the effects of multiple factors were evaluated on the basis of different statistical models such as Plackett–Burman and response surface methodology (Central Composite Design). On the basis of Plackett–Burman analysis, it was determined that the model was significant, and the factors of molasses concentration, temperature and incubation time were found significant. Other factors such as potassium di-hydrogen phosphate, ammonium sulphate, magnesium sulphate, yeast extract, pH, immobilized yeast, agitation and pretreated hydrolysate were found to be not significant. This means that all these factors are acceptable at their minimum levels as compared to the significant factors. The reason for this could be that since the cells were immobilized and already in the stationary phase, growth factors and nutrients such as KH2PO4, (NH4)2SO4, MgSO4 and yeast extract were not required in higher concentration. Since the organisms were immobilized, pH did not adversely affect the rate of bioethanol production. Pretreated hydrolysate, which was added (5%) to observe the effect of furfural compound on S. cerevisiae, also did not affect the rate of bioethanol production. On the basis of response surface methodology—central composite design, it was determined that the quadratic model was significant, and the factors of molasses concentration and incubation time were found to be significant. Temperature was not found to be a significant factor. The reason for this could be that since the organisms were immobilized, there was less effect of temperature on the immobilized cells. The immobilized cells could be reused for more than 120 days, retaining its original activity.
Molasses is the current major source for bioethanol production and it is available cheaply due to it being a waste by-product of sugar mills. Molasses is more preferred over lignocellulosic substrates because despite being cheaper than molasses, such substrates require additional treatment before they can be utilized for bioethanol production. Also, molasses has sugars which are readily degraded by microorganisms. When lignocellulosic substrates are given acid treatment, hydroxymethylfurfural (HMF) is produced, which is inhibitory to the production of ethanol by microorganisms.
The main advantage of immobilized system for large-scale industrial production of bioethanol is that it is economically beneficial because it eliminates the need for a separate process of cell removal from the product stream. Also, in this study, the effect of hydroxymethylfurfural (HMF) on the activity of immobilized cells was observed and it was found that immobilized cells could tolerate 5% concentration of HMF. Therefore, immobilized yeast cells can be used for the production of bioethanol from sources which contain diluted sugar such as effluent from paper–pulp industry as well as acid-treated lignocellulose substrates. Further studies using yeast immobilized in CAMB are needed such as continuous fermentation, further scale up and bioethanol production in a magnetically stabilized fluidized bed reactor (MSFBR), where the position of the beads in the system can be controlled and maintained by the application of oscillating electric field.
References
Abdul Rahman I, Ayob MTM, Radiman S (2014) Enhanced photocatalytic performance of NiO-decorated ZnO nanowhiskers for methylene blue degradation. J Nanotechnol 212694:8. https://doi.org/10.1155/2014/212694
Al-Bahry SN, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ, Al-Makhmari HS, Al-Sulaimani HS (2013) Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int Biodeterior Biodegradation 81:141–146
Alexandre H, Rousseaux I, Charpentier C (1994) Ethanol adaptation mechanisms in Saccharomyces cerevisiae. Biotechnol Appl Biochem 20(2):173–183
Amin G, De Mot R, Van Dijck K, Verachtert H (1985) Direct alcoholic fermentation of starchy biomass using amylolytic yeast strains in batch and immobilized cell systems. Appl Microbiol Biotechnol 22(4):237–245
Bajaj BK, Taank V, Thakur RL (2003) Characterization of yeasts for ethanolic fermentation of molasses with high sugar concentrations. J Sci Ind Res 62(11):1079–1085
Bajaj BK, Yousuf S, Thakur RL (2001) Selection and characterization of yeasts for desirable fermentation characteristics. Indian J Microbiol 41(2):107–110
Bajpai PK, Margaritis A (1985) Kinetics of ethanol production by immobilized cells of Zymomonas mobilis at varying D-glucose concentrations. Enzyme Microb Technol 7(9):462–464
Baptista CMSG, Cóias JMA, Oliveira ACM, Oliveira NMC, Rocha JMS, Dempsey MJ, Benson PS (2006) Natural immobilisation of microorganisms for continuous ethanol production. Enzyme Microb Technol 40(1):127–131
Beaven MJ, Charpentier C, Rose AH (1982) Production and tolerance of ethanol in relation to phospholipid fatty-acyl composition in Saccharomyces cerevisiae NCYC 431. J Gen Microbiol 128(7):1447–1455
Bekers M, Ventina E, Karsakevich A, Vina I, Rapoport A, Upite D, Linde R (1999) Attachment of yeast to modified stainless steel wire spheres, growth of cells and ethanol production. Process Biochem 35(5):523–530
Berry CC, Curtis AS (2003) Functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36(13):R198
Bisson LF (1999) Stuck and sluggish fermentations. Am J Enol Vitic 50(1):107–119
Black GM, Webb C, Matthews TM, Atkinson B (1984) Practical reactor systems for yeast cell immobilization using biomass support particles. Biotechnol Bioeng 26(2):134–141
Borzani W (2001) Variation of the ethanol yield during oscillatory concentrations changes in undisturbed continuous ethanol fermentation of sugar-cane blackstrap molasses. World J Microbiol Biotechnol 17(3):253–258
Borzani W, Gerab A, De La Higuera GA, Pires MH, Piplovic R (1993) Batch ethanol fermentation of molasses: a correlation between the time necessary to complete the fermentation and the initial concentrations of sugar and yeast cells. World J Microbiol Biotechnol 9(2):265–268
Cassidy MB, Lee H, Trevors JT (1996) Environmental applications of immobilized microbial cells: a review. J Ind Microbiol 16(2):79–101
Chen JC, Chou CC (1993) Cane sugar handbook: a manual for cane sugar manufacturers and their Chemists. Wiley, New York
Cohen Y (2001) Biofiltration–the treatment of fluids by microorganisms immobilized into the filter bedding material: a review. Biores Technol 77(3):257–274
De Carvalho JCM, Aquarone E, Sato S, Brazzach ML, Moraes DA, Borzani W (1993) Fed-batch alcoholic fermentation of sugar cane blackstrap molasses: influence of the feeding rate on yeast yield and productivity. Appl Microbiol Biotechnol 38(5):596–598
Del Borghi M, Converti A, Parisi F, Ferraiolo G (1985) Continuous alcohol fermentation in an immobilized cell rotating disk reactor. Biotechnol Bioeng 27(6):761–768
Dubois M, Gilles KA, Amilton JK (1956) Colorimetric determination of sugars and related substances. Anal Chem 28:350–356
El Ghandoor H, Zidan HM, Khalil MM, Ismail MIM (2012) Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int J Electrochem Sci 7:5734–5745
El-Gendy NS, Madian HR, Amr SSA (2013) Design and optimization of a process for sugarcane molasses fermentation by Saccharomyces cerevisiae using response surface methodology. Int J Microbiol 2013
Fujimura T, Kaetsu I (1985) Nature of yeast-cells immobilized by radiation polymerization activity dependence on the molecular-motion of polymer carriers. Zeitschrift Fur Naturforschung Ca J Biosci 40(7–8):576–579
Ghareib M, Youssef KA, Khalil AA (1988) Ethanol tolerance of Saccharomyces cerevisiae and its relationship to lipid content and composition. Folia Microbiol 33(6):447–452
Göksungur Y, Zorlu N (2001) Production of ethanol from beet molasses by Ca-alginate immobilized yeast cells in a packed-bed bioreactor. Turk J Biol 25(3):265–275
Gray WD (1941) Studies on the alcohol tolerance of yeasts. J Bacteriol 42(5):561
Gray WD (1948) Further studies on the alcohol tolerance of yeast: its relationship to cell storage products. J Bacteriol 55(1):53
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Hansen AC, Zhang Q, Lyne PW (2005) Ethanol–diesel fuel blends—a review. Biores Technol 96(3):277–285
Haynes WC, Wickerham LJ, Hesseltine CW (1955) Maintenance of cultures of industrially important microorganisms. Appl Microbiol 3(6):361
Huang SH, Liao MH, Chen DH (2003) Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol Prog 19(3):1095–1100
Ibeas JI, Jimenez J (1997) Mitochondrial DNA loss caused by ethanol in Saccharomyces flor yeasts. Appl Environ Microbiol 63(1):7–12
Ingale S, Joshi SJ, Gupte A (2014) Production of bioethanol using agricultural waste: banana pseudo stem. Braz J Microbiol 45(3):885–892
Ingram LO (1976) Adaptation of membrane lipids to alcohols. J Bacteriol 125(2):670–678
Ivanova V, Petrova P, Hristov J (2011) Application in the ethanol fermentation of immobilized yeast cells in matrix of alginate/magnetic nanoparticles, on chitosan-magnetite microparticles and cellulose-coated magnetic nanoparticles. Int Rev Chem Eng 3(3):289–299
Joshi S, Yamazaki H (1984) Film fermenter for ethanol production by yeast immobilized on cotton cloth. Biotech Lett 6(12):797–802
Joshi S, Bharucha C, Jha S, Yadav S, Nerurkar A, Desai AJ (2008) Biosurfactant production using molasses and whey under thermophilic conditions. Biores Technol 99(1):195–199
Joshi S, Yadav S, Nerurkar A, Desai AJ (2007) Statistical optimization of medium components for the production of biosurfactant by Bacillus licheniformis K51. J Microbiol Biotechnol 17(2):313
Kshirsagar SD, Waghmare PR, Loni PC, Patil SA, Govindwar SP (2015) Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv 5(58):46525–46533
Limtong S, Sringiew C, Yongmanitchai W (2007) Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Biores Technol 98(17):3367–3374
López A, Lázaro N, Marqués AM (1997) The interphase technique: a simple method of cell immobilization in gel-beads. J Microbiol Methods 30(3):231–234
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15(4):377–390
Marcelle A, de Vos Betty-Jayne, Visser MS (2007) The preparation, assay and certification of aqueous ethanol reference solutions. Accred Qual Assur 12:188–193
McGhee JE, Carr ME, St Julian G (1984) Continuous bioconversion of starch to ethanol by calcium-alginate immobilized enzymes and yeasts. Cereal Chem (US) 61(5)
Miller GL (1959) Use of DNS reagent for the measurement of reducing sugar. Anal Chem 31(1):426–428
Mino AK (2010) Ethanol production from sugarcane in India: viability, constraints and implications, Doctoral dissertation, University of Illinois at Urbana–Champaign
Mishra P, Prasad R (1989) Relationship between ethanol tolerance and fatty acyl composition of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 30(3):294–298
Moreno-Garrido I (2008) Microalgae immobilization: current techniques and uses. Biores Technol 99(10):3949–3964
Muthukrishnan S, Bhakya S, Kumar TS, Rao MV (2015) Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegia thwaitesii–An endemic species. Ind Crops Prod 63:119–124
Nagashima M, Azuma M, Noguchi S (1983) Technology developments in biomass alcohol production in Japan: continuous alcohol production with immobilized microbial cells. Ann NY Acad Sci 413(1):457–468
Najafpour G, Younesi H, Ismail KSK (2004) Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Biores Technol 92(3):251–260
Nigam JN, Gogoi BK, Bezbaruah RL (1998) Alcoholic fermentation by agar-immobilized yeast cells. World J Microbiol Biotechnol 14(3):457–459
Nofemele Z, Shukla P, Trussler A, Permaul K, Singh S (2012) Improvement of ethanol production from sugarcane molasses through enhanced nutrient supplementation using Saccharomyces cerevisiae. J Brew Distilling 3(2):29–35
Nyirő-Kósa I, Rečnik A, Pósfai M (2012) Novel methods for the synthesis of magnetite nanoparticles with special morphologies and textured assemblages. J Nanopart Res 14(10):1–10
Okita WB, Bonham DB, Gainer JL (1985) Covalent coupling of microorganisms to a cellulosic support. Biotechnol Bioeng 27(5):632–637
Osawemwenze LA, Adogbo GM (2013) Ethanol synthesis using yeast anchored on calcium alginate and clay support. Int J Sci Eng Res 4(4):479–484
Padman AJ, Henderson J, Hodgson S, Rahman PK (2014) Biomediated synthesis of silver nanoparticles using Exiguobacterium mexicanum. Biotech Lett 36:2079–2084
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36(13):R167
Prakasham RS, Kuriakose B, Ramakrishna SV (1999) The influence of inert solids on ethanol production by Saccharomyces cerevisiae. Appl Biochem Biotechnol 82(2):127–134
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16(8):675–729
Priyadarshini E, Pradhan N, Sukla LB, Panda PK (2014) Controlled synthesis of gold nanoparticles using Aspergillus terreus IF0 and its antibacterial potential against gram negative pathogenic bacteria. J Nanotechnol 653198:9. https://doi.org/10.1155/2014/653198
Raheem A, Wakg WA, Yap YT, Danquah MK, Harun R (2015) Optimization of the microalgae Chlorella vulgaris for syngas production using central composite design. RSC Adv 5(88):71805–71815
Raju SS, Shinoj P, Joshi PK (2009) Sustainable development of biofuels: prospects and challenges. Econ Polit Wkly 65–72
Ray S, Goldar A, Miglani S (2012) The ethanol blending policy in India. Econ Political Wkly 47(1):23–35
Roy K, Sarkar CK, Ghosh CK (2014) Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Appl Nanosci 1–7
Šafařı́k I, Šafařı́ková M (1999) Use of magnetic techniques for the isolation of cells. J Chromatogr B Biomed Sci Appl 722(1):33–53
Šafařík I, Šafaříková M (2002) Magnetic nanoparticles and biosciences. Springer, Vienna, pp 1–23
Šafaříková M, Šafařik I (2001) Immunomagnetic separation of Escherichia coli O26, O111 and O157 from vegetables. Lett Appl Microbiol 33(1):36–39
Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA (2015) Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol 99:4579–4593
Tartaj P, Morales MP, Veintemillas-Verdaguer S, Gonzalez-Carreño T, Serna CJ (2006) Synthesis, properties and biomedical applications of magnetic nanoparticles. Handb Magn Mater 16(5):403–482
Turhan O, Isci A, Mert B, Sakiyan O, Donmez S (2015) Optimization of ethanol production from microfluidized wheat straw by response surface methodology. Prep Biochem Biotechnol 45(8):785–795
Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, Malarkodi C, Sivakavinesan M, Annadurai G (2014) Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg Chem Appl 742346. https://doi.org/10.1155/2014/742346
Wang LH, Hsie MC, Chang CY, Kuo YC, Sang SL, Hsiao HD, Chen HC (1984) Improvement of ethanol productivity from cane molasses by a process using a high yeast cell concentration. ASPAC, Food & Fertilizer Technology Center
Wyman CE, Hinman ND (1990) Ethanol. Appl Biochem Biotechnol 24(1):735–753
Acknowledgements
SI and VP would like to thank ARIBAS and CVM, and SJ would like to acknowledge Sultan Qaboos University for providing the research facility.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ingale, S., Parnandi, V.A., Joshi, S.J. (2019). Bioethanol Production Using Saccharomyces cerevisiae Immobilized in Calcium Alginate–Magnetite Beads and Application of Response Surface Methodology to Optimize Bioethanol Yield. In: Srivastava, N., Srivastava, M., Mishra, P., Upadhyay, S., Ramteke, P., Gupta, V. (eds) Sustainable Approaches for Biofuels Production Technologies. Biofuel and Biorefinery Technologies, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-319-94797-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-94797-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94796-9
Online ISBN: 978-3-319-94797-6
eBook Packages: EnergyEnergy (R0)